Surface Characteristics and Color Stability of Gingiva-Colored Resin Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Surface Composition and Microstructure
2.3. Degree of Conversion
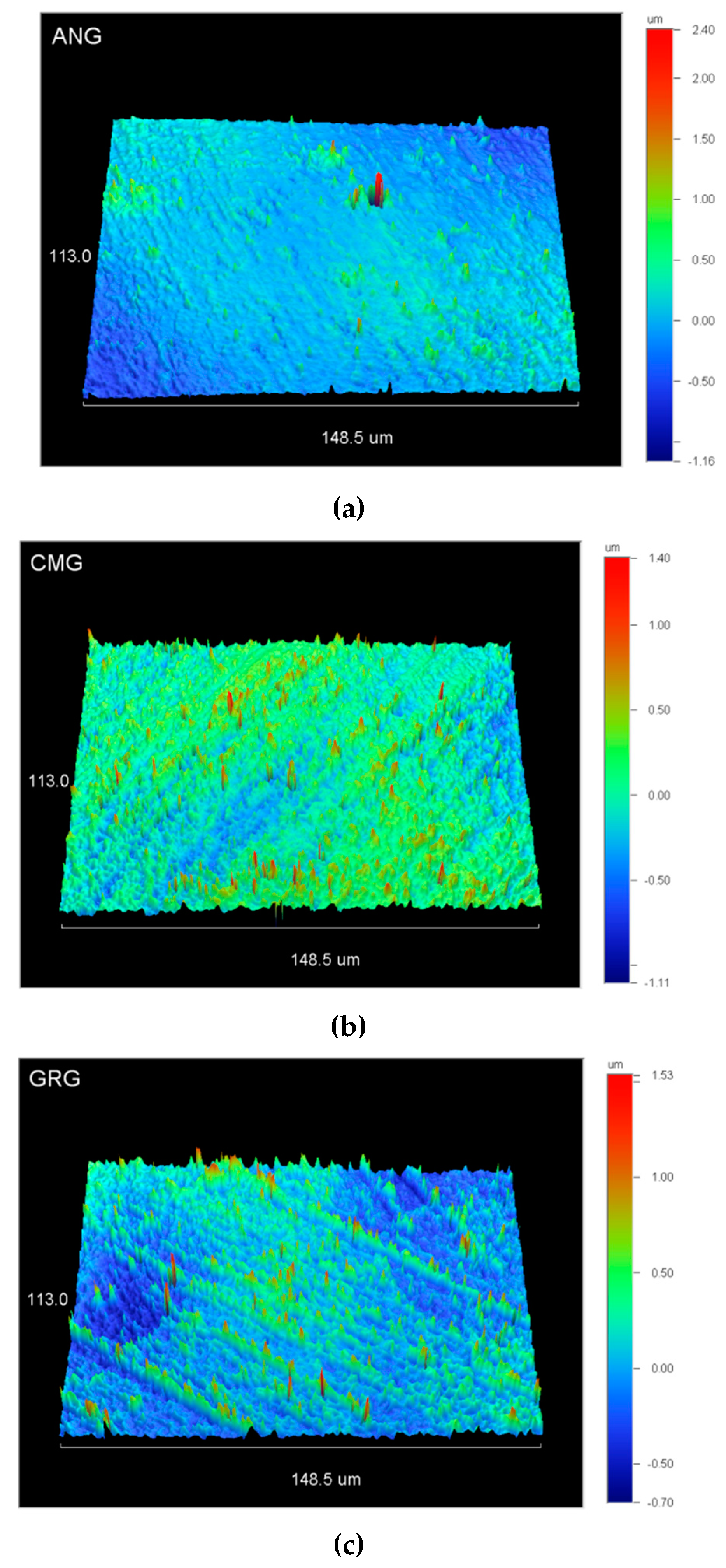
2.4. Surface Roughness
2.5. Color Stability
2.6. Hardness
2.7. Statistical Analysis
3. Results
3.1. Composition and Microstructure
3.2. Degree of Conversion
3.3. Surface Roughness
3.4. Color Stability
3.5. Hardness
4. Discussion
5. Conclusions
- The gingiva-colored resin composites were susceptible to staining, with coffee producing the greatest color changes in all the materials tested.
- There was no statistically significant difference in the degree of conversion between the materials. Hardness was not affected by the immersion media. Nevertheless, the hardest inorganic particle-filled material demonstrated the highest Sdr values and ΔE changes in coffee and wine.
