Development of Graphene Oxide-Based Nonedible Cottonseed Nanofluids for Power Transformers
Abstract
:1. Introduction
2. Experimental Process
2.1. Materials and Characterization Techniques
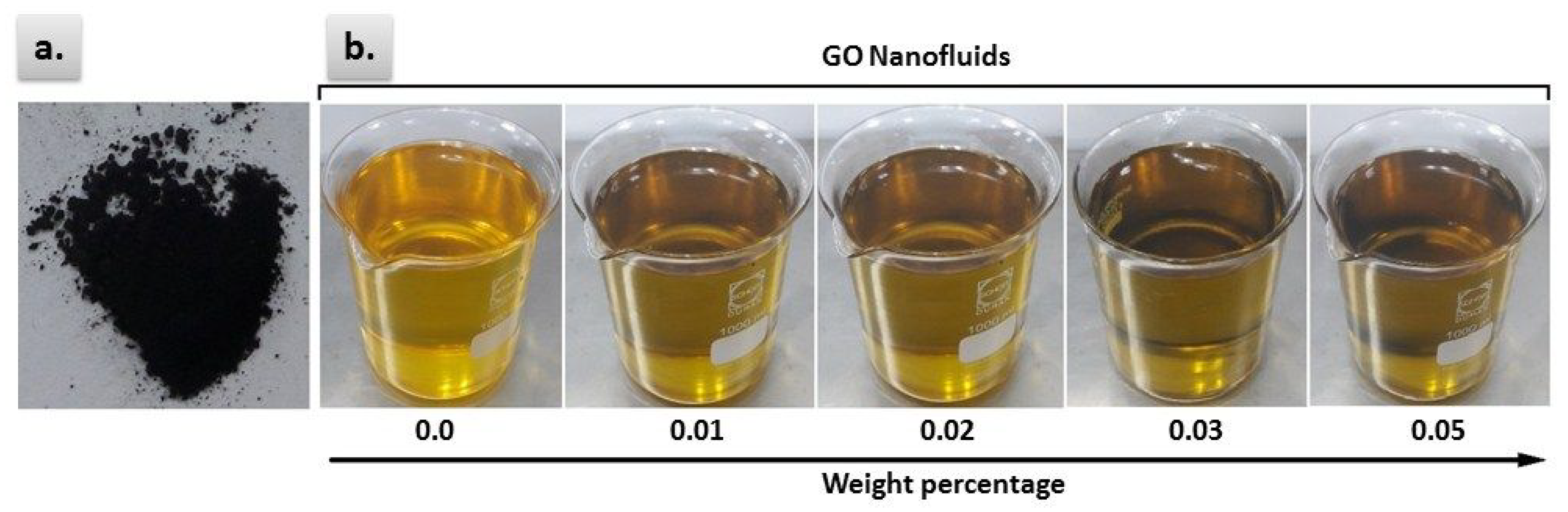
2.2. Preparation of Nanofluids
