Application of Pulsed Electric Field in Antifouling Treatment of Sodium Gluconate Mother Liquor by Electrodialysis
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Setup
2.3. Experimental Operational Conditions
2.4. Analytical Methods
2.4.1. Measurement of Stack Resistance
2.4.2. Production Energy Consumption and Salt Flux
2.4.3. Current Density
2.4.4. Conductivity Control
2.5. Fouling Analysis
2.5.1. Membrane Electric Resistance
2.5.2. Scanning Electron Microscope
3. Results and Discussion
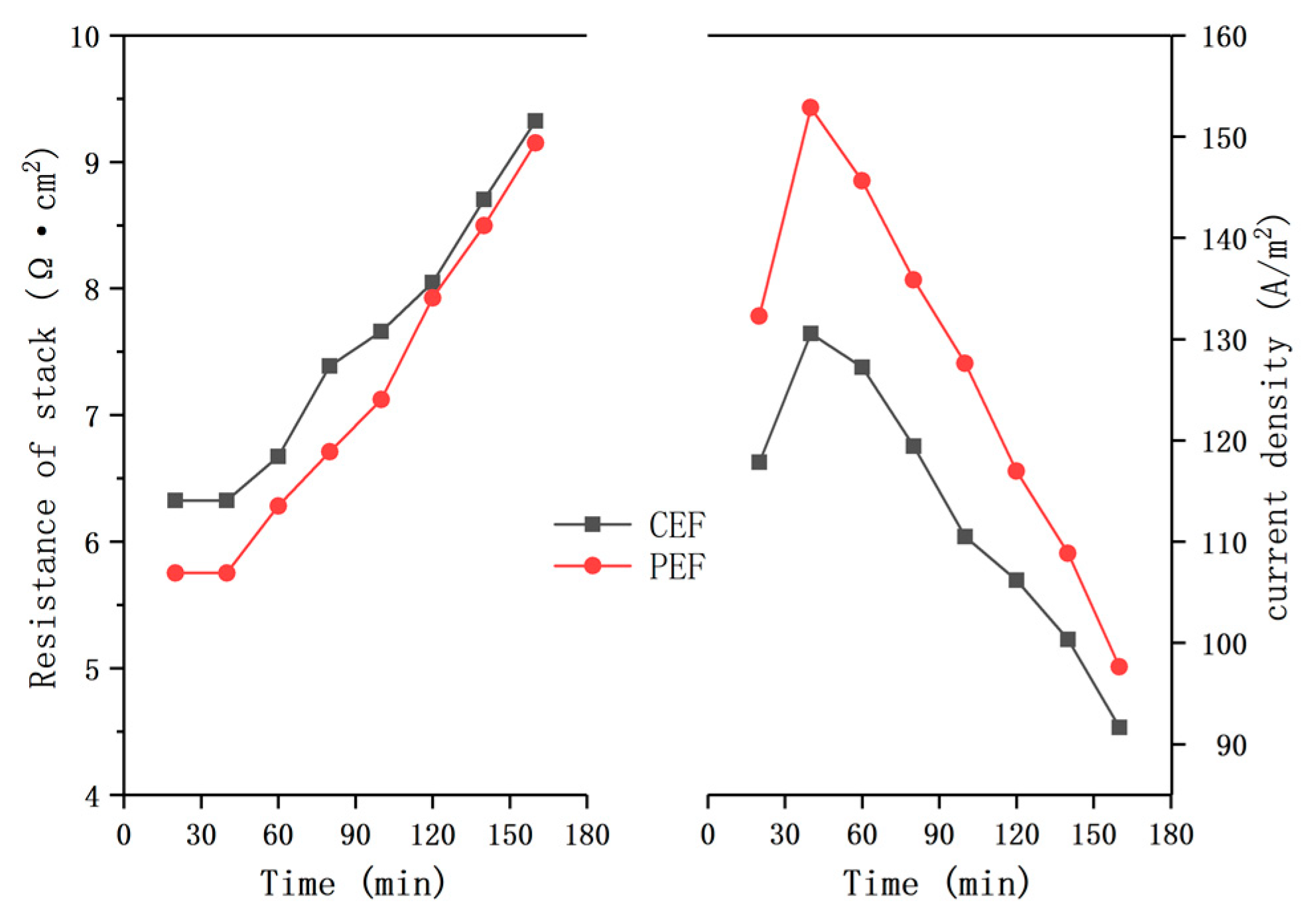
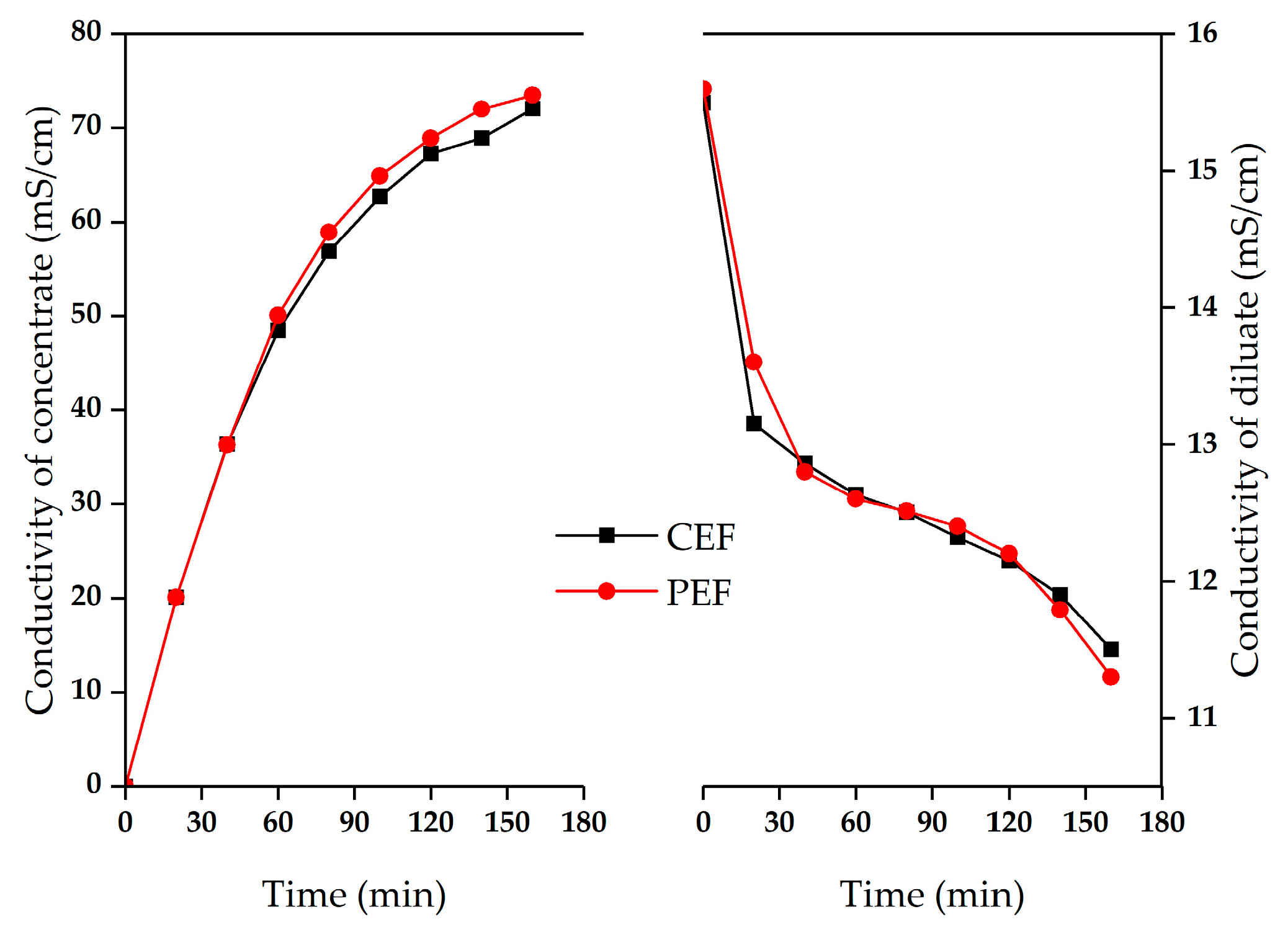
3.1. Effect of Two Electric Fields on ED Treatment of Mother Liquor
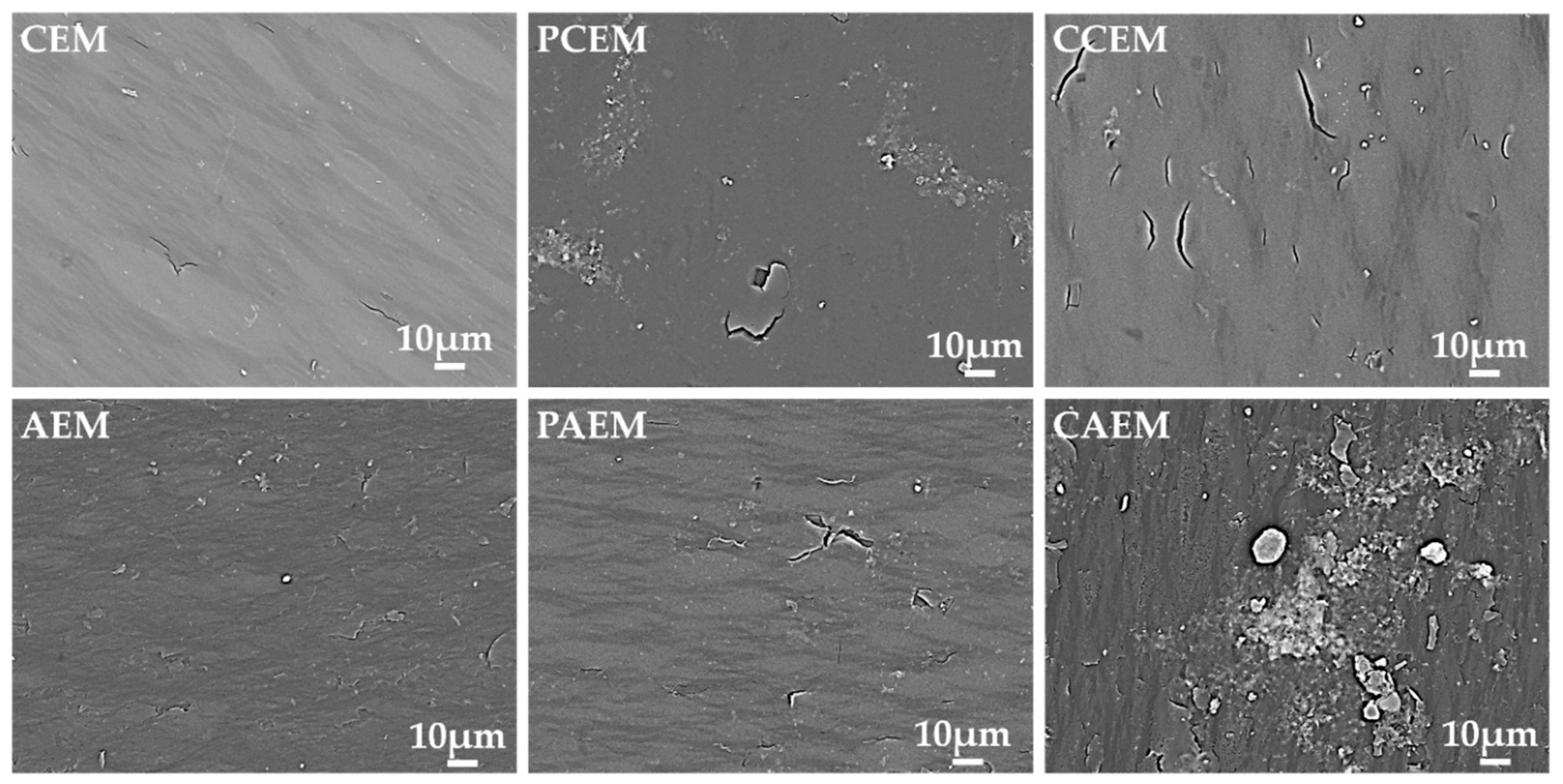
3.2. Fouling Phenomena in ED of Sodium Gluconate Mother Liquor
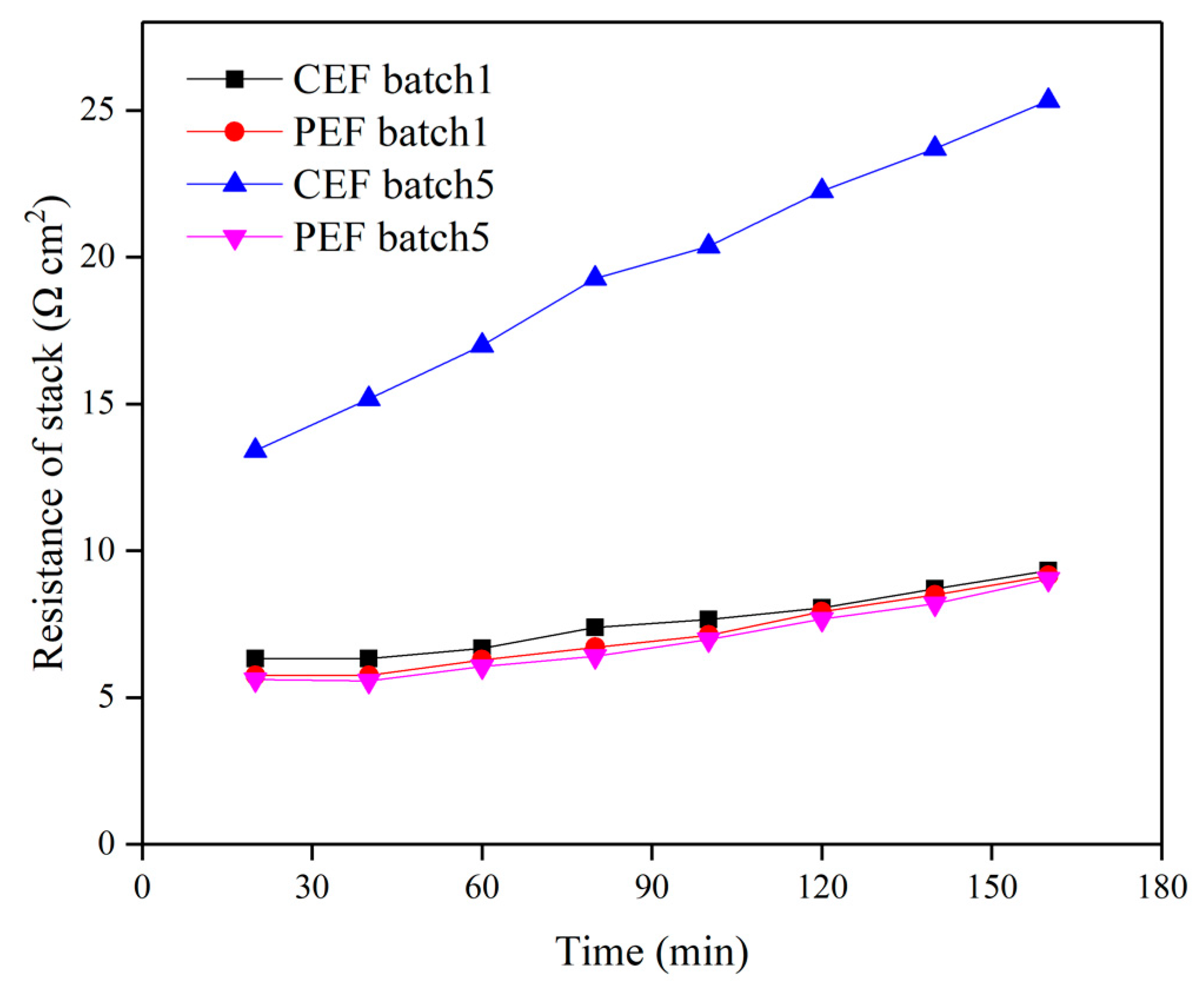
3.3. Pulse ED Treatment of Sodium Gluconate Mother Liquor to Reduce Fouling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ED | Electrodialysis |
| PEF | Pulsed electric field |
| CEF | Conventional electric field |
| AEM | Anion exchange membrane |
| CEM | Cation exchange membrane |
| CAEM | Anion exchange membranes under conventional electric field conditions |
| PAEM | Anion exchange membranes under pulsed electric field conditions |
| CCEM | Cation exchange membranes under conventional electric field conditions |
| PCEM | Cation exchange membranes under pulsed electric field conditions |
| SEM | Scanning electron microscopy |
| IEC | Ion exchange capacity |
References
