Investigation of Surface Modification of Polystyrene by a Direct and Remote Atmospheric-Pressure Plasma Jet Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
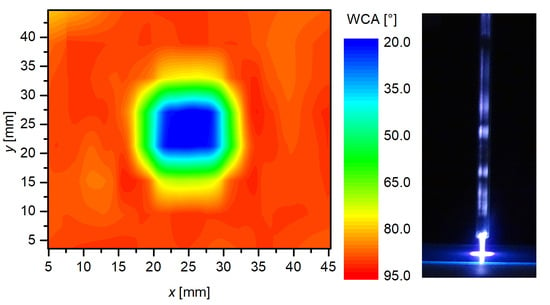
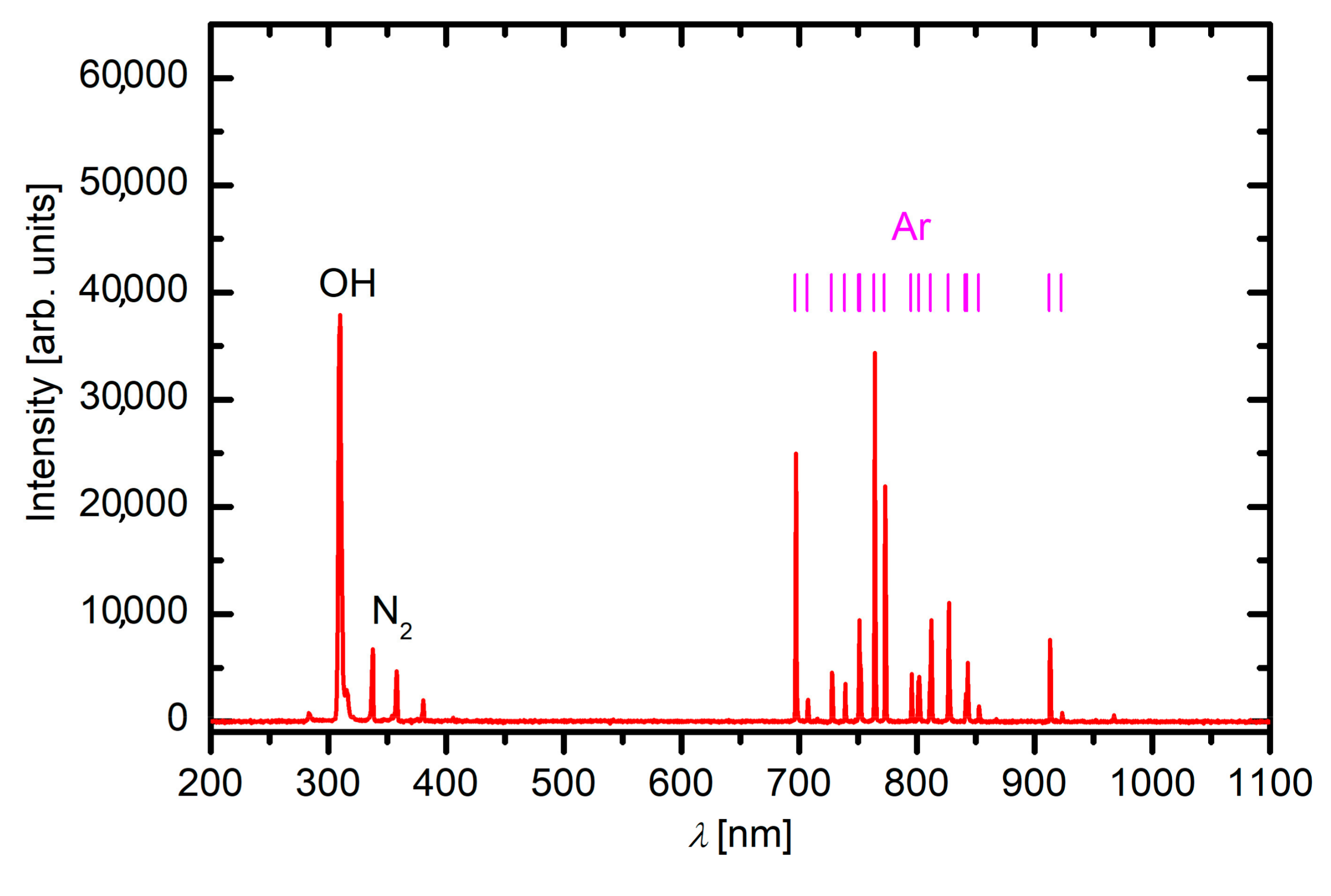
3.1. Plasma Characterization
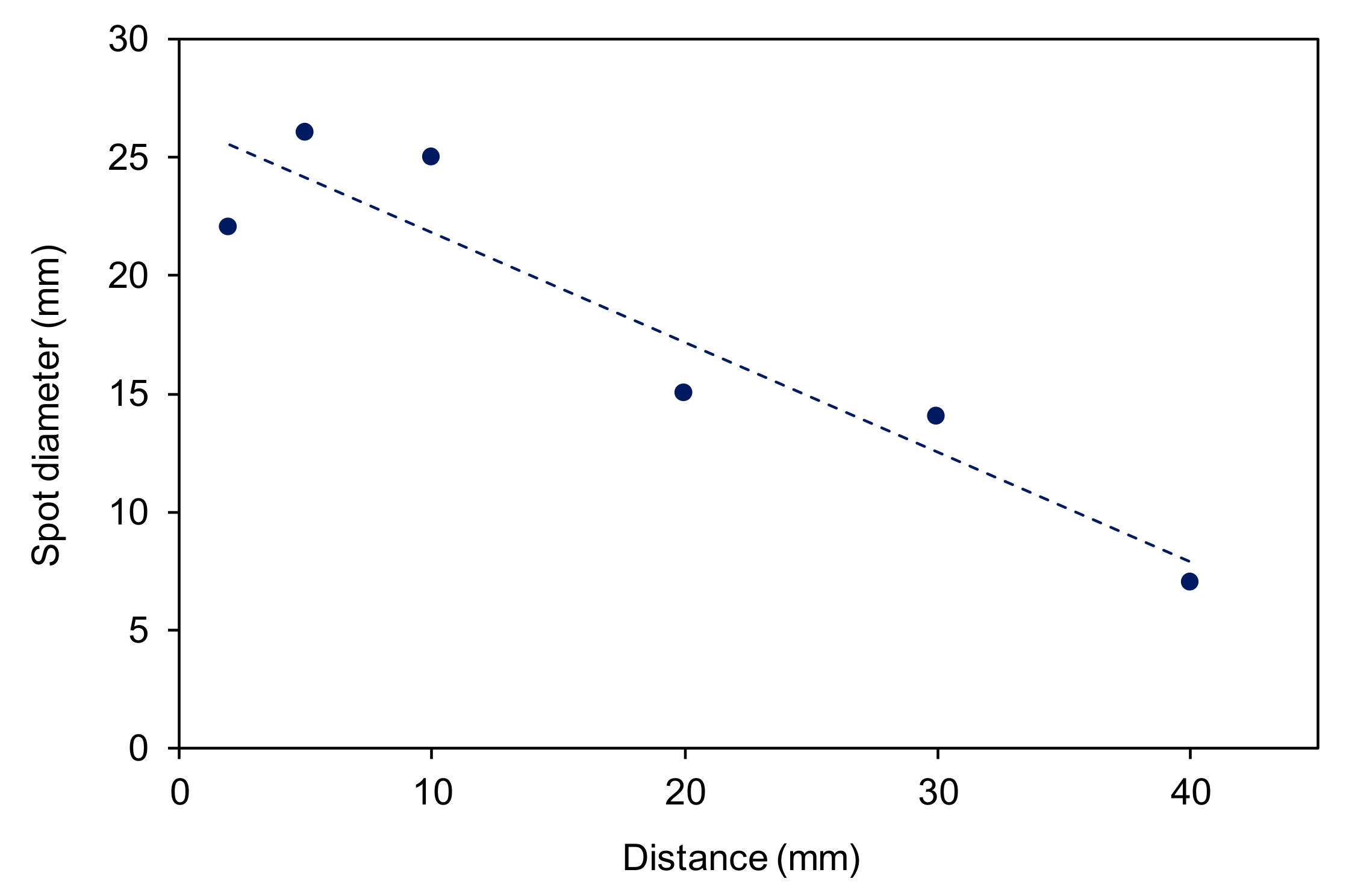
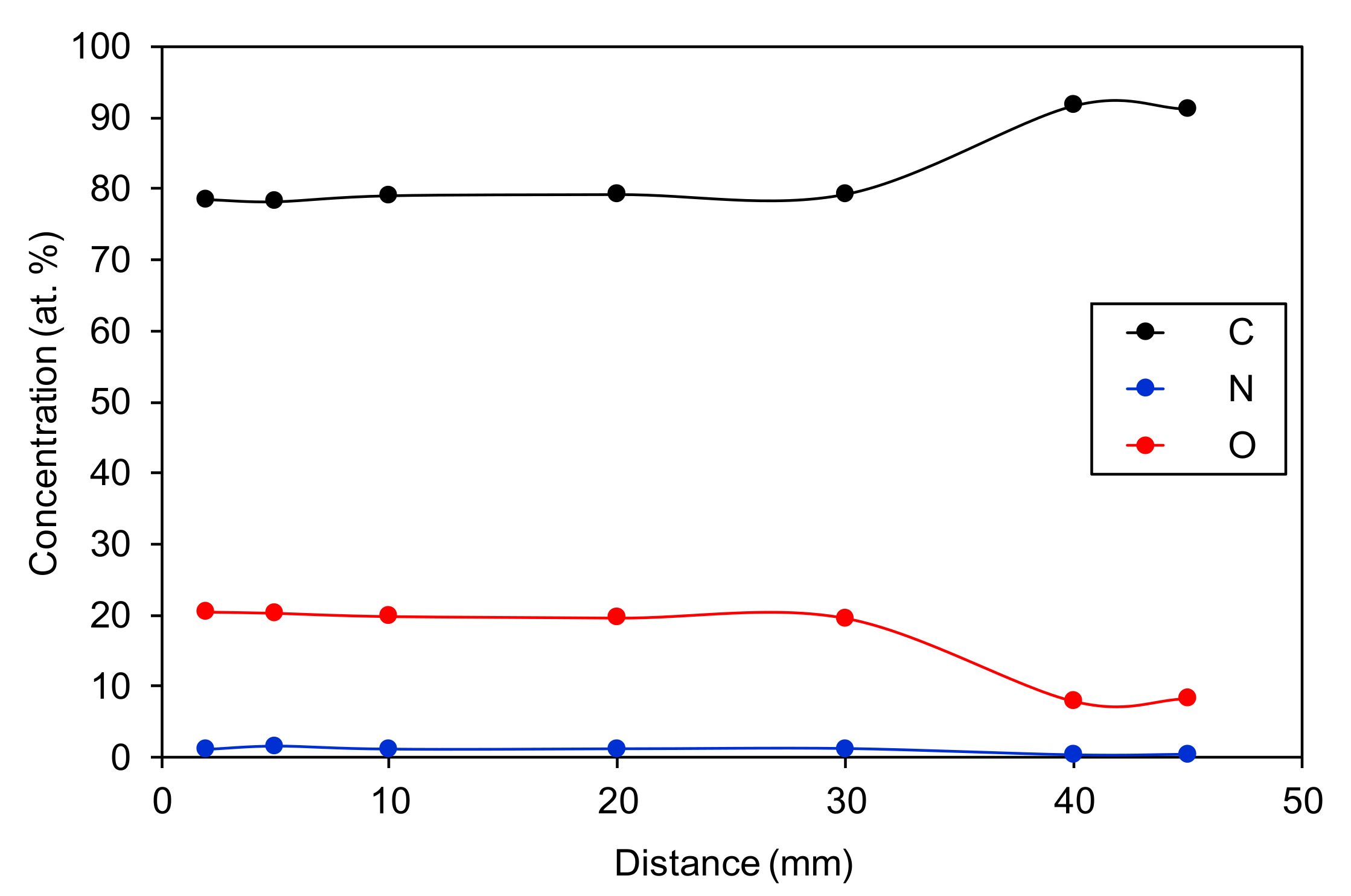
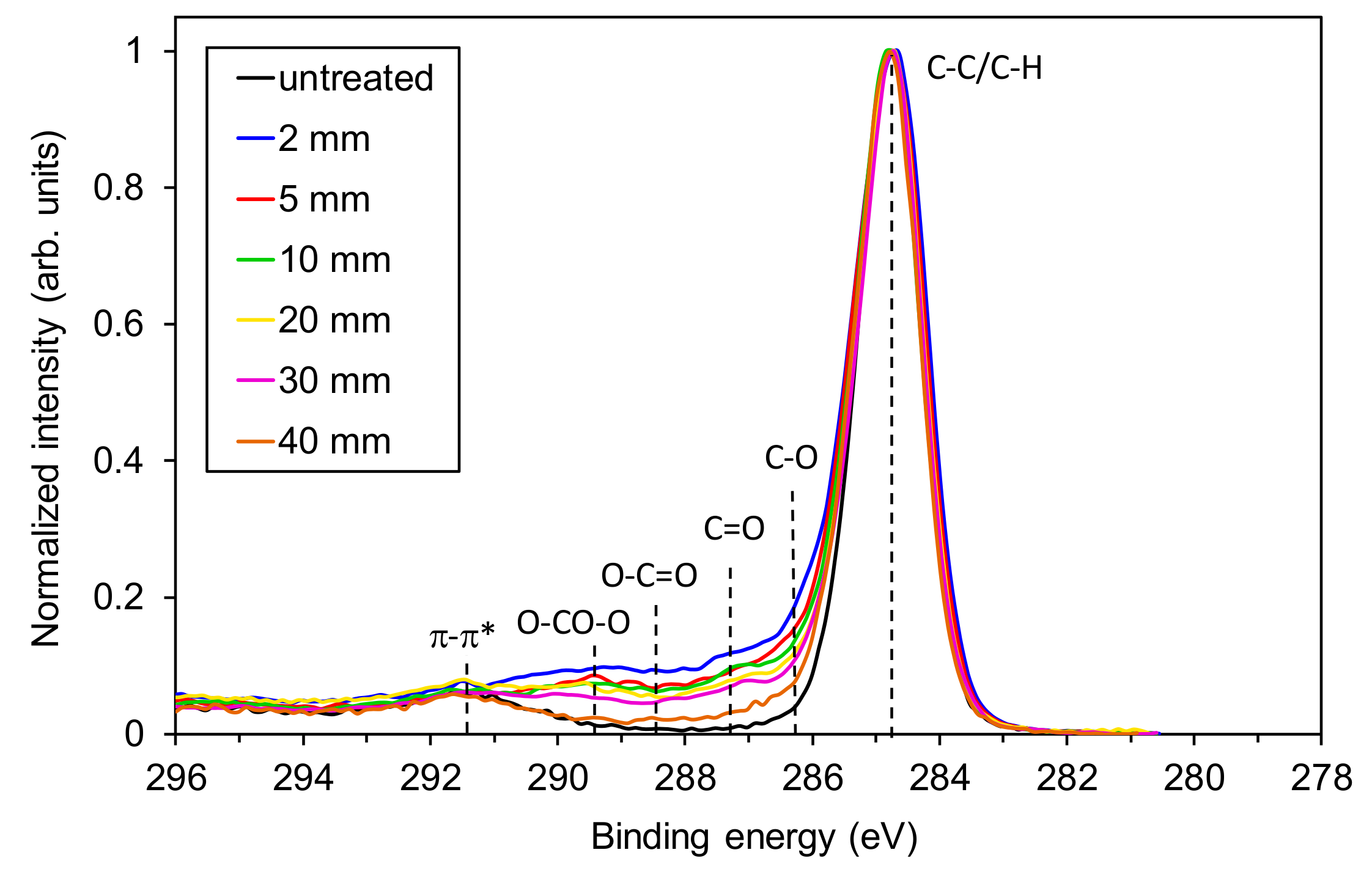
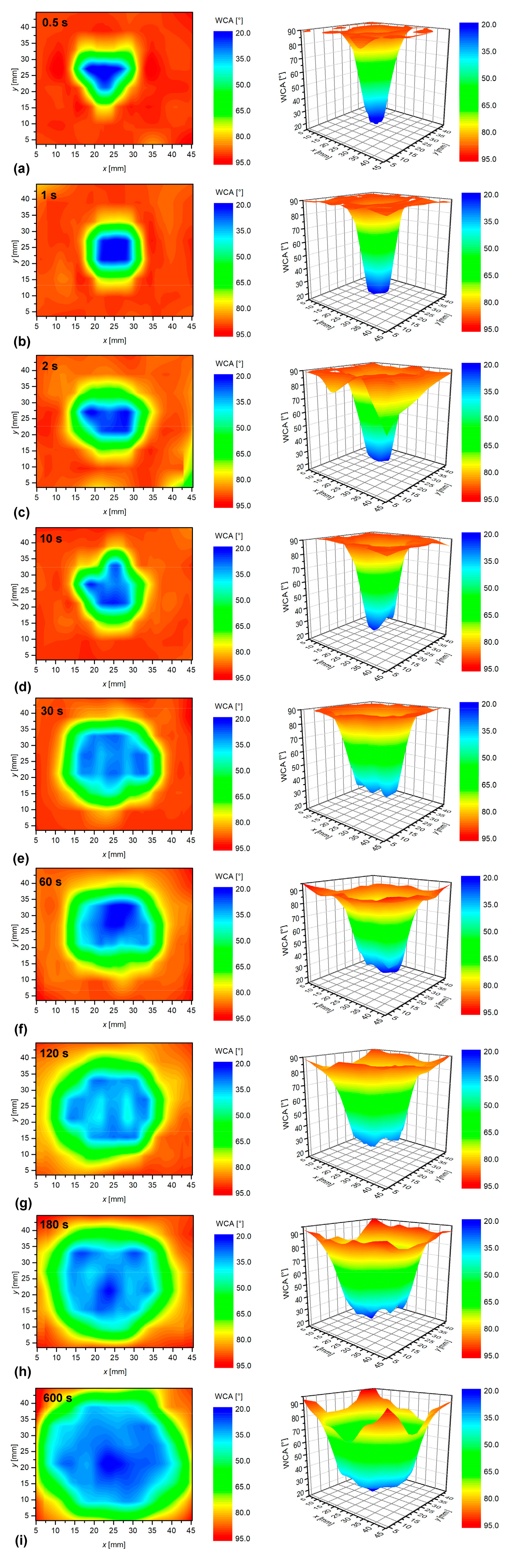
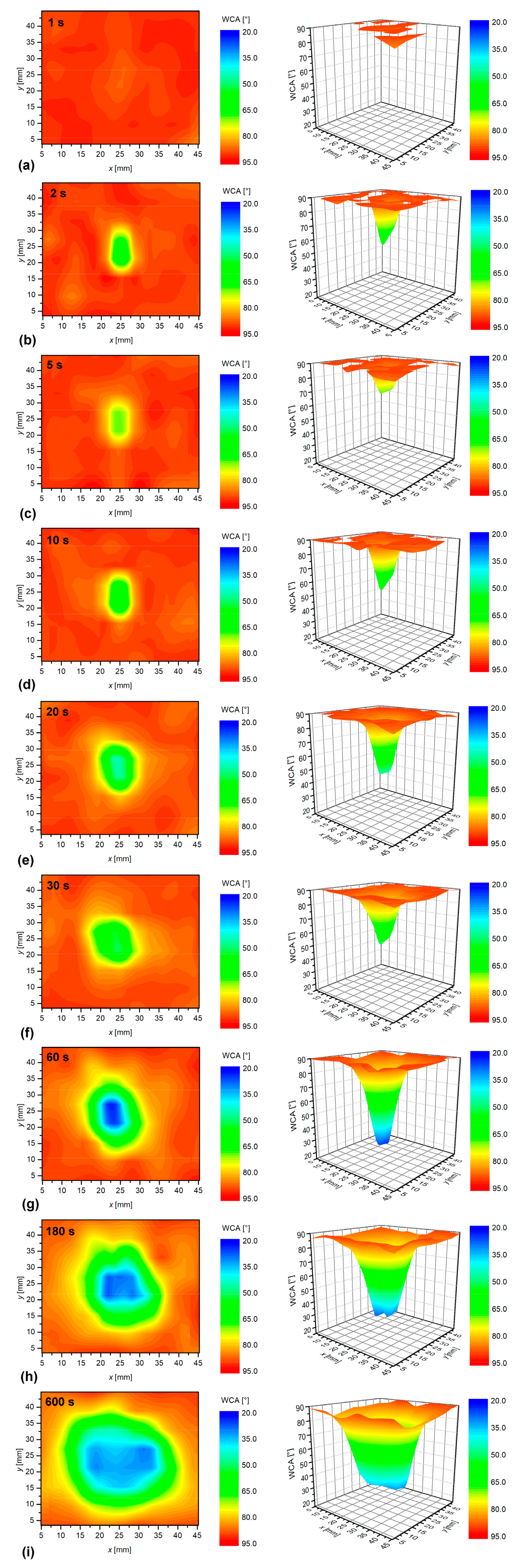
3.2. The Effect of the Sample-to-Nozzle Distance
3.3. Effect of Treatment Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Golda, J.; Biskup, B.; Layes, V.; Winzer, T.; Benedikt, J. Vacuum ultraviolet spectroscopy of cold atmospheric pressure plasma jets. Plasma Process. Polym. 2020. [Google Scholar] [CrossRef]
- Schulz-von der Gathen, V.; Buck, V.; Gans, T.; Knake, N.; Niemi, K.; Reuter, S.; Schaper, L.; Winter, J. Optical diagnostics of micro discharge jets. Contrib. Plasma Phys. 2007, 47, 510–519. [Google Scholar] [CrossRef]