- From the surface roughness parameters tested, only the functional parameter Sc, expressing the volume retention capacity of a surface, showed correlations with ΔL*, Δb* and ΔE.
Author Contributions
Funding
Conflicts of Interest
References
- Coachman, C.; Salama, M.; Garber, D.; Calamita, M.; Salama, H.; Cabral, G. Prosthetic Gingival Reconstruction in a Fixed Partial Restoration. Part 1: Introduction to Artificial Gingiva as an Alternative Therapy. Int. J. Periodontics Restor. Dent. 2009, 29, 471–477. [Google Scholar]
- Thoma, D.S.; Mόhlemann, S.; Jung, R.E. Critical Soft-Tissue Dimensions with Dental Implants and Treatment Concepts. Periodontology 2000 2014, 66, 106–118. [Google Scholar] [CrossRef]
- Coachman, C.; Salama, M.; Garber, D.; Calamita, M.; Salama, H.; Cabral, G. Prosthetic Gingival Reconstruction in Fixed Partial Restorations. Part 3: Laboratory Procedures and Maintenance. Int. J. Periodontics Restor. Dent. 2010, 30, 19–29. [Google Scholar]
- Mackert, J.R.; Williams, A.L. Microcracks in Dental Porcelain and Their Behavior during Multiple Firing. J. Dent. Res. 1996, 75, 1484–1490. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Nakajima, K.; Tsuge, T.; McGlumphy, E.A. The Use of Customized Implant Frameworks with Gingiva-Colored Composite Resin to Restore Deficient Gingival Architecture. J. Prosthet. Dent. 2007, 97, 112–117. [Google Scholar] [CrossRef]
- Malσ, P.; de Araϊjo Nobre, M.; Borges, J.; Almeida, R. Retrievable Metal Ceramic Implant-Supported Fixed Prostheses with Milled Titanium Frameworks and All-Ceramic Crowns: Retrospective Clinical Study with up to 10 Years of Follow-Up. J. Prosthodont. Implant Esthet. Reconstr. Dent. 2012, 21, 256–264. [Google Scholar] [CrossRef]
- Petropoulou, A.; Pappa, E.; Pelekanos, S. Esthetic Considerations When Replacing Missing Maxillary Incisors with Implants: A Clinical Report. J. Prosthet. Dent. 2013, 109, 140–144. [Google Scholar] [CrossRef]
- Geckili, O.; Bilhan, H.; Ceylan, G.; Cilingir, A. Edentulous Maxillary Arch Fixed Implant Rehabilitation Using a Hybrid Prosthesis Made of Micro-Ceramic-Composite: Case Report. J. Oral Implant. 2013, 39, 115–120. [Google Scholar] [CrossRef]
- Petropoulou, A.; Pantzari, F.; Nomikos, N.; Chronopoulos, V.; Kourtis, S. The Use of Indirect Resin Composites in Clinical Practice: A Case Series. Dentistry 2013, 3. [Google Scholar] [CrossRef]
- An, H.-S.; Park, J.-M.; Park, E.-J. Evaluation of Shear Bond Strengths of Gingiva-Colored Composite Resin to Porcelain, Metal and Zirconia Substrates. J. Adv. Prosthodont. 2011, 3, 166–171. [Google Scholar] [CrossRef]
- Williams, B.; Braden, M. Characteristics of fissure sealants. J. Dent. Res. 1981, 60, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.S.Q.S.; Labruna Moreira, A.D.; de Albuquerque, P.P.A.C.; de Menezes, L.R.; Pfeifer, C.S.; Schneider, L.F.J. Effect of Monomer Type on the CC Degree of Conversion, Water Sorption and Solubility, and Color Stability of Model Dental Composites. Dent. Mater. 2017, 33, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of Water Sorption, Solubility and Modulus of Elasticity of Light-Cured Dimethacrylate-Based Dental Resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Gajewski, V.E.S.; Pfeifer, C.S.; Frσes-Salgado, N.R.G.; Boaro, L.C.C.; Braga, R.R. Monomers Used in Resin Composites: Degree of Conversion, Mechanical Properties and Water Sorption/Solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Ren, Y.-F.; Feng, L.; Serban, D.; Malmstrom, H.S. Effects of Common Beverage Colorants on Color Stability of Dental Composite Resins: The Utility of a Thermocycling Stain Challenge Model in Vitro. J. Dent. 2012, 40, e48–e56. [Google Scholar] [CrossRef]