- Zhang, T.; Zhao, X.; Zhang, J.; Tian, Y.; Liu, J. The composition analysis of the residual sugar from the sodium gluconate fermentation by Aspergillus niger. China Brew. 2016, 34, 88–91. [Google Scholar]
- Lu, F.; Wang, Z.; Zhao, W.; Chu, J.; Zhuang, Y. A simple novel approach for real-time monitoring of sodium gluconate production by on-line physiological parameters in batch fermentation by Aspergillus niger. Bioresour. Technol. 2016, 202, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Zheng, C.; Mondal, A.N.; Hossain, M.M.; Wu, B.; Emmanuel, K.; Liang, W.; Xu, T. Preparation of anion exchange membranes from BPPO and dimethylethanolamine for electrodialysis. Desalination 2017, 402, 10–18. [Google Scholar] [CrossRef]
- Han, L.; Galier, S.; Balmann, R.D. Transfer of neutral organic solutes during desalination by electrodialysis: Influence of the salt composition. J. Membr. Sci. 2016, 511, 207–218. [Google Scholar] [CrossRef]
- Lei, C.; Li, Z.; Gao, Q.; Fu, R.; Wang, W.; Li, Q.; Liu, Z. Comparative study on the production of gluconic acid by electrodialysis and bipolar membrane electrodialysis: Effects of cell configurations. J. Membr. Sci. 2020, 608, 118192. [Google Scholar] [CrossRef]
- Wang, W.; Fu, R.; Liu, Z.; Wang, H. Low-resistance anti-fouling ion exchange membranes fouled by organic foulants in electrodialysis. Desalination 2017, 417, 1–8. [Google Scholar] [CrossRef]
- Lee, H.-J.; Oh, S.J.; Moon, S.-H. Removal of hardness in fermentation broth by electrodialysis. J. Chem. Technol. Biot. 2002, 77, 1005–1012. [Google Scholar] [CrossRef]
- Li, H.; Xia, H.; Mei, Y. Modeling organic fouling of reverse osmosis membrane: From adsorption to fouling layer formation. Desalination 2016, 386, 25–31. [Google Scholar] [CrossRef]