- Guo, H.; Liu, J.; Yang, B.; Chen, X.; Yang, C. Localized etching of polymer films using an atmospheric pressure air microplasma jet. J. Micromech. Microeng. 2014, 25, 015010. [Google Scholar] [CrossRef]
- Szili, E.J.; Al-Bataineh, S.A.; Bryant, P.M.; Short, R.D.; Bradley, J.W.; Steele, D.A. Controlling the spatial distribution of polymer surface treatment using atmospheric-pressure microplasma jets. Plasma Process. Polym. 2011, 8, 38–50. [Google Scholar] [CrossRef]
- Kehrer, M.; Duchoslav, J.; Hinterreiter, A.; Mehic, A.; Stehrer, T.; Stifter, D. Surface functionalization of polypropylene using a cold atmospheric pressure plasma jet with gas water mixtures. Surf. Coat. Technol. 2020, 384, 125170. [Google Scholar] [CrossRef]
- Luan, P.; Knoll, A.J.; Bruggeman, P.J.; Oehrlein, G.S. Plasma–surface interaction at atmospheric pressure: A case study of polystyrene etching and surface modification by Ar/O2 plasma jet. J. Vac. Sci. Technol. A 2017, 35, 05C315. [Google Scholar] [CrossRef]
- Luan, P.; Kondeti, V.S.S.K.; Knoll, A.J.; Bruggeman, P.J.; Oehrlein, G.S. Effect of water vapor on plasma processing at atmospheric pressure: Polymer etching and surface modification by an Ar/H2O plasma jet. J. Vac. Sci. Technol. A 2019, 37, 031305. [Google Scholar] [CrossRef]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef]
- Bartis, E.A.; Luan, P.; Knoll, A.J.; Hart, C.; Seog, J.; Oehrlein, G.S. Polystyrene as a model system to probe the impact of ambient gas chemistry on polymer surface modifications using remote atmospheric pressure plasma under well-controlled conditions. Biointerphases 2015, 10, 029512. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Evolution of the surface wettability of PET polymer upon treatment with an atmospheric-pressure plasma jet. Polymers 2020, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Onyshchenko, I.; Yu Nikiforov, A.; De Geyter, N.; Morent, R. Local analysis of PET surface functionalization by an atmospheric pressure plasma jet. Plasma Process. Polym. 2015, 12, 466–476. