Magnesium Oxychloride Cement Composites with Silica Filler and Coal Fly Ash Admixture
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
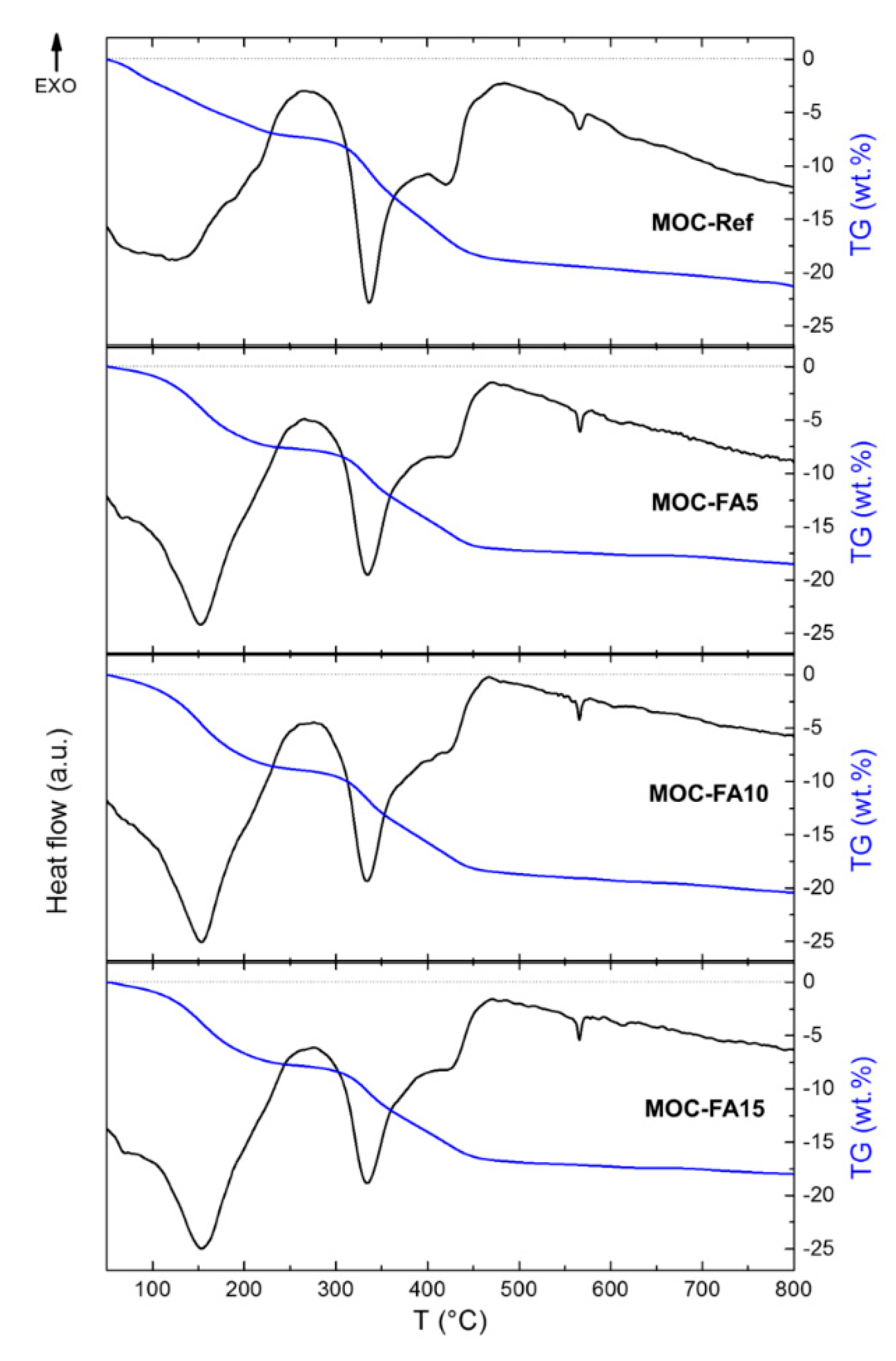
- The main precipitated phases in hardened composites were magnesium hydroxide (brucite), MOC Phase 5 and iowaite;
- The use of FA led to a significant decrease in porosity and microstructure refinement compared to the control material with silica sand as only filler;
- For 5% replacement of silica sand with FA the spread diameter slightly increased; the workability of composites with a higher dosage of FA was slightly worse than that measured for control mixture MOC-Ref;
- The mechanical resistance of MOC-FA composites was high; the compressive strength was for 5% silica sand substitution even higher than that of the reference composite;
- The water transport and storage were greatly reduced by the use of FA in composite mixes;
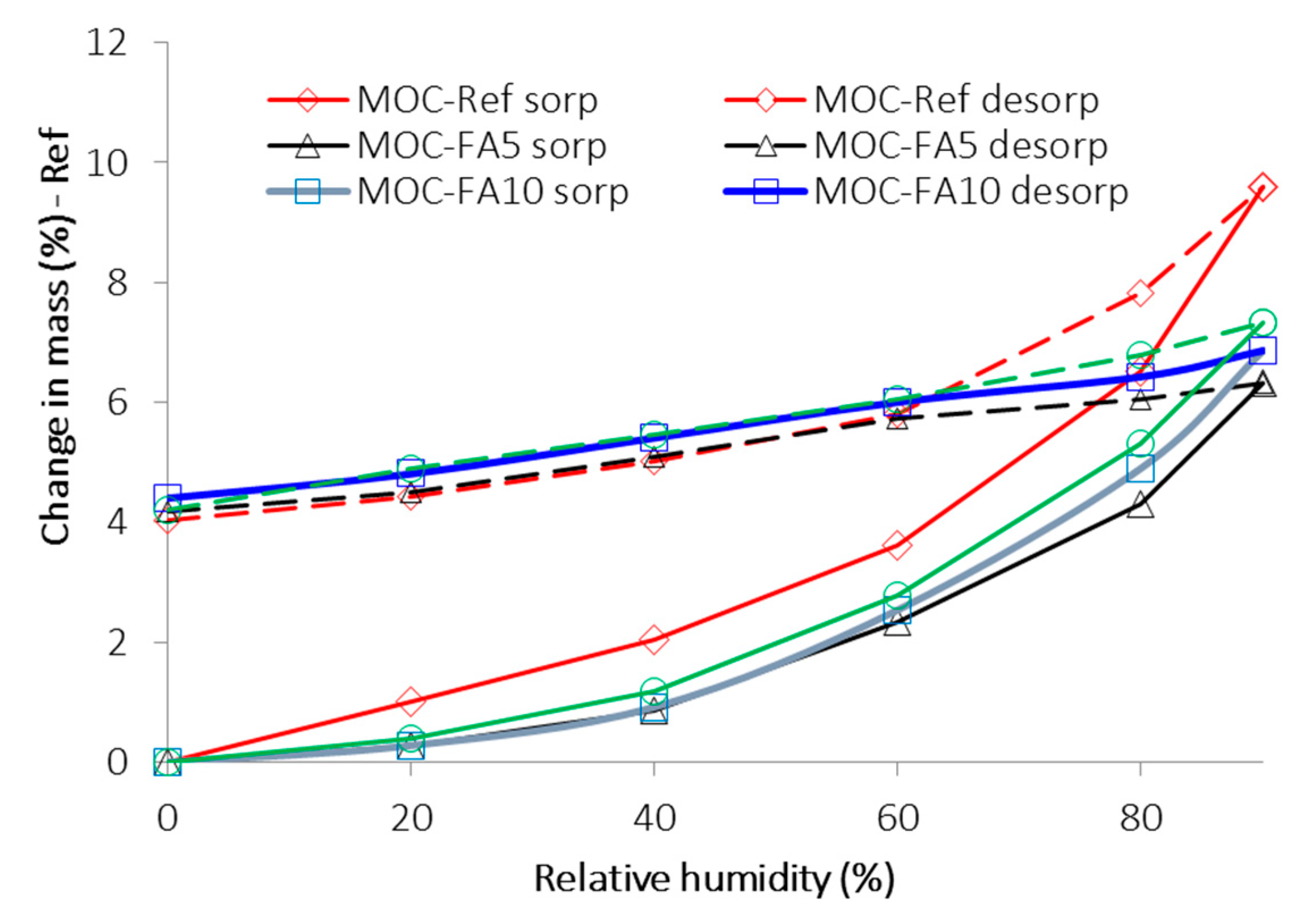
- The water vapor sorption capacity of materials with FA decreased compared to reference material; contrary to that all tested composites yielded similar and high hysteresis in desorption;
- Heat transport and accumulation in designed composites were high as a result of porosity and the thermal behavior of incorporated FA.
Author Contributions
Funding
Conflicts of Interest
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Bhagatg Singh, G.V.P.; Subramaniam, K.V.L. Production and characterization of low-energy Portland composite cement from post-industrial waste. J. Clean. Prod. 2019, 239, 118024. [Google Scholar] [CrossRef]
- Lippiatt, N.; Ling, T.Ch.; Pan, S.Y. Towards carbon-neutral construction materials: Carbonation of cement-based materials and the future perspective. J. Build. Eng. 2020, 28, 101062. [Google Scholar] [CrossRef]
- Giesekam, J.; Barrett, J.R.; Taylor, P. Construction sector views on low carbon building materials. Build. Res. Inf. 2016, 44, 423–444. [Google Scholar] [CrossRef]
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Provis, J.L.; Tsang, D.C.W.; Poon, Ch.S. Accelerated carbonation of reactive MgO and Portland cement blends under flowing CO2 gas. Cem. Concr. Compos. 2020, 106, 103489. [Google Scholar] [CrossRef]
- Unluer, C. Investigating the Carbonation and Mechanical Performance of Reactive MgO Cement Based Concrete Mixes. Nano Hybrids Compos. 2018, 19, 23–33. [Google Scholar] [CrossRef]