- Nohwa, L.; Gary, A.; Jean-Philippe, C.; Herve, B. Identification and understanding of fouling in low-pressure membrane (MF/UF) filtration by natural organic matter (NOM). Water Res. 2004, 38, 4511–4523. [Google Scholar]
- Lindstrand, V.; Jönsson, A.S.; Sundström, G. Organic fouling of electrodialysis membranes with and without applied voltage. Desalination 2000, 130, 73–84. [Google Scholar] [CrossRef]
- Tanaka, N.; Nagase, M.; Higa, M. Organic fouling behavior of commercially available hydrocarbon-based anion-exchange membranes by various organic-fouling substances. Desalination 2012, 296, 81–86. [Google Scholar] [CrossRef]
- Lee, H.-J.; Oh, S.-J.; Moon, S.-H. Recovery of ammonium sulfate from fermentation waste by electrodialysis. Water Res. 2003, 37, 1091–1099. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, S.H.; Tsai, S.P. Effects of pulsed electric fields on membrane fouling in electrodialysis of NaCl solution containing humate. Sep. Purif. Technol. 2002, 27, 89–95. [Google Scholar] [CrossRef]
- Merkel, A.; Ashrafi, A.M. An Investigation on the Application of Pulsed Electrodialysis Reversal in Whey Desalination. Int. J. Mol. Sci. 2019, 20, 1918. [Google Scholar] [CrossRef] [PubMed]
- Mishchuk, N.A.; Koopal, L.K.; Gonzalez-Caballero, F. Intensification of electrodialysis by applying a non-stationary electric field. Colloid. Surf. A 2001, 176, 195–212. [Google Scholar] [CrossRef]