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wagner, R.; Kostov, G.K. Study of modified area of polymer samples exposed to a He atmospheric pressure plasma jet using different treatment conditions. Polymers 2020, 12, 1028. [Google Scholar] [CrossRef] [PubMed]
- Ellerweg, D.; Benedikt, J.; von Keudell, A.; Knake, N.; Schulz-von der Gathen, V. Characterization of the effluent of a He/O2 microscale atmospheric pressure plasma jet by quantitative molecular beam mass spectrometry. New J. Phys. 2010, 12, 013021. [Google Scholar] [CrossRef]
- Sousa, J.S.; Niemi, K.; Cox, L.J.; Algwari, Q.T.; Gans, T.; O’Connell, D. Cold atmospheric pressure plasma jets as sources of singlet delta oxygen for biomedical applications. J. Appl. Phys. 2011, 109, 123302. [Google Scholar] [CrossRef]
- Reuter, S.; Niemi, K.; Schulz-von der Gathen, V.; Döbele, H.F. Generation of atomic oxygen in the effluent of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2009, 18, 015006. [Google Scholar] [CrossRef]
- Reuter, S.; Winter, J.; Schmidt-Bleker, A.; Schroeder, D.; Lange, H.; Knake, N.; Schulz-von der Gathen, V.; Weltmann, K.D. Atomic oxygen in a cold argon plasma jet: TALIF spectroscopy in ambient air with modelling and measurements of ambient species diffusion. Plasma Sources Sci. Technol. 2012, 21, 024005. [Google Scholar] [CrossRef]
- Jiang, J.; Luo, Y.; Moldgy, A.; Aranda Gonzalvo, Y.; Bruggeman, P.J. Absolute spatially and time-resolved O, O3, and air densities in the effluent of a modulated RF-driven atmospheric pressure plasma jet obtained by molecular beam mass spectrometry. Plasma Process. Polym. 2019, e1900163. [Google Scholar] [CrossRef]
- Schröter, S.; Wijaikhum, A.; Gibson, A.R.; West, A.; Davies, H.L.; Minesi, N.; Dedrick, J.; Wagenaars, E.; de Oliveira, N.; Nahon, L.; et al. Chemical kinetics in an atmospheric pressure helium plasma containing humidity. Phys. Chem. Chem. Phys. 2018, 20, 24263–24286. [Google Scholar] [CrossRef]
- Fuh, C.A.; Clark, S.M.; Wu, W.; Wang, C. Electronic ground state OH(X) radical in a low-temperature atmospheric pressure plasma jet. J. Appl. Phys. 2016, 120, 163303. [Google Scholar] [CrossRef]