2.3. Measurement Techniques
3. Structural Characterization and Stability Analysis
3.1. Structural Characterization
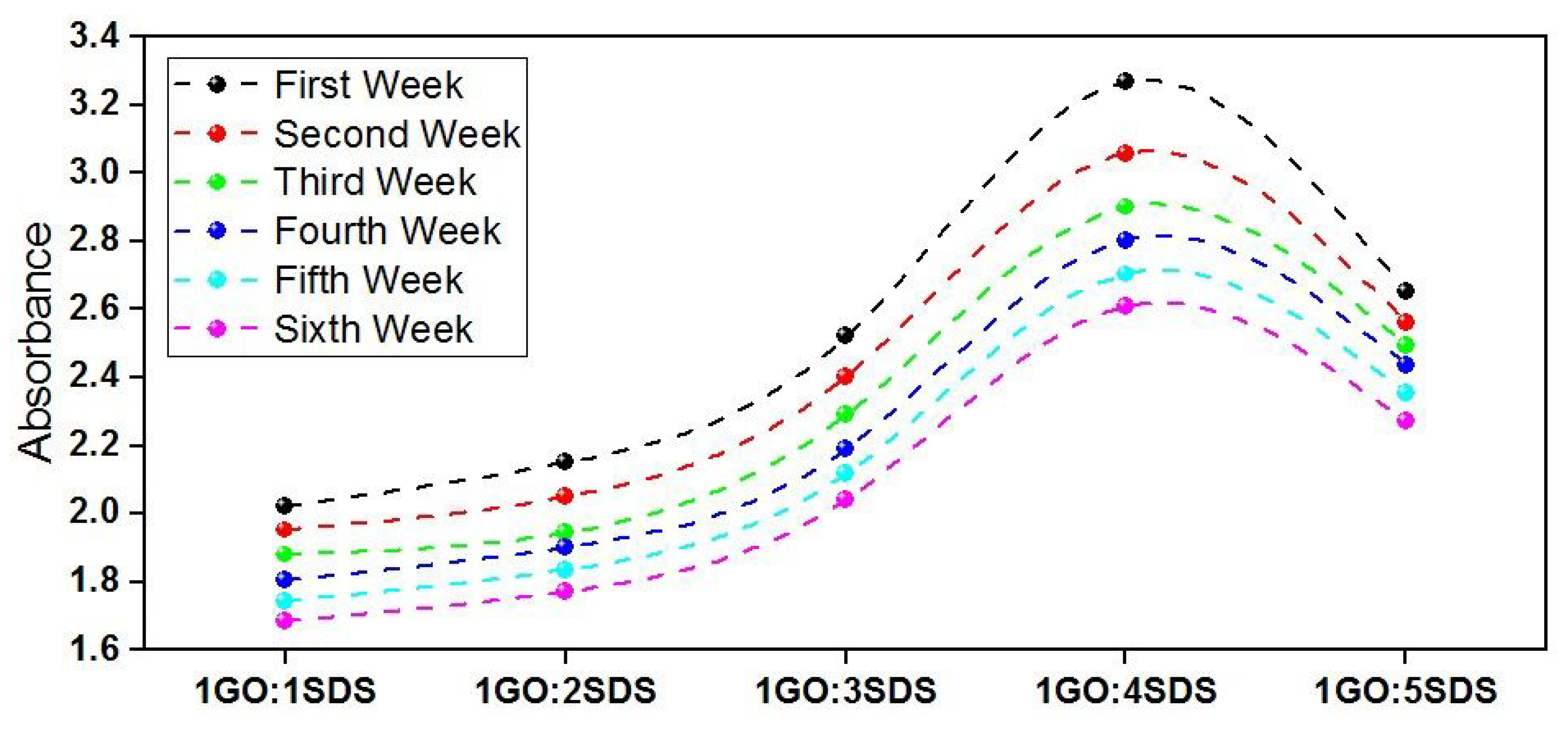
3.2. Stability Analysis of GO-Based CSO Nanofluids
4. Dielectric Properties of GO-Based CSO Nanofluids