- Tang, S.; Hu, Y.; Ren, W.; Yu, P.; Huang, Q.; Qi, X.; Li, Y.; Chen, E. Modeling on the hydration and leaching of eco-friendly magnesium oxychloride cement paste at the microscale. Constr. Build. Mater. 2019, 204, 684–690. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Accelerated carbonation — A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Pu, L.; Unluer, C. Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. Constr. Build. Mater. 2016, 120, 349–363. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Lauermannová, A.; Antončík, F.; Pavlíková, M.; Pavlík, Z.; Sedmidubský, D. Carbon Dioxide Uptake by MOC-Based Materials. App. Sci. 2020, 10, 2254. [Google Scholar] [CrossRef] [Green Version]
- Vandeperre, L.J.; Al-Tabbaa, A. Accelerated carbonation of reactive MgO cements. Adv. Cem. Res. 2007, 19, 67–79. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Francis, P.S. Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cem. Concr. Compos. 2017, 78, 97–107. [Google Scholar] [CrossRef]
- Sorel, S. On a New Magnesium Cement. CR Acad. Sci. 1867, 65, 102–104. [Google Scholar]
- Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z.; Pavlíková, M. Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg(OH)2∙MgCl2∙8H2O. Materials 2020, 13, 767. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Yuan, Q.; Deng, D. The role of phosphoric acid in improving the strength of magnesium oxychloride cement pastes with large molar ratios of H2O/MgCl2. Cem. Concr. Compos. 2019, 97, 379–386. [Google Scholar] [CrossRef]
- Jiříčková, A.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Jankovský, O. Synthesis, structure, and thermal stability of magnesium oxychloride 5Mg(OH)2∙MgCl2∙8H2O. Appl. Sci. 2020, 10, 1683. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Chen, H.; Li, Z.; Li, H. Simulation of the properties of MgO-MgfCl2-H2O system by thermodynamic method. Cem. Concr. Res. 2015, 68, 105–111. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Pivák, A.; Pavlík, Z. Experimental Analysis of MOC Composite with a Waste-Expanded Polypropylene-Based Aggregate. Materials 2018, 11, 931. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yu, H.; Zheng, L.; Wen, J.; Wu, C.; Tan, Y. Compressive strength of fly ash magnesium oxychloride cement containing granite wastes. Constr. Build. Mater. 2013, 38, 1–7. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Antončík, F.; Pivák, A.; Pavlík, Z. Influence of waste plastic aggregate and water-repellent additive on the properties of lightweight magnesium oxychloride cement composite. Appl. Sci. 2019, 9, 5463. [Google Scholar]
- Dehua, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar]