- Cowperthwaite, G.F.; Foy, J.J.; Malloy, M.A. The Nature of the Crosslinking Matrix Found in Dental Composite Filling Materials and Sealants. In Biomedical and Dental Applications of Polymers; Gebelein, C.G., Koblitz, F.F., Eds.; Polymer Science and Technology; Springer: Boston, MA, USA, 1981; pp. 379–385. [Google Scholar] [CrossRef]
- Burchill, P.J.; Mathys, G.; Stacewicz, R.H. Analysis and Properties of Some Commercial Poly(Methylmethacrylate)-Based Materials. J. Mater. Sci. 1987, 22, 483–487. [Google Scholar] [CrossRef]
- Stansbury, J.W. Dimethacrylate Network Formation and Polymer Property Evolution as Determined by the Selection of Monomers and Curing Conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef]
- Wada, K.; Ikeda, E.; Wada, J.; Inoue, G.; Miyasaka, M.; Miyashin, M. Wear Characteristics of Trimethylolpropane Trimethacrylate Filler-Containing Resins for the Full Crown Restoration of Primary Molars. Dent. Mater. J. 2016, 35, 585–593. [Google Scholar] [CrossRef]
- da Silva, E.M.; Miragaya, L.; Noronha-Filho, J.D.; Amaral, C.M.; Poskus, L.T.; Guimarγes, J.G.A. Characterization of an Experimental Resin Composite Organic Matrix Based on a Tri-Functional Methacrylate Monomer. Dent. Mater. J. 2016, 35, 159–165. [Google Scholar] [CrossRef]
- Barnes, N.; Feinman, R.; Waknine, S.; Alpert, B.H. Gum-Colored Dental Composite and Dental Restoration Kit. US Patent 5,094,619, 4 July 1995. [Google Scholar]
- Ruyter, I.E. Methacrylate-Based Polymeric Dental Materials: Conversion and Related Properties. Summary and Review. Acta Odontol. Scand. 1982, 40, 359–376. [Google Scholar] [CrossRef]
- Pereira, S.G.; Nunes, T.G.; Kalachandra, S. Low Viscosity Dimethacrylate Comonomer Compositions [Bis-GMA and CH3Bis-GMA] for Novel Dental Composites; Analysis of the Network by Stray-Field MRI, Solid-State NMR and DSC & FTIR. Biomaterials 2002, 23, 3799–3806. [Google Scholar] [CrossRef] [PubMed]
- Silikas, N.; Kavvadia, K.; Eliades, G.; Watts, D. Surface Characterization of Modern Resin Composites: A Multitechnique Approach. Am. J. Dent. 2005, 18, 95–100. [Google Scholar] [PubMed]
- Ferracane, J.L.; Mitchem, J.C.; Condon, J.R.; Todd, R. Wear and Marginal Breakdown of Composites with Various Degrees of Cure. J. Dent. Res. 1997, 76, 1508–1516. [Google Scholar] [CrossRef]
- Bollen, C.M.; Lambrechts, P.; Quirynen, M. Comparison of Surface Roughness of Oral Hard Materials to the Threshold Surface Roughness for Bacterial Plaque Retention: A Review of the Literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Teranaka, A.; Tomiyama, K.; Ohashi, K.; Miyake, K.; Shimizu, T.; Hamada, N.; Mukai, Y.; Hirayama, S.; Nihei, T. Relevance of Surface Characteristics in the Adhesiveness of Polymicrobial Biofilms to Crown Restoration Materials. J. Oral Sci. 2018, 60, 129–136. [Google Scholar] [CrossRef]