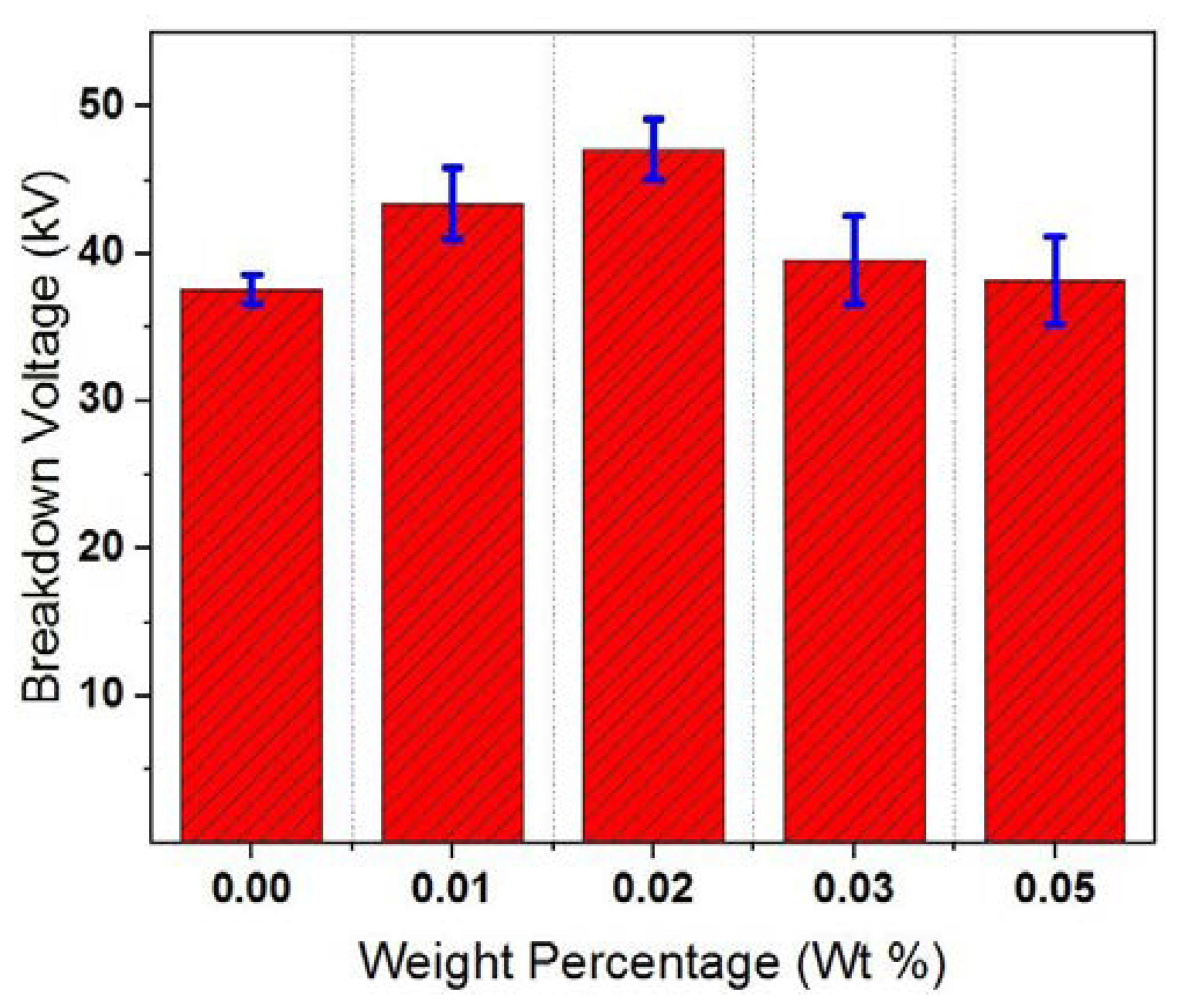
4.1. Breakdown Voltage
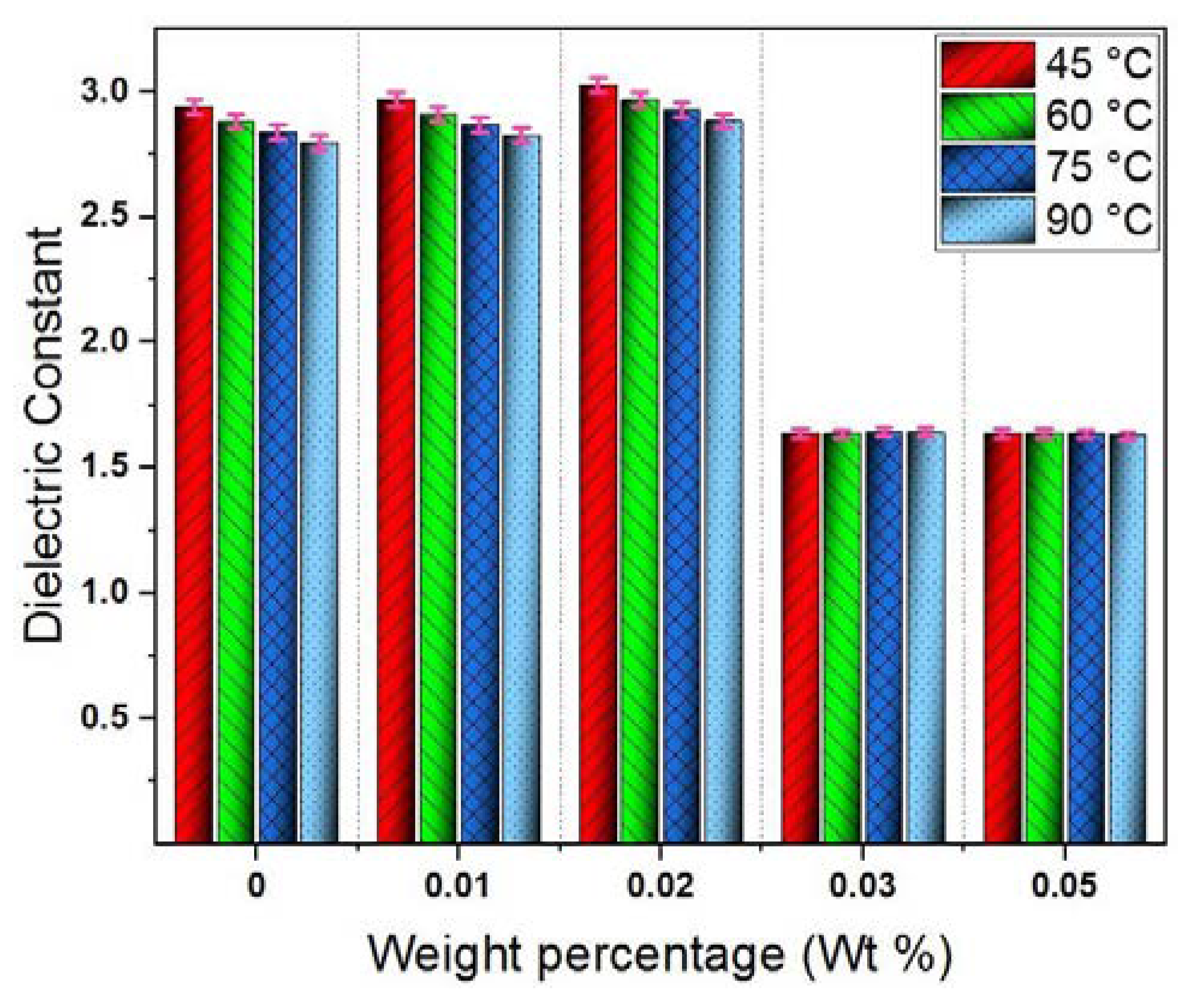
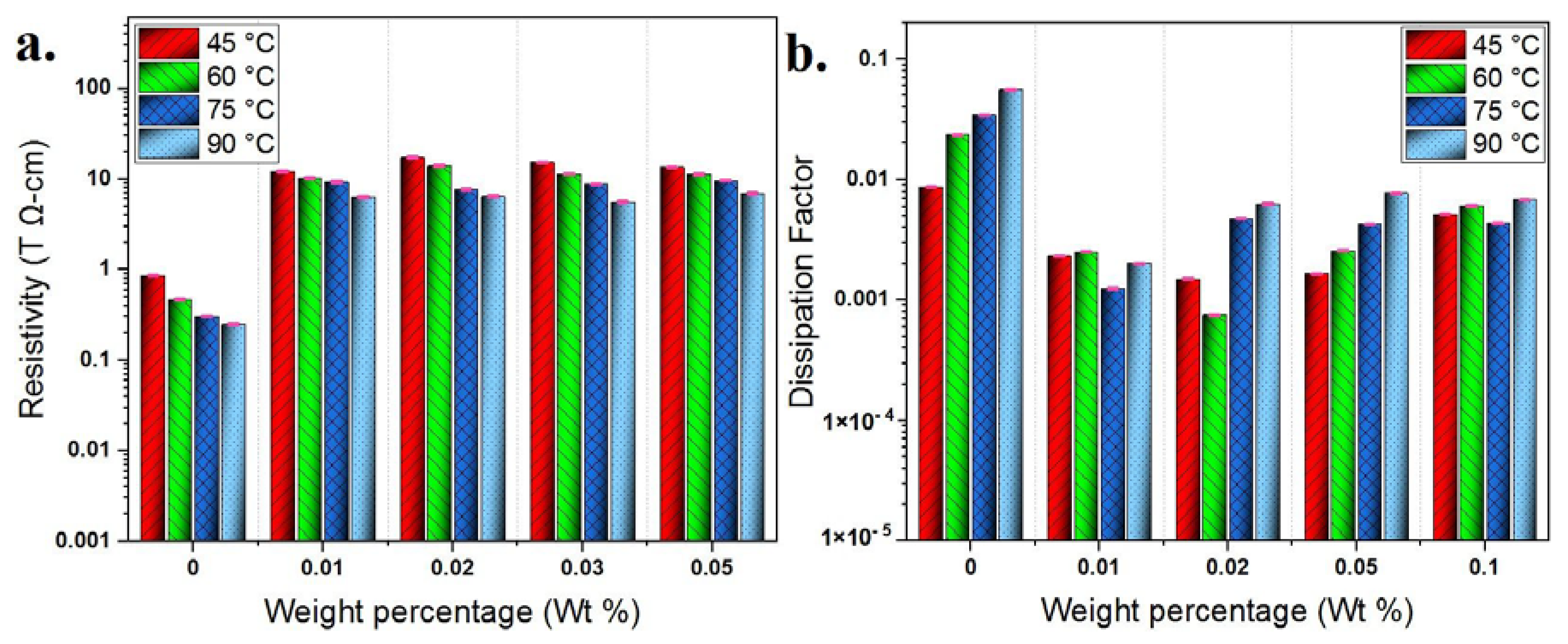
4.2. Dielectric Constant, Loss Tangent and Resistivity