- Sushkov, V.; Herrendorf, A.-P.; Hippler, R. Metastable argon atom density in complex argon/acetylene plasmas determined by means of optical absorption and emission spectroscopy. J. Phys. D Appl. Phys. 2016, 49, 425201. [Google Scholar] [CrossRef]
- Birer, Ö. Reactivity zones around an atmospheric pressure plasma jet. Appl. Surf. Sci. 2015, 354, 420–428. [Google Scholar] [CrossRef]
- Nikiforov, A.Y.; Sarani, A.; Leys, C. The influence of water vapor content on electrical and spectral properties of an atmospheric pressure plasma jet. Plasma Sources Sci. Technol. 2011, 20, 015014. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/H2O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17, 063504. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.J.; Joh, H.M.; Chung, T.H.; Bae, S.H.; Leem, S.H. Optical and electrical characterization of an atmospheric pressure microplasma jet with a capillary electrode. Phys. Plasmas 2010, 17, 033502. [Google Scholar] [CrossRef]
- Olabanji, O.T.; Bradley, J.W. Side-on surface modification of polystyrene with an atmospheric pressure microplasma jet. Plasma Process. Polym. 2012, 9, 929–936. [Google Scholar] [CrossRef]
- Dowling, D.P.; O’Neill, F.T.; Langlais, S.J.; Law, V.J. Influence of DC pulsed atmospheric pressure plasma jet processing conditions on polymer activation. Plasma Process. Polym. 2011, 8, 718–727. [Google Scholar] [CrossRef]
- Fricke, K.; Duske, K.; Quade, A.; Nebe, B.; Schroder, K.; Weltmann, K.-D.; von Woedtke, T. Comparison of nonthermal plasma processes on the surface properties of polystyrene and their impact on cell growth. IEEE Trans. Plasma Sci. 2012, 40, 2970–2979. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Kovac, J.; Mozetic, M. Initial stages in functionalization of polystyrene upon treatment with oxygen plasma late flowing afterglow. Plasma Sources Sci. Technol. 2018, 27, 094005. [Google Scholar] [CrossRef]
- Vesel, A. Modification of polystyrene with a highly reactive cold oxygen plasma. Surf. Coat. Technol. 2010, 205, 490–497. [Google Scholar] [CrossRef]