- Malek, P.; Ortiz, J.M.; Richards, B.S.; Schäfer, A.I. Electrodialytic removal of NaCl from water: Impacts of using pulsed electric potential on ion transport and water dissociation phenomena. J. Membr. Sci. 2013, 435, 99–109. [Google Scholar] [CrossRef]
- Sistat, P.; Huguet, P.; Ruiz, B.; Pourcelly, G.; Mareev, S.A.; Nikonenko, V.V. Effect of pulsed electric field on electrodialysis of a NaCl solution in sub-limiting current regime. Electrochim. Acta 2015, 164, 267–280. [Google Scholar] [CrossRef]
- Ruiz, B.; Sistat, P.; Huguet, P.; Pourcelly, G.; Arayafarias, M.; Bazinet, L. Application of relaxation periods during electrodialysis of a casein solution: Impact on anion-exchange membrane fouling. J. Membr. Sci. 2007, 287, 41–50. [Google Scholar] [CrossRef]
- Mishchuk, N.A.; Verbich, S.V.; Gonzales-Caballero, F. Concentration Polarization and Specific Selectivity of Membranes in Pulse Mode. Colloid J. 2001, 63, 586–595. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Pourcelly, G.; Bazinet, L. Impact of pulsed electric field on electrodialysis process performance and membrane fouling during consecutive demineralization of a model salt solution containing a high magnesium/calcium ratio. J. Colloid. Interface Sci. 2011, 361, 79–89. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pourcelly, G.; Bazinet, L. Intensification of demineralization process and decrease in scaling by application of pulsed electric field with short pulse/pause conditions. J. Membr. Sci. 2014, 468, 389–399. [Google Scholar] [CrossRef]