5. Thermal Properties of GO-Based CSO Nanofluids
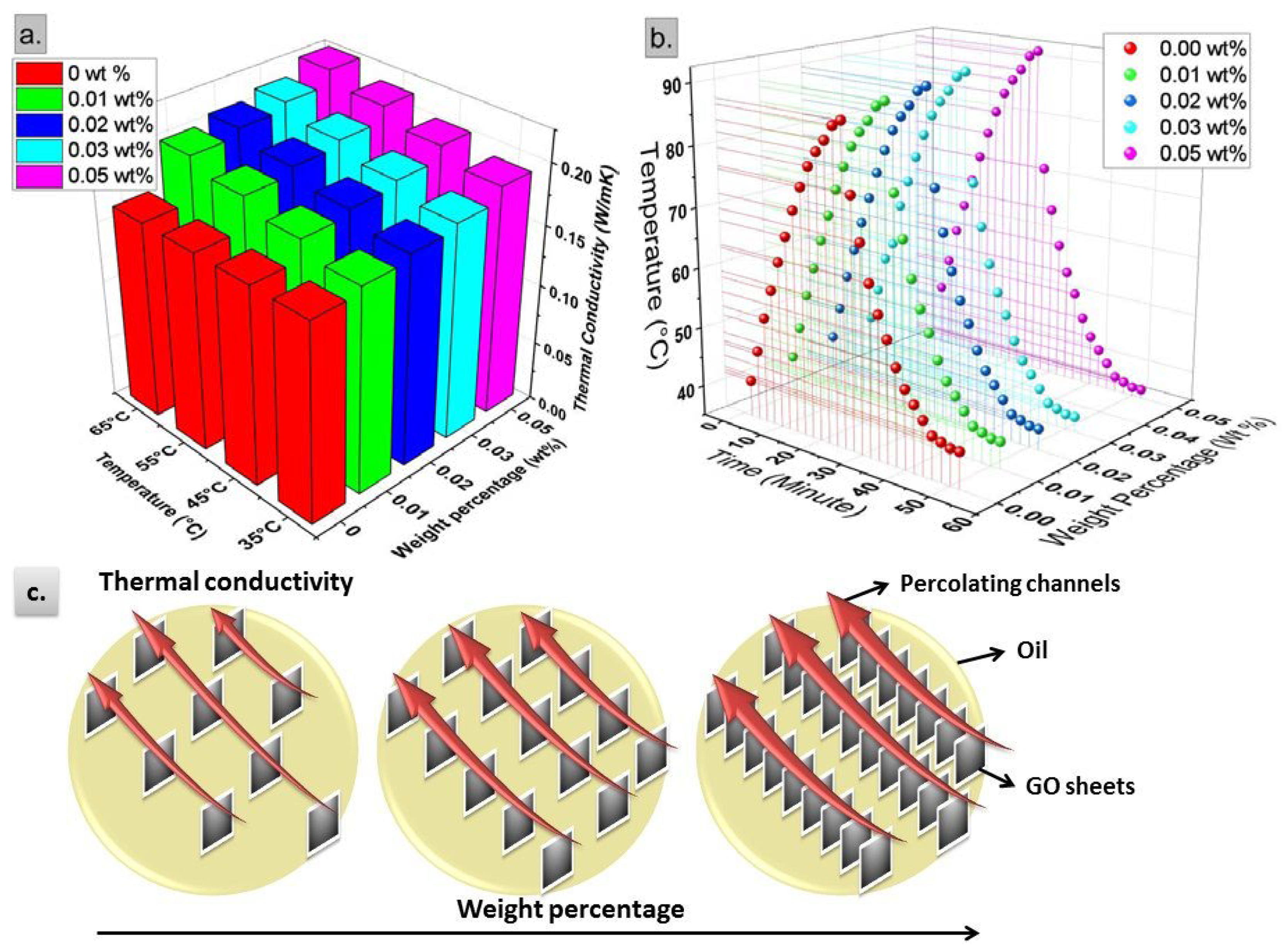
5.1. Thermal Conductivity and Thermal Response
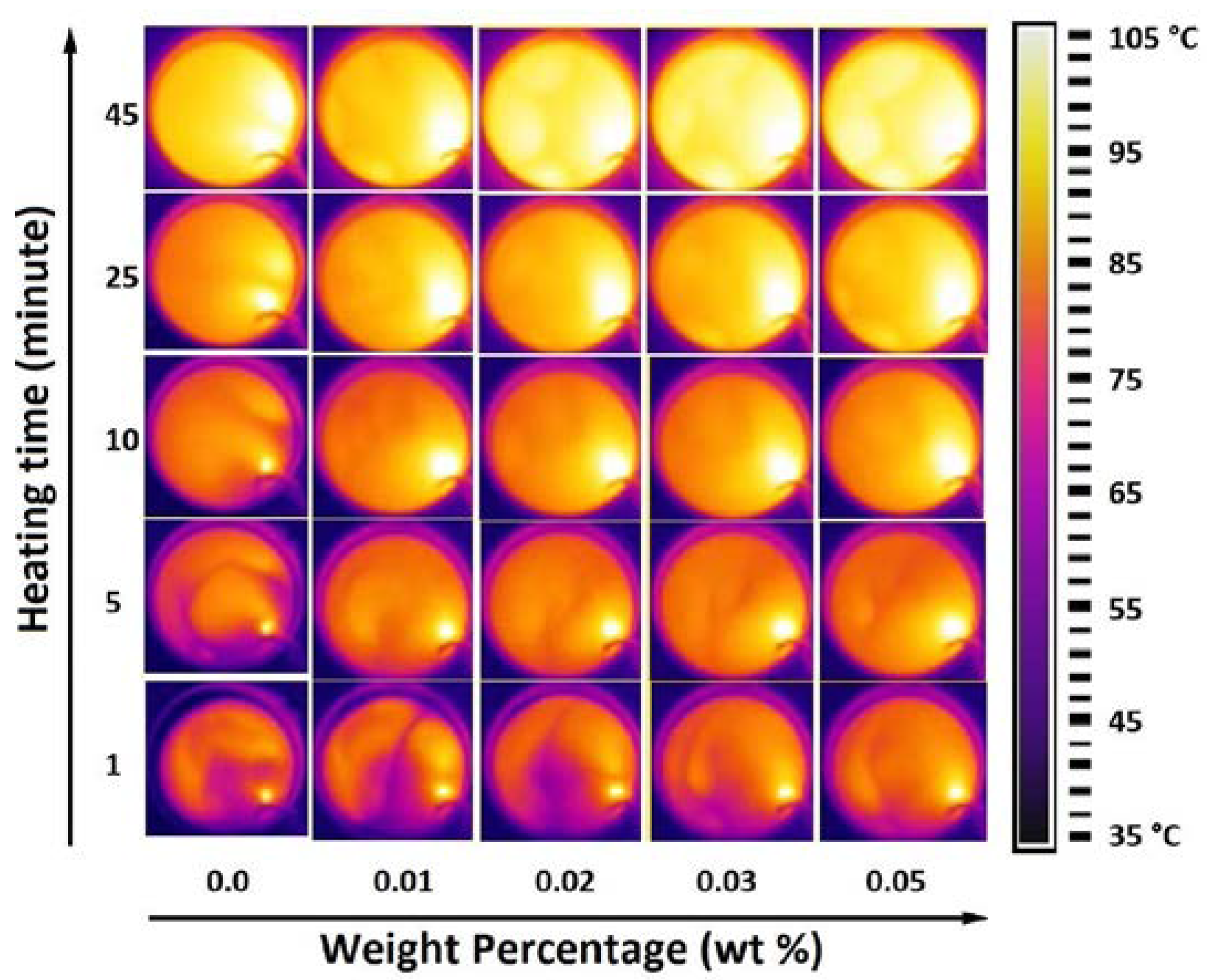
5.2. Thermogram Analysis
6. Conclusions
- Stability analysis of GO-based CSO nanofluids were performed using UV–Vis optical spectroscopy over a wavelength ranging between 200 nm and 800 nm. For all samples, the UV-Vis spectra peaked at a wavelength of 250 nm. The 1GO:1SDS ratio was adopted, where it exhibited the lowest absorbance drop.