- Lu, H.; Roeder, L.B.; Lei, L.; Powers, J.M. Effect of Surface Roughness on Stain Resistance of Dental Resin Composites. J. Esthet. Restor. Dent. 2005, 17, 102–108; discussion 109. [Google Scholar] [CrossRef]
- Ereifej, N.S.; Oweis, Y.G.; Eliades, G. The Effect of Polishing Technique on 3-D Surface Roughness and Gloss of Dental Restorative Resin Composites. Oper. Dent. 2013, 38, E1–E12. [Google Scholar] [CrossRef]
- Ruivo, M.A.; Pacheco, R.R.; Sebold, M.; Giannini, M. Surface Roughness and Filler Particles Characterization of Resin-Based Composites. Microsc. Res. Tech. 2019, 82, 1756–1767. [Google Scholar] [CrossRef]
- Heintze, S.D.; Forjanic, M.; Ohmiti, K.; Rousson, V. Surface Deterioration of Dental Materials after Simulated Toothbrushing in Relation to Brushing Time and Load. Dent. Mater. 2010, 26, 306–319. [Google Scholar] [CrossRef]
- Khashayar, G. Color Science in Dentistry. Ph.D. Thesis, Free University, Amsterdam, The Netherlands, 2013; pp. 137–142. [Google Scholar]
- Kentrou, C.; Papadopoulos, T.; Lagouvardos, P. Color Changes in Staining Solutions of Four Light-Cured Indirect Resin Composites. Odontology 2014, 102, 189–196. [Google Scholar] [CrossRef]
- Poggio, C.; Ceci, M.; Beltrami, R.; Mirando, M.; Wassim, J.; Colombo, M. Color Stability of Esthetic Restorative Materials: A Spectrophotometric Analysis. Acta Biomater. Odontol. Scand. 2016, 2, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Seghi, R.R.; Gritz, M.D.; Kim, J. Colorimetric Changes in Composites Resulting from Visible-Light-Initiated Polymerization. Dent. Mater. 1990, 6, 133–137. [Google Scholar] [CrossRef]
- Park, J.-K.; Kim, T.-H.; Ko, C.-C.; Garcνa-Godoy, F.; Kim, H.-I.; Kwon, Y.H. Effect of Staining Solutions on Discoloration of Resin Nanocomposites. Am. J. Dent. 2010, 23, 39–42. [Google Scholar] [PubMed]
- Asmussen, E.; Peutzfeldt, A. Influence of Pulse-Delay Curing on Softening of Polymer Structures. J. Dent. Res. 2001, 80, 1570–1573. [Google Scholar] [CrossRef]
- Ardu, S.; Braut, V.; Gutemberg, D.; Krejci, I.; Dietschi, D.; Feilzer, A.J. A Long-Term Laboratory Test on Staining Susceptibility of Esthetic Composite Resin Materials. Quintessence Int. Berl. Ger. 1985 2010, 41, 695–702. [Google Scholar]
- Anfe, T.E.d.A.; Agra, C.M.; Vieira, G.F. Evaluation of the Possibility of Removing Staining by Repolishing Composite Resins Submitted to Artificial Aging. J. Esthet. Restor. Dent. 2011, 23, 260–267. [Google Scholar] [CrossRef]
- Milleding, P.; Ahlgren, F.; Wennerberg, A.; Ortengren, U.; Karlsson, S. Microhardness and Surface Topography of a Composite Resin Cement after Water Storage. Int. J. Prosthodont. 1998, 11, 21–26. [Google Scholar]
- Sφderholm, K.-J.; Zigan, M.; Ragan, M.; Fischlschweiger, W.; Bergman, M. Hydrolytic Degradation of Dental Composites. J. Dent. Res. 2016. [Google Scholar] [CrossRef]





| Material (Code) | Composition* | Finishing/Polishing Procedure* | Manufacturer |
|---|---|---|---|
| AnaxGUM (ANG) Shade: Light Pink | Resin: UDMA, BDDMA, BisGMA Filler: Glass, pyrogenic SiO2 (74 wt%, 0.7 μm) | Polishing brush and Pasta Grigia II | Anaxdent GmbH Stuttgard, Germany |
| Ceramage Body (CMG) Shade: Gum-L | Resin: UDMA, dimethacrylates Filler: ZrSiO4 (73 wt% progressed fine structure filler) | CompoMaster (diamond impregnated silicone points coarse, hight-lustre) Dura-Polish, Dura-Polish DIA (alumina pastes) | Shofu Kyoto, Japan |