- Andreeva, M.A.; Gil, V.V.; Pismenskaya, N.D.; Dammak, L.; Kononenko, N.A.; Larchet, C.; Grande, D.; Nikonenko, V.V. Mitigation of membrane scaling in electrodialysis by electroconvection enhancement, pH adjustment and pulsed electric field application. J. Membr. Sci. 2018, 549, 129–140. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, P.; Cong, W. Cation-exchange membrane fouling and cleaning in bipolar membrane electrodialysis of industrial glutamate production wastewater. Sep. Purif. Technol. 2011, 79, 103–113. [Google Scholar] [CrossRef]
- Casademont, C.; Farias, M.A.; Pourcelly, G.; Bazinet, L. Impact of electrodialytic parameters on cation migration kinetics and fouling nature of ion-exchange membranes during treatment of solutions with different magnesium/calcium ratios. J. Membr. Sci. 2008, 325, 570–579. [Google Scholar] [CrossRef]
- Casademont, C.; Sistat, P.; Ruiz, B.; Pourcelly, G.; Bazinet, L. Electrodialysis of model salt solution containing whey proteins: Enhancement by pulsed electric field and modified cell configuration. J. Membr. Sci. 2009, 328, 238–245. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Positive Impact of Pulsed Electric Field on Lactic Acid Removal, Demineralization and Membrane Scaling during Acid Whey Electrodialysis. Int. J. Mol. Sci. 2019, 20, 797. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, L.; Gaudreau, H.; Lavigne, D.; Martin, N. Partial demineralization of maple sap by electrodialysis: Impact on syrup sensory and physicochemical characteristics. J. Sci. Food Agric. 2007, 87, 1691–1698. [Google Scholar] [CrossRef]
- Pérez, A.; Andrés, L.J.; Alvarez, R.; Coca, J.; Hill, C.G., Jr. Electrodialysis of whey permeates and retentates 372 obtained by ultrafiltration. J. Food Process Eng. 1994, 17, 177–190. [Google Scholar] [CrossRef]