- The AC BDV has been enhanced by about 25% at GO weight percentage of 0.02 wt%. This enhancement was explained in terms of the role of EDL acts in traps and capturing the brisk electrons produced under high electric field.
- The dielectric constant indicated a slight increase against weight percentage of GO nanosheets up to 0.02 wt%, followed by a remarkable decrease at 0.03 wt% and 0.05 wt%. This was explained considering polarizability of liquid molecules and inner polarizability of solid particles in addition to polarizability at EDL.
- The resistivity has been increased and the dissipation factor has been decreased against weight percentage of GO nanosheets up to 0.02 wt%. This was attributed to the role of EDL in capturing and trapping charge carriers and storing them on the surface of GO nanosheets.
- Above weight percentage 0.02 wt%, a decrement has been occurred in BDV and dielectric properties due to possible overlapping between adjacent EDLs, thereby reducing their effectiveness.
- The thermal properties, investigated by thermal conductivity, thermal response and thermogram proved continuous enhancement in thermal performance against weight percentage up to the maximum considered weight percentage of 0.05 wt%.
- Nanofluids behaved as smart fluids capable of dissipating more heat at higher temperatures, where the highest enhancement at 65 °C was 36.4% compared to 15.8% at 35 °C.
- Phonon transport through GO nanosheets, their Brownian motion, and their interparticle interactions due to EDL were proposed as the governing mechanism behind thermal performance enhancement.
Author Contributions
Funding
Conflicts of Interest
References
- Daghrah, M.; Wang, Z.; Liu, Q.; Hilker, A.; Gyore, A. Experimental Study of the Influence of Different Liquids on the Transformer Cooling Performance. IEEE Trans. Power Del. 2019, 34, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Rao, U.M.; Fofana, I.; Jaya, T.; Rodriguez-Celis, E.M.; Jalbert, J.; Picher, P. Alternative dielectric fluids for transformer insulation system: Progress, challenges, and future prospects. IEEE Access 2019, 7, 184552–184571. [Google Scholar]
- Salama, M.M.M.; Mansour, D.A.; Daghrah, M.; Abdelkasoud, S.M.; Abbas, A.A. Thermal performance of transformers filled with environmentally friendly oils under various loading conditions. Int. J. Electr. Power Energy Syst. 2020, 118, 105743. [Google Scholar] [CrossRef]
- IEEE. IEEE Guide for Acceptance and Maintenance of Natural Ester Insulating Liquid in Transformers; C57.147-2018; IEEE Std: New York, NY, USA, 2018. [Google Scholar]
- Wang, X.; Wang, Z.D. Study of dielectric behavior of ester transformer liquids under ac voltage. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1916–1925. [Google Scholar] [CrossRef]
- Abdelmalik, A.A. Analysis of thermally aged insulation paper in a natural ester-based dielectric fluid. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2408–2414. [Google Scholar] [CrossRef]
- Fernández, O.H.A.; Fofana, I.; Jalbert, J.; Gagnon, S.; Rodriguez-Celis, E.; Duchesne, S.; Ryadi, M. Aging characterization of electrical insulation papers impregnated with synthetic ester and mineral oil: Correlations between mechanical properties, depolymerization and some chemical markers. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 217–227. [Google Scholar]
- Raymon, A.; Pakianathan, P.S.; Rajamani, M.P.E.; Karthik, R. Enhancing the critical characteristics of natural esters with antioxidants for power transformer applications. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 899–912. [Google Scholar] [CrossRef]
- Madavan, R.; Balaraman, S. Comparison of antioxidant influence on mineral oil and natural ester properties under accelerated aging conditions. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2800–2808. [Google Scholar] [CrossRef]
- Federation of Indian Chambers of Commerce & Industry. COTTON 2020 Roadmap for Sustainable Production; FICCI: New Delhi, India, 2012. [Google Scholar]
- Durak, E.; Karaosmanoǧlu, F. Using of cottonseed oil as an environmentally accepted lubricant additive. Energy Sources 2004, 26, 611–625. [Google Scholar] [CrossRef]
- Liu, Q.; Singh, S.P.; Green, A.G. High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol. 2002, 129, 1732–1743. [Google Scholar] [CrossRef] [Green Version]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cottonseed oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- Raymon, A.; Sakthibalan, S.; Cinthal, C.; Subramaniaraja, R.; Yuvaraj, M. Enhancement and comparison of nano-ester insulating fluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 892–900. [Google Scholar] [CrossRef]