- Chen, X.; Zhang, T.; Bi, W.; Cheeseman, Ch. Effect of tartaric acid and phosphoric acid on the water resistance of magnesium oxychloride (MOC) cement. Constr. Build. Mater. 2019, 213, 528–536. [Google Scholar] [CrossRef]
- Xu, K.; Xi, J.; Guo, Y.; Dong, S. Effects of a new modifier on the water-resistance of magnesia cement tiles. Solid. State. Sci. 2012, 14, 10–14. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Soe, K.; Hutchinson, W.D.; Timmers, H.; Poblete, M.R. Effect of fly ash on mechanical properties of magnesium cement under water attack. Struct. Concr. 2019, 1–19. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cem. Concr. Res. 2017, 94, 115–122. [Google Scholar] [CrossRef]
- Chau, C.K.; Chan, J.; Li, Z. Influence of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Pavlík, Z.; Jankovský, O.; Pokorný, J.; Tydlitát, V.; Svora, P.; Černý, R. Physical and chemical characterization of technogenic pozzolans for the application in blended cements. Constr. Build. Mater. 2018, 160, 106–116. [Google Scholar]
- Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete, ASTM C618-19; ASTM International: West Conshohocken, PA, USA, 2019.
- Methods of Testing Cement — Part 6: Determination of Fineness, EN 196-6; European Committee for Standardization: Brussels, Belgium, 2010.
- Tests for Geometrical Properties of Aggregates—Part 1: Determination of Particle Size Distribution—Sieving Method, EN 933-1; European Committee for Standardization: Brussels, Belgium, 2012.
- Binders for Magnesite Screeds—Caustic Magnesia and Magnesium Chloride—Part 2: Test Methods, EN 14016-2; European Committee for Standardization: Brussels, Belgium, 2004.
- Methods of Test for Mortar for Masonry—Part 3: Determination of Consistence of Fresh Mortar (by Flow Table), EN 1015-3; European Committee for Standardization: Brussels, Belgium, 1999.
- Methods of Test for Mortar for Masonry—Part 10: Determination of Dry Bulk Density of Hardened Mortar, EN 1015-10; European Committee for Standardization: Brussels, Belgium, 1999.
- Pavlík, Z.; Pokorný, J.; Pavlíková, M.; Zemanová, L.; Záleská, M.; Vyšvařil, M.; Žižlavský, T. Mortars with Crushed Lava Granulate for Repair of Damp Historical Buildings. Materials 2019, 12, 3557. [Google Scholar] [CrossRef]
- Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar, EN 1015-11; European Committee for Standardization: Brussels, Belgium, 1999.
- Natural Stone Test Methods—Determination of Water Absorption at Atmospheric Pressure, EN 13755; European Committee for Standardization: Brussels, Belgium, 2008.