- Lee, H.J.; Moon, S.H. Enhancement of electrodialysis performances using pulsing electric fields during extended period operation. J. Colloid Interf. Sci. 2005, 287, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid. Interface 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Bonnélye, V.; Guey, L.; Castillo, J.D. UF/MF as RO pre-treatment: The real benefit. Desalination 2008, 222, 59–65. [Google Scholar] [CrossRef]





| Membrane Type | Thickness/Wet (µm) | IEC a (Ion Exchange Capacity, meq·g−1) | Area Resistance b (Ω·cm2) | Water Uptake c (%) | Transport Number d |
|---|---|---|---|---|---|
| AEM | 40–50 | 0.90–1.10 | ≤2.5 | 15–20 | ≥0.98 |
| CEM | 40–50 | 0.90–1.10 | ≤3.3 | 15–20 | ≥0.97 |
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | |
|---|---|---|---|---|---|
| Current efficiency | 46.5% | 48.2% | 51.7% | 39.7% | 37.0% |
| Average current density (A/m2) | 113.3 | 114.6 | 116.1 | 69.5 | 49.6 |
| Demineralization rate | 25.80% | 24.58% | 25.67% | 12.90% | 5.80% |
| Production energy consumption (kWh/t) | 185.1 | 178.7 | 165.4 | 226.9 | 252.4 |
| Resistance of the membrane stack (Ω·cm2) | 9.3 | 9.2 | 9.1 | 21.0 | 25.3 |
| Electric Resistance (Ω cm2) | AEM | CEM | |
|---|---|---|---|
| Conventional electric field (CEF) | R01 | 2.34 | 3.24 |
| R12 | 4.24 | 3.17 | |
| ΔR14 | 81.20% | −2.16% | |
| R23 | 2.98 | 3.15 | |
| ΔR25 | 27.35% | −2.78% | |
| Pulsed electric field (PEF) | R0 | 2.45 | 3.21 |
| R1 | 3.01 | 3.13 | |