| Gradia Gum (GRG) Shade: G23 | Resin: UDMA, NGDMA, TMPTMA Filler: Trimodal (pre-polymerized particles, AlBSiO4, SiO2, 75 wt%) | CompoMaster (diamond impregnated silicone points coarse, hight-lustre) Gradia Diapolisher paste | GC Europe NV Leuven, Belgium |
| Material | Elemental Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Si | Al | Zr | Na | K | |
| ANG | 55.4 | 21.5 | 19.8 | 3.3 | - | - | - |
| CMG | 31.9 | 31.7 | 25.6 | 0.7 | 9.4 | 0.7 | - |
| GRG | 55.7 | 22.9 | 18.2 | 2.2 | - | - | 0.9 |
| Material | DC% | Sa (nm) | Sz (μm) | Sdr (%) | Sc (nm3/nm2) |
|---|---|---|---|---|---|
| ANG | 55 (2.6)a | 221.5 (131.6)a | 2.1 (1.2)a | 3.7 (1.3)a | 310.4 (174.7)a |
| CMG | 54.6 (2.9)a | 306.4 (177.9)a | 3.2 (1.6)a | 9.2 (3.6)b | 388.8 (203.4)a |
| GRG | 50.4 (5.9)a | 188.3 (49)a | 2.0 (0.2)a | 4.3 (1.7)a | 267.7 (99.4)a |
| GROUPS | ANG | CMG | GRG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | ||||
| Reference | 50.58 (0.78) A | 13.91 (0.46) A | −3.19 (0.59) A | 57.33 (1.56) B | 23.21 (0.84) B | 5.78 (0.22) B | 50.01 (0.9) A | 21.2 (0.57) C | 5.58 (0.28) B | 50.58 (0.78) A | ||
| After 30d immersion | ΔL* | Δa* | Δb* | ΔE* | ΔL* | Δa* | Δb* | ΔE* | ΔL* | Δa* | Δb* | ΔE* |
| Water | 0.73 (0.87) a,A | −2.83 (1.06) a,A | −2.50 (0.82) a,A | 3.99 (1.16) a,A | 1.24 (1.50) a,A | 0.07 (0.78) a,B | −0.36 (0.27) a,B | 1.79 (1.12) a,B | 0.33 (0.91) a,A | −1.13 (0.90) a,C | −0.37 (0.34) a,B | 1.63 (0.72) a,B |
| Coffee | 1.49 (1.68) a,A | −1.30 (1.17) a,b,A | 7.41 (1.30) b,A,B | 7.88 (1.58) b,A | −4.18 (1.97) b,B | −3.69 (1.39) b,B,C | 9.64 (2.06) b,B | 11.37 (2.12) b,B | −0.53 (0.99) b,A | −3.09 (1.15) b,B, | 5.05 (0.94) b,A | 6.09 (1.11) b,C |
| Wine | 0.93 (1.48) a,A,B | −0.72 (0.60) b,A | 3.50 (0.32) c,A | 3.99 (0.34) a,A | 0.08 (1.64) a,A | −2.08 (1.03) b,B | 6.93 (1.30) b,B | 7.51 (1.04) c,B | 1.40 (0.78) a,B | −1.38 (1.15) a,b,A,B | 1.42 (0.30) c,A | 2.67 (0.79) a,C |
| GROUPS | ANG | CMG | GRG |
|---|---|---|---|
| Reference | 50.3 (46.6–54) a,A | 75.1 (68.9–75.3) a,B | 37 (35.8–38.1) a,C |
| Water | 56.2 (54–58.4) a,A | 75.3 (75.3–82.5) a,B | 38.2 (33.6–40.7) a,C |
| Coffee | 48.4 (46.6–50.1) a,A | 80.1 (60.9–84.9) a,B | 40.7 (40.7–43.5) a,C |
| Wine | 45.4 (40.7–58.4) a,A | 70.1 (66.3–74.9) a,B | 38.1 (38.1–40.7) a,C |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petropoulou, A.; Dimitriadi, M.; Zinelis, S.; Sarafianou, A.; Eliades, G. Surface Characteristics and Color Stability of Gingiva-Colored Resin Composites. Materials 2020, 13, 2540. https://doi.org/10.3390/ma13112540
Petropoulou A, Dimitriadi M, Zinelis S, Sarafianou A, Eliades G. Surface Characteristics and Color Stability of Gingiva-Colored Resin Composites. Materials. 2020; 13(11):2540. https://doi.org/10.3390/ma13112540
Chicago/Turabian StylePetropoulou, Aikaterini, Maria Dimitriadi, Spiros Zinelis, Aspasia Sarafianou, and George Eliades. 2020. "Surface Characteristics and Color Stability of Gingiva-Colored Resin Composites" Materials 13, no. 11: 2540. https://doi.org/10.3390/ma13112540
APA StylePetropoulou, A., Dimitriadi, M., Zinelis, S., Sarafianou, A., & Eliades, G. (2020). Surface Characteristics and Color Stability of Gingiva-Colored Resin Composites. Materials, 13(11), 2540. https://doi.org/10.3390/ma13112540