- Minea, A.A. A review on electrical conductivity of nanoparticle-enhanced fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Li, J.; Yao, W.; Wang, F.; Wan, F.; Tan, Y.; Mehmood, M.A. Electrical and thermal properties of insulating oil-based nanofluids: A comprehensive overview. IET Nanodielectr. 2019, 2, 27–40. [Google Scholar] [CrossRef]
- Geng, Y.; Khodadadi, H.; Karimipour, A.; Safaei, M.R.; Nguyen, T.K. A comprehensive presentation on nanoparticles electrical conductivity of nanofluids: Statistical study concerned effects of temperature, nanoparticles type and solid volume concentration. Physica A 2020, 542, 123432. [Google Scholar] [CrossRef]
- Qiu, L.; Zhu, N.; Feng, Y.; Michaelides, E.E.; Żyła, G.; Jing, D.; Zhang, X.; Norris, P.M.; Markides, C.N.; Mahian, O. Review of recent advances in thermophysical properties at the nanoscale: From solid state to colloids. Phys. Rep. 2020, 843, 1–81. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Ghazali, N.N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Akram, N. The effect of nano-additives in diesel-biodiesel fuel blends: A comprehensive review on stability, engine performance and emission characteristics. Energy Convers. Manag. 2018, 178, 146–177. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Nik-Ghazali, N.N.; Kalam, M.A.; Badruddin, I.A.; Banapurmath, N.R.; Khan, T.Y.; Bashir, M.N.; Akram, N.; Farade, R.; Afzal, A. The effects of graphene oxide nanoparticle additive stably dispersed in dairy scum oil biodiesel-diesel fuel blend on CI engine: Performance, emission and combustion characteristics. Fuel 2019, 257, 116015. [Google Scholar] [CrossRef]
- Ahmad, F.; Khan, A.A.; Khan, Q.; Hussain, M.R. State-of-art in nano-based dielectric oil: A review. IEEE Access 2019, 7, 13396–13410. [Google Scholar] [CrossRef]
- Mansour, D.A.; Shaalan, E.M.; Ward, S.A.; El Dein, A.Z.; Karaman, H.S.; Ahmed, H.M. Multiple nanoparticles for improvement of thermal and dielectric properties of oil nanofluids. IET Sci. Meas. Technol. 2019, 13, 968–974. [Google Scholar] [CrossRef]
- Charalampakos, V.P.; Peppas, G.D.; Pyrgioti, E.C.; Bakandritsos, A.; Polykrati, A.D.; Gonos, I.F. Dielectric insulation characteristics of natural ester fluid modified by colloidal iron oxide ions and silica nanoparticles. Energies 2019, 12, 3259. [Google Scholar] [CrossRef] [Green Version]
- Aljure, M.; Becerra, M.; Karlsson, M.E. Streamer inception from ultra-sharp needles in mineral oil based nanofluids. Energies 2018, 11, 2064. [Google Scholar] [CrossRef] [Green Version]
- Fal, J.; Mahian, O.; Żyła, G. Nanofluids in the service of high voltage transformers: Breakdown properties of transformer oils with nanoparticles, a Review. Energies 2018, 11, 2942. [Google Scholar] [CrossRef] [Green Version]
- Fontes, D.H.; Ribatski, G.; Filho, E.P.B. Experimental evaluation of thermal conductivity, viscosity and breakdown voltage AC of nanofluids of carbon nanotubes and diamond in transformer oil. Diam. Relat. Mater. 2015, 58, 115–121. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Tie, P. The effect of surfactants on heat transfer feature of nanofluids. Exp. Therm. Fluid Sci. 2013, 46, 259–262. [Google Scholar] [CrossRef]
- Williams, O.A.; Hees, J.; Dieker, C.; Jäger, W.; Kirste, L.; Nebel, C.E. Size-dependent reactivity of diamond nanoparticles. ACS Nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef]
- Álvarez-Regueiro, E.; Vallejo, J.P.; Fernández-Seara, J.; Fernández, J.; Lugo, L. Experimental convection heat transfer analysis of a nano-enhanced industrial coolant. Nanomaterials 2019, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Laguna, M.R.; Gómez-Romero, P.; Torres, C.M.S.; Chavez-Angel, E. Modification of the Raman spectra in graphene-based nanofluids and its correlation with thermal properties. Nanomaterials 2019, 9, 804. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Zhang, M.; Ji, J.; Pang, X.; Liu, R. Three-dimensional heterostructured reduced graphene oxide-hexagonal boron nitride-stacking material for silicone thermal grease with enhanced thermally conductive properties. Nanomaterials 2019, 9, 938. [Google Scholar] [CrossRef] [Green Version]
- Warner, J.H.; Schaffel, F.; Bachmatiuk, A.; Rummeli, M.H. Chapter 6: Applications of Graphene. In Graphene: Fundamentals and Emergent Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Asadi, A.; Aberoumand, S.; Moradikazerouni, A.; Pourfattah, F.; Żyła, G.; Estellé, P.; Mahian, O.; Wongwises, S.; Nguyen, H.M.; Arabkoohsar, A. Recent advances in preparation methods and thermophysical properties of oil-based nanofluids: A state-of-the-art review. Powder Technol. 2019, 352, 209–226. [Google Scholar] [CrossRef]
- Valantina, S.R.; Jayalatha, K.A.; Angeline, D.R.P.; Uma, S.; Ashvanth, B. Synthesis and characterisation of electro-rheological property of novel eco-friendly rice bran oil and nanofluid. J. Mol. Liq. 2018, 256, 256–266. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Bao, D. Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology 2010, 21, 055705. [Google Scholar] [CrossRef]