- Roels, S.; Carmeliet, J.; Hens, H.; Adan, O.; Brocken, H.; Cerny, R.; Pavlik, Z.; Hall, C.; Kumaran, K.; Pel, L.; et al. Interlaboratory Comparison of Hygric Properties of Porous Building Materials. J. Therm. Envel. Build. Sci. 2004, 27, 307–325. [Google Scholar] [CrossRef]
- Methods of Test for Mortar for Masonry—Part 18: Determination of Water-Absorption Coefficient Due to Capillary Action of Hardened Mortar, EN 1015-18; European Committee for Standardization: Brussels, Belgium, 2002.
- Kumaran, M.K. Moisture diffusivity of building materials from water absorption measurements. J. Therm. Envel. Build. Sci. 1999, 22, 349–355. [Google Scholar] [CrossRef]
- Pavlík, Z.; Černý, R. Determination of moisture diffusivity as a function of both moisture and temperature. Int. J. Thermophys. 2012, 33, 1704–1714. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Wang, L.; Wang, J. Effect of Magnesium oxychloride Cement on Stabilization/Solidification of Sewage Sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar]
- Hall, D.A.; Stevens, R.; El-Jazairi, B. The effect of retarders on the microstructure and mechanical properties of magnesia–phosphate cement mortar. Cem. Concr. Res. 2001, 31, 455–465. [Google Scholar] [CrossRef]
- Biel, T.D.; Lee, H. Magnesium Oxychloride Cement Concrete with Recycled Tire Rubber. Transp. Res. Rec. 1996, 1561, 6–12. [Google Scholar] [CrossRef]