| ΔR1 | 22.86% | −2.94% | |
| R2 | 2.96 | 3.09 | |
| ΔR2 | 20.81% | −3.74% | |
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | Batch 5 | |
|---|---|---|---|---|---|
| Current efficiency | 42.6% | 46.4% | 46.3% | 47.5% | 46.8% |
| Average current density (A/m2) | 127.5 | 128.2 | 129.7 | 130.4 | 131.3 |
| Demineralization rate | 28.60% | 27.09% | 26.4% | 24.51% | 19.35% |
| Production energy consumption (kWh/t) | 199.1 | 194.0 | 195.6 | 190.0 | 193.5 |
| Resistance of the membrane stack (Ω·cm2) | 9.2 | 9.2 | 9.1 | 9.0 | 9.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Li, Z.; Lei, C.; Fu, R.; Wang, W.; Li, Q.; Liu, Z. Application of Pulsed Electric Field in Antifouling Treatment of Sodium Gluconate Mother Liquor by Electrodialysis. Materials 2020, 13, 2501. https://doi.org/10.3390/ma13112501
Gao Q, Li Z, Lei C, Fu R, Wang W, Li Q, Liu Z. Application of Pulsed Electric Field in Antifouling Treatment of Sodium Gluconate Mother Liquor by Electrodialysis. Materials. 2020; 13(11):2501. https://doi.org/10.3390/ma13112501
Chicago/Turabian StyleGao, Qi, Zichao Li, Chunxiao Lei, Rongqiang Fu, Wei Wang, Qun Li, and Zhaoming Liu. 2020. "Application of Pulsed Electric Field in Antifouling Treatment of Sodium Gluconate Mother Liquor by Electrodialysis" Materials 13, no. 11: 2501. https://doi.org/10.3390/ma13112501
APA StyleGao, Q., Li, Z., Lei, C., Fu, R., Wang, W., Li, Q., & Liu, Z. (2020). Application of Pulsed Electric Field in Antifouling Treatment of Sodium Gluconate Mother Liquor by Electrodialysis. Materials, 13(11), 2501. https://doi.org/10.3390/ma13112501