- Long, J.; Li, S.; Liang, J.; Wang, Z.; Liang, B. Preparation and characterization of graphene oxide and it application as a reinforcement in polypropylene composites. Polym. Compos. 2019, 40, 425–843. [Google Scholar] [CrossRef]
- Zainy, M.; Huang, N.M.; Kumar, S.V.; Lim, H.N.; Chia, C.H.; Harrison, I. Simple and scalable preparation of reduced graphene oxide-silver nanocomposites via rapid thermal treatment. Mater. Lett. 2012, 89, 180–183. [Google Scholar] [CrossRef]
- Nuncira, J.; Seara, L.M.; Sinisterra, R.D.; Caliman, V.; Silva, G.G. Long-term colloidal stability of graphene oxide aqueous nanofluids. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 407–417. [Google Scholar] [CrossRef]
- Choi, C.; Yoo, H.S.; Oh, J.M. Preparation and heat transfer properties of nanoparticle-in-transformer oil dispersions as advanced energy-efficient coolants. Curr. Appl. Phys. 2008, 8, 710–712. [Google Scholar] [CrossRef]
- Ise, N.; Sogami, I.S. Structure Formation in Solution: Ionic Polymers and Colloidal Particles; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Mansour, D.A.; Elsaeed, A.M.; Izzularab, M.A. The role of interfacial zone in dielectric properties of transformer oil-based nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3364–3372. [Google Scholar] [CrossRef]
- Bhunia, M.M.; Panigrahi, K.; Das, S.; Chattopadhyay, K.K.; Chattopadhyay, P. Amorphous graphene—Transformer oil nanofluids with superior thermal and insulating properties. Carbon 2018, 139, 1010–1019. [Google Scholar] [CrossRef]
- Sima, W.; Shi, J.; Yang, Q.; Huang, S.; Cao, X. Effects of conductivity and permittivity of nanoparticle on transformer oil insulation performance: Experiment and theory. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 380–390. [Google Scholar] [CrossRef]
- Raju, G.G. Dielectrics in Electric Fields, 2nd ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Kochetov, R.; Andritsch, T.; Morshuis, P.H.F.; Smit, J.J. Anomalous behaviour of the dielectric spectroscopy response of nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 107–117. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Two-dimensional phonon transport in graphene. J. Phys. Condens. Matter 2012, 24, 233203. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.A.; Majumdar, A. Transient ballistic and diffusive phonon heat transport in thin films. J. Appl. Phys. 1993, 74, 31. [Google Scholar] [CrossRef]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity in graphene: Prospects for thermal management application in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Shukla, K.N.; Koller, T.M.; Rausch, M.H.; Fröba, A.P. Effective thermal conductivity of nanofluids—A new model taking into consideration Brownian motion. Int. J. Heat Mass Transf. 2016, 99, 532–540. [Google Scholar] [CrossRef]
- Liu, J.M.; Liu, Z.H.; Chen, Y.J. Experiment and calculation of the thermal conductivity of nanofluid under electric field. Int. J. Heat Mass Transf. 2017, 107, 6–12. [Google Scholar] [CrossRef]
- Mansour, D.A.; Emara, R.F.; El-Nemr, M.K. Heat transport characteristics of oil-based nanofluids with different types of nanoparticles. In Proceedings of the IEEE 19th International Conference on Dielectric Liquids (ICDL), Manchester, UK, 25–29 June 2017; pp. 1–4. [Google Scholar]
- Jung, J.Y.; Yoo, J.Y. Thermal conductivity enhancement of nanofluids in conjunction with electrical double layer (EDL). Int. J. Heat Mass Transf. 2009, 52, 525–528. [Google Scholar] [CrossRef]
- Rodríguez-Laguna, M.D.R.; Castro-Alvarez, A.; Sledzinska, M.; Maire, J.; Costanzo, F.; Ensing, B.; Pruneda, M.; Ordejón, P.; Torres, C.S.; Gómez-Romero, P.; et al. Mechanisms behind the enhancement of thermal properties of graphene nanofluids. Nanoscale 2018, 10, 15402–15409. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farade, R.A.; Abdul Wahab, N.I.; Mansour, D.-E.A.; Azis, N.B.; bt. Jasni, J.; Soudagar, M.E.M.; Siddappa, V. Development of Graphene Oxide-Based Nonedible Cottonseed Nanofluids for Power Transformers. Materials 2020, 13, 2569. https://doi.org/10.3390/ma13112569
Farade RA, Abdul Wahab NI, Mansour D-EA, Azis NB, bt. Jasni J, Soudagar MEM, Siddappa V. Development of Graphene Oxide-Based Nonedible Cottonseed Nanofluids for Power Transformers. Materials. 2020; 13(11):2569. https://doi.org/10.3390/ma13112569
Chicago/Turabian StyleFarade, Rizwan A., Noor Izzri Abdul Wahab, Diaa-Eldin A. Mansour, Norhafiz B. Azis, Jasronita bt. Jasni, Manzoore Elahi M. Soudagar, and Vasudevamurthy Siddappa. 2020. "Development of Graphene Oxide-Based Nonedible Cottonseed Nanofluids for Power Transformers" Materials 13, no. 11: 2569. https://doi.org/10.3390/ma13112569