- Vesel, A.; Primc, G.; Zaplotnik, R.; Mozetič, M. Applications of highly non-equilibrium low-pressure oxygen plasma for treatment of polymers and polymer composites on an industrial scale. Plasma Phys. Control. Fusion 2020, 62, 024008. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A.; Primc, G. Investigation of Surface Modification of Polystyrene by a Direct and Remote Atmospheric-Pressure Plasma Jet Treatment. Materials 2020, 13, 2435. https://doi.org/10.3390/ma13112435
Vesel A, Primc G. Investigation of Surface Modification of Polystyrene by a Direct and Remote Atmospheric-Pressure Plasma Jet Treatment. Materials. 2020; 13(11):2435. https://doi.org/10.3390/ma13112435
Chicago/Turabian StyleVesel, Alenka, and Gregor Primc. 2020. "Investigation of Surface Modification of Polystyrene by a Direct and Remote Atmospheric-Pressure Plasma Jet Treatment" Materials 13, no. 11: 2435. https://doi.org/10.3390/ma13112435
APA StyleVesel, A., & Primc, G. (2020). Investigation of Surface Modification of Polystyrene by a Direct and Remote Atmospheric-Pressure Plasma Jet Treatment. Materials, 13(11), 2435. https://doi.org/10.3390/ma13112435