- Misra, A.K.; Mathur, R. Magnesium oxychloride cement concrete. Bull. Mater. Sci. 2007, 30, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Shehab, H.K.; Eisa, A.S.; Wahba, A.M. Mechanical properties of fly ash based geopolymer concrete with full and partial cement replacement. Constr. Build. Mater. 2016, 126, 560–565. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete; Prentice Hall: Engelwood Cliffs, NJ, USA, 2002. [Google Scholar]
- Mehta, P.K. Concrete Structure Properties and Materials; Prentice Hall: Engelwood Cliffs, NJ, USA, 1986. [Google Scholar]
- Brunauer, S.; Emmet, P.H.; Teller, W.E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, E.W.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Hansen, K.K. Sorption Isotherms—A Catalogue, Technical Report 162/86; TU Denmark: Lyngby, Danmark, 1986. [Google Scholar]
- Derluyn, H.; Derome, D.; Carmeliet, J.; Stora, E.; Barbarulo, R. Hysteretic moisture behavior of concrete: modelling and analysis. Cem. Concr. Res. 2012, 42, 1379–1388. [Google Scholar] [CrossRef]
- Fredriksson, M.; Thybring, E.E. Scanning or desorption isotherms? Characterising sorption hysteresis of wood. Cellulose 2018, 25, 4477–4485. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Xing, P.; Gao, S. Studies on the basic compounds of magnesia cement: the thermal behaviour of magnesium chlorides. Thermochim. Acta 1991, 183, 349–363. [Google Scholar] [CrossRef]
- Dinnebier, R.E.; Halasz, I.; Freyer, D. The crystal structures of two anhydrous magnesium hydroxychloride phases from in situ synchrotron powder diffraction data. Z. Anorg. Allg. Chem. 2011, 637, 1458–1462. [Google Scholar] [CrossRef]
- He, P.; Poon, Ch.S.; Tsang, D.C.W. Comparison of glass powder and pulverized fuel ash for improving the water resistance of magnesium oxychloride cement. Cem. Concr. Compos. 2018, 86, 98–109. [Google Scholar] [CrossRef]
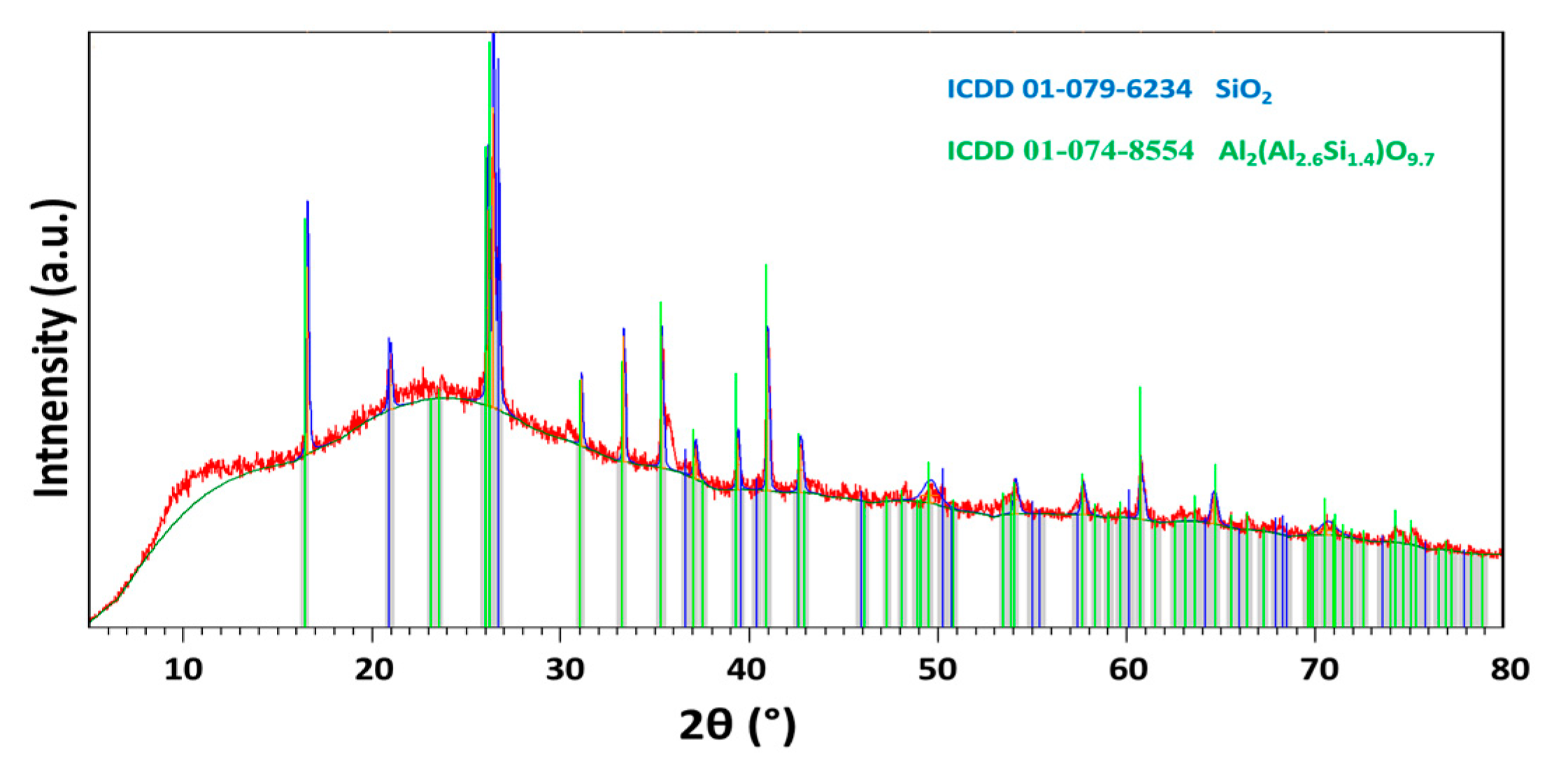

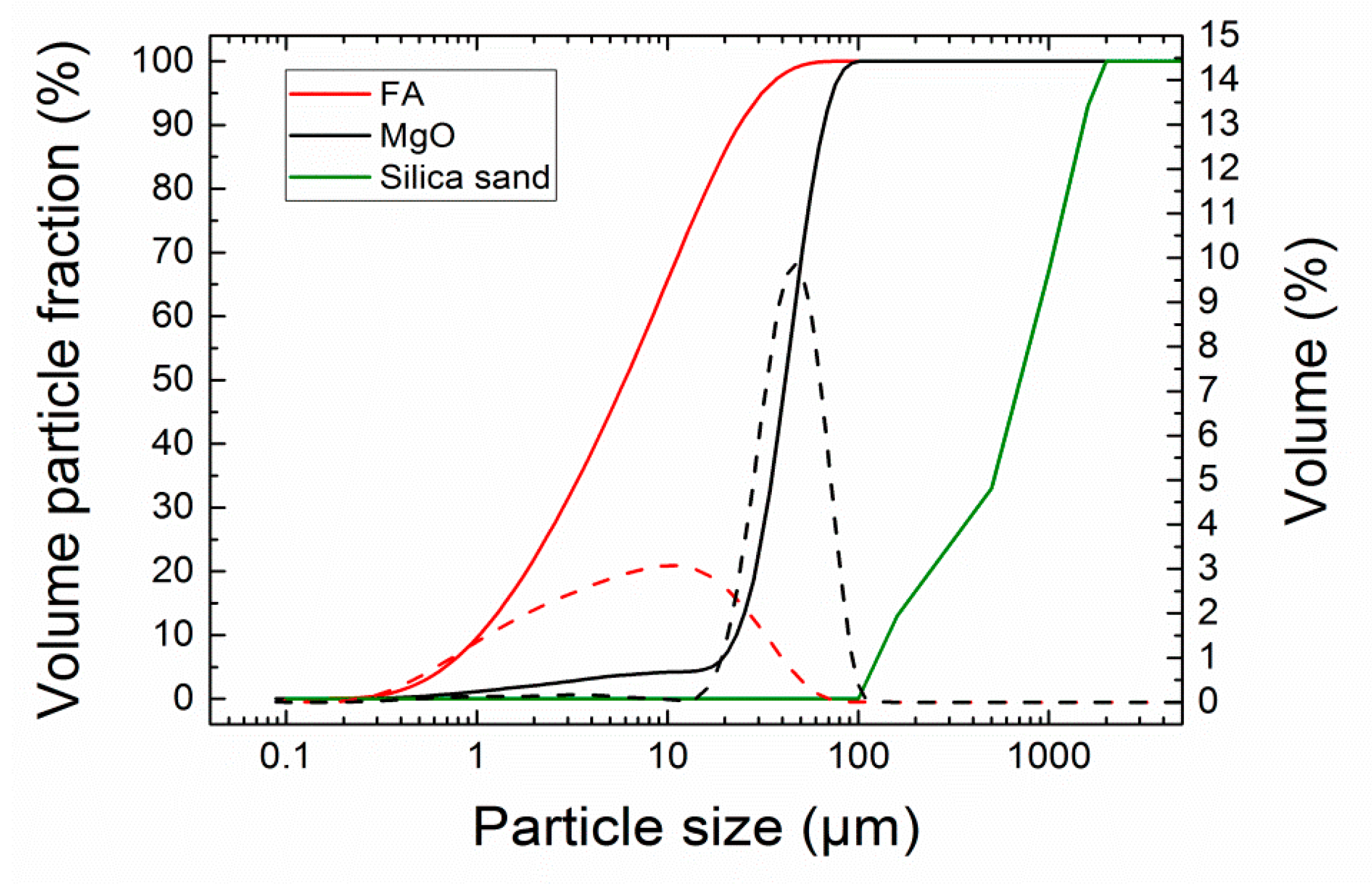
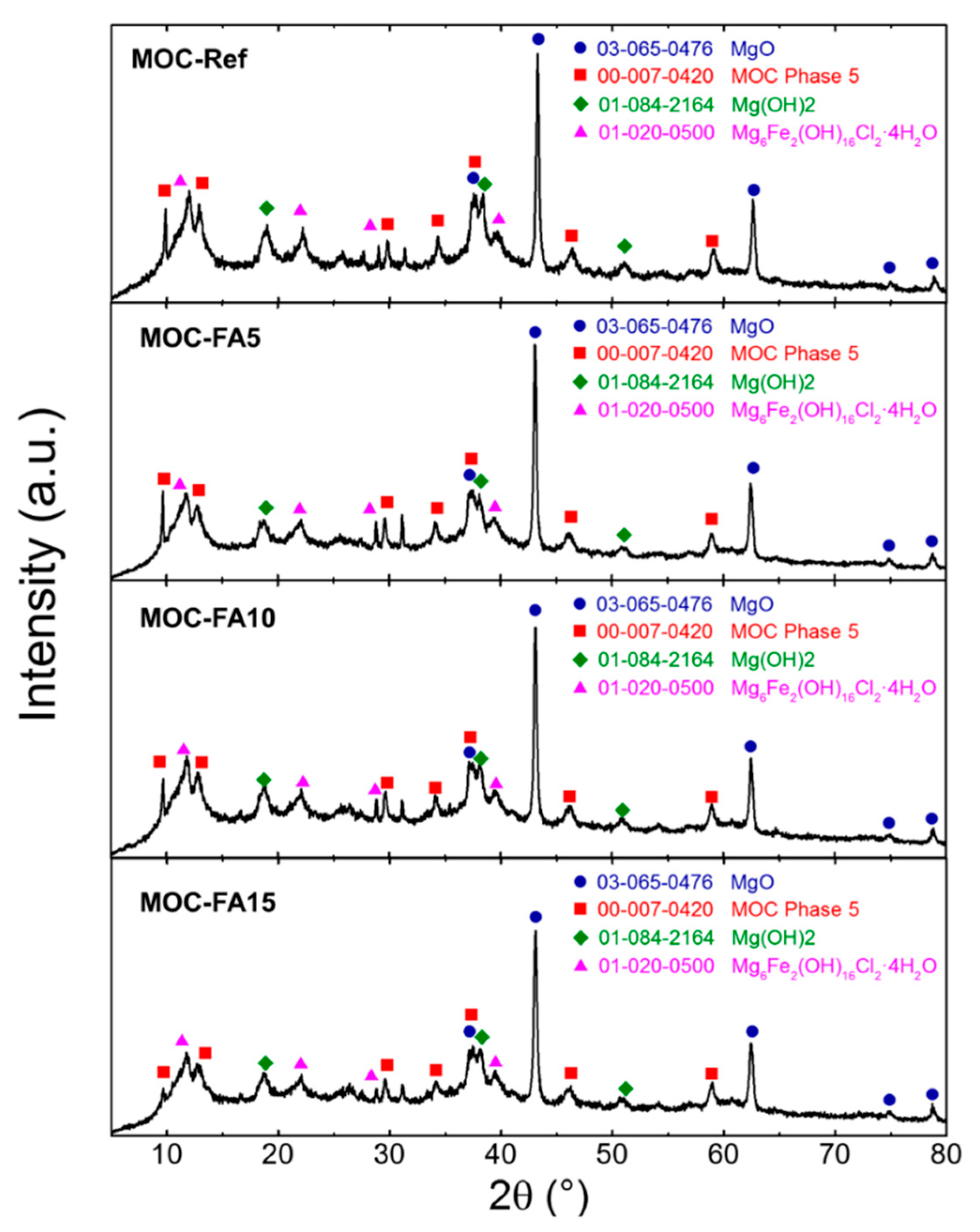
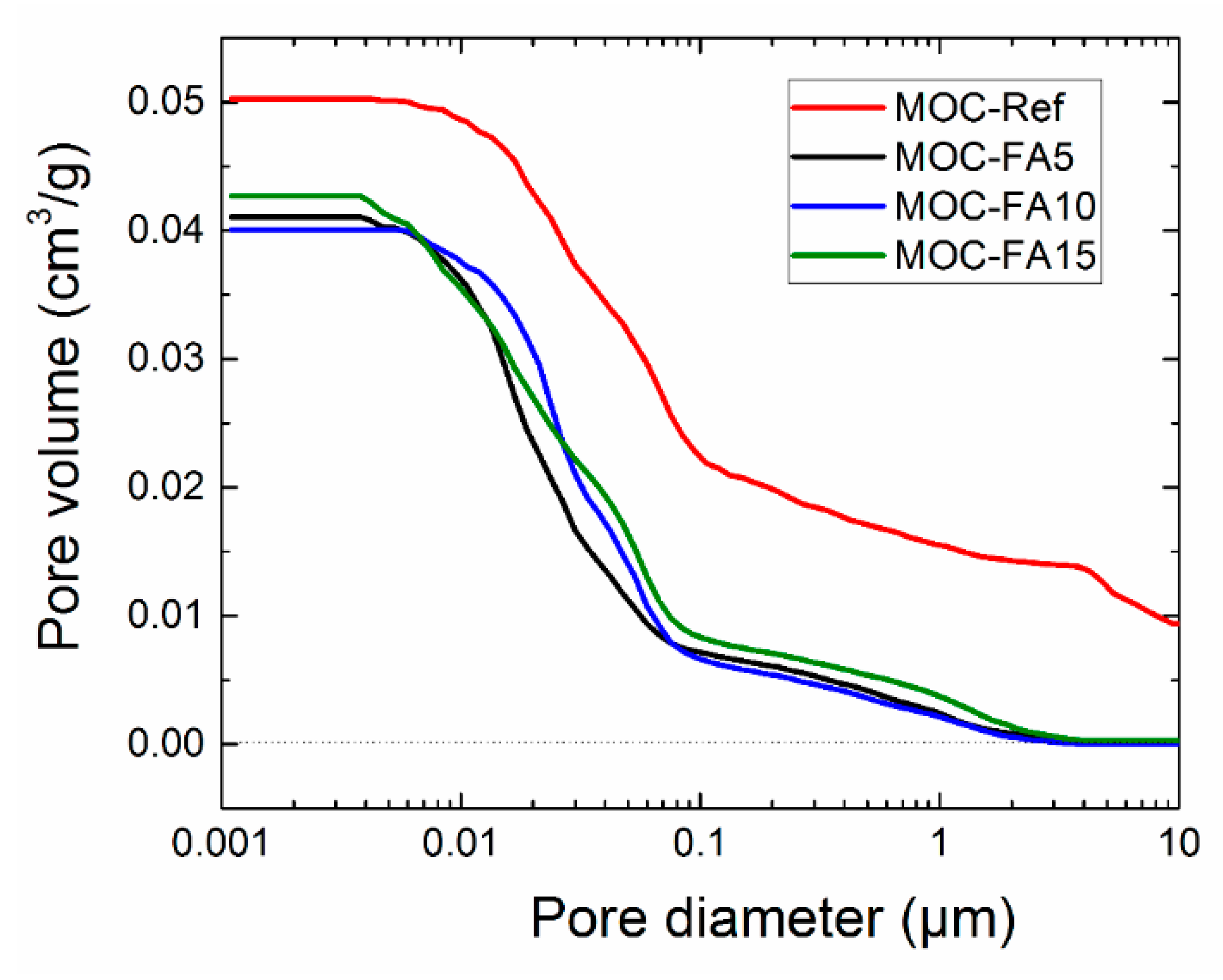
| Substance | MgO | Silica Sand | FA |
|---|---|---|---|
| SiO2 | 3.8 | 96.3 | 50.3 |
| CaO | 5.2 | – | 1.8 |
| Al2O3 | 6.0 | 3.1 | 35.4 |
| MgO | 80.5 | 0.4 | 1.4 |
| Fe2O3 | 3.8 | – | 6.8 |
| K2O | – | – | 1.7 |
| SO3 | 0.4 | – | 0.4 |
| Na2O | – | – | 0.1 |
| TiO2 | – | 0.1 | 1.5 |
| P2O5 | – | – | – |
| ∑ | 99.7 | 99.9 | 99.4 |
| Material | Loose Bulk Density (kg∙m−3) | Specific Density (kg∙m−3) | Blaine Specific Surface (m2∙kg−1) |
|---|---|---|---|
| MgO | 841 | 3338 | 701 |
| Silica sand | 1660 | 2650 | – |
| Fly ash | 793 | 2061 | 407 |
| Material | MgO | MgCl2 | Water | Silica Sand (PG1, PG2, PG3) | Fly Ash |
|---|---|---|---|---|---|
| MOC-Ref | 1541.25 | 682.85 | 567.25 | 3 × 1125.00 | – |
| MOC-FA5 | 1541.25 | 682.85 | 567.25 | 3 × 1068.75 | 95.54 |
| MOC-FA10 | 1541.25 | 682.85 | 567.25 | 3 × 1012.50 | 191.07 |
| MOC-FA15 | 1541.25 | 682.85 | 567.25 | 3 × 956.25 | 286.61 |
| Material | Spread Diameter (mm) | ρb (kg∙m−3) | ρs (kg∙m−3) | P (%) |
|---|---|---|---|---|
| MOC-Ref | 165/165 ± 5 | 2124 ± 30 | 2430 ± 29 | 12.6 ± 0.3 |
| MOC-FA5 | 170/170 ± 5 | 2170 ± 30 | 2280 ± 27 | 4.8 ± 0.1 |
| MOC-FA10 | 160/160 ± 5 | 2137 ± 30 | 2257 ± 27 | 5.3 ± 0.1 |
| MOC-FA15 | 160/160 ± 5 | 2124 ± 30 | 2250 ± 27 | 5.6 ± 0.1 |
| Material | Rf (MPa) | Rc (MPa) | Ed (GPa) |
|---|---|---|---|
| MOC-Ref | 22.9 | 77.7 | 40.3 |
| MOC-FA5 | 22.1 | 81.0 | 37.5 |
| MOC-FA10 | 22.0 | 77.6 | 37.4 |
| MOC-FA15 | 19.9 | 77.2 | 36.9 |
| Pore Diameter Range (μm) | Specific Vol. (cm3/g) | Specific Vol. (%) | Relative Vol. (%) |
|---|---|---|---|
| MOC-Ref | |||
| 100–10 | 0.00936 | 18.59 | 18.59 |
| 10–1 | 0.01542 | 30.60 | 12.01 |
| 1–0.1 | 0.02186 | 43.40 | 12.80 |
| 0.1–0.01 | 0.04845 | 96.18 | 52.78 |
| 0.01–0.001 | 0.05027 | 99.79 | 3.61 |
| MOC-FA5 | |||
| 100–10 | 0.00007 | 0.16 | 0.16 |
| 10–1 | 0.00220 | 5.33 | 5.17 |
| 1–0.1 | 0.00706 | 17.13 | 11.80 |
| 0.1–0.01 | 0.03566 | 86.51 | 69.38 |
| 0.01–0.001 | 0.04106 | 99.61 | 13.10 |
| MOC-FA10 | |||
| 100–10 | 0.00005 | 0.12 | 0.12 |
| 10–1 | 0.00197 | 4.89 | 4.77 |
| 1–0.1 | 0.00649 | 16.14 | 11.25 |
| 0.1–0.01 | 0.03717 | 92.51 | 76.37 |
| 0.01–0.001 | 0.04008 | 99.78 | 7.27 |
| MOC-FA15 | |||
| 100–10 | 0.00027 | 0.63 | 0.63 |
| 10–1 | 0.00354 | 8.30 | 7.67 |
| 1–0.1 | 0.00817 | 19.13 | 10.83 |
| 0.1–0.01 | 0.03490 | 81.69 | 62.56 |
| 0.01–0.001 | 0.04268 | 99.91 | 18.22 |
| Material | Aw (kg∙m−2∙s−1/2) | κ (m2∙s−1) | Wa (%) |
|---|---|---|---|
| MOC-Ref | 0.061 | 4.20 × 10−9 | 3.21 |
| MOC-FA5 | 0.028 | 6.66 × 10−10 | 2.06 |
| MOC-FA10 | 0.030 | 8.64 × 10−10 | 2.16 |
| MOC-FA15 | 0.033 | 1.14 × 10−9 | 2.31 |
| Material | λ (W∙m−1∙K−1) | a × 10−6 (m2∙s−1) | cv × 106 (J∙m−3∙K−1) |
|---|---|---|---|
| MOC-Ref | 3.030 | 1.318 | 2.302 |
| MOC-FA5 | 3.173 | 1.484 | 2.139 |
| MOC-FA10 | 2.996 | 1.315 | 2.273 |
| MOC-FA15 | 2.920 | 1.448 | 1.996 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivák, A.; Pavlíková, M.; Záleská, M.; Lojka, M.; Jankovský, O.; Pavlík, Z. Magnesium Oxychloride Cement Composites with Silica Filler and Coal Fly Ash Admixture. Materials 2020, 13, 2537. https://doi.org/10.3390/ma13112537
Pivák A, Pavlíková M, Záleská M, Lojka M, Jankovský O, Pavlík Z. Magnesium Oxychloride Cement Composites with Silica Filler and Coal Fly Ash Admixture. Materials. 2020; 13(11):2537. https://doi.org/10.3390/ma13112537
Chicago/Turabian StylePivák, Adam, Milena Pavlíková, Martina Záleská, Michal Lojka, Ondřej Jankovský, and Zbyšek Pavlík. 2020. "Magnesium Oxychloride Cement Composites with Silica Filler and Coal Fly Ash Admixture" Materials 13, no. 11: 2537. https://doi.org/10.3390/ma13112537
APA StylePivák, A., Pavlíková, M., Záleská, M., Lojka, M., Jankovský, O., & Pavlík, Z. (2020). Magnesium Oxychloride Cement Composites with Silica Filler and Coal Fly Ash Admixture. Materials, 13(11), 2537. https://doi.org/10.3390/ma13112537









