Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Groups
2.2. Treatment Groups
2.3. Bone Marrow-Derived Mononuclear Cells (BMC)
2.4. Surgical Procedures: Generation of the Induced Membrane, Implantation of Decellularized Dermal Graft and β-TCP Scaffolds
2.5. Evaluation
2.5.1. µCT Analysis
2.5.2. Biomechanical Testing
2.5.3. Histological Analysis
2.6. Statistics
3. Results
3.1. Animal Care/Complications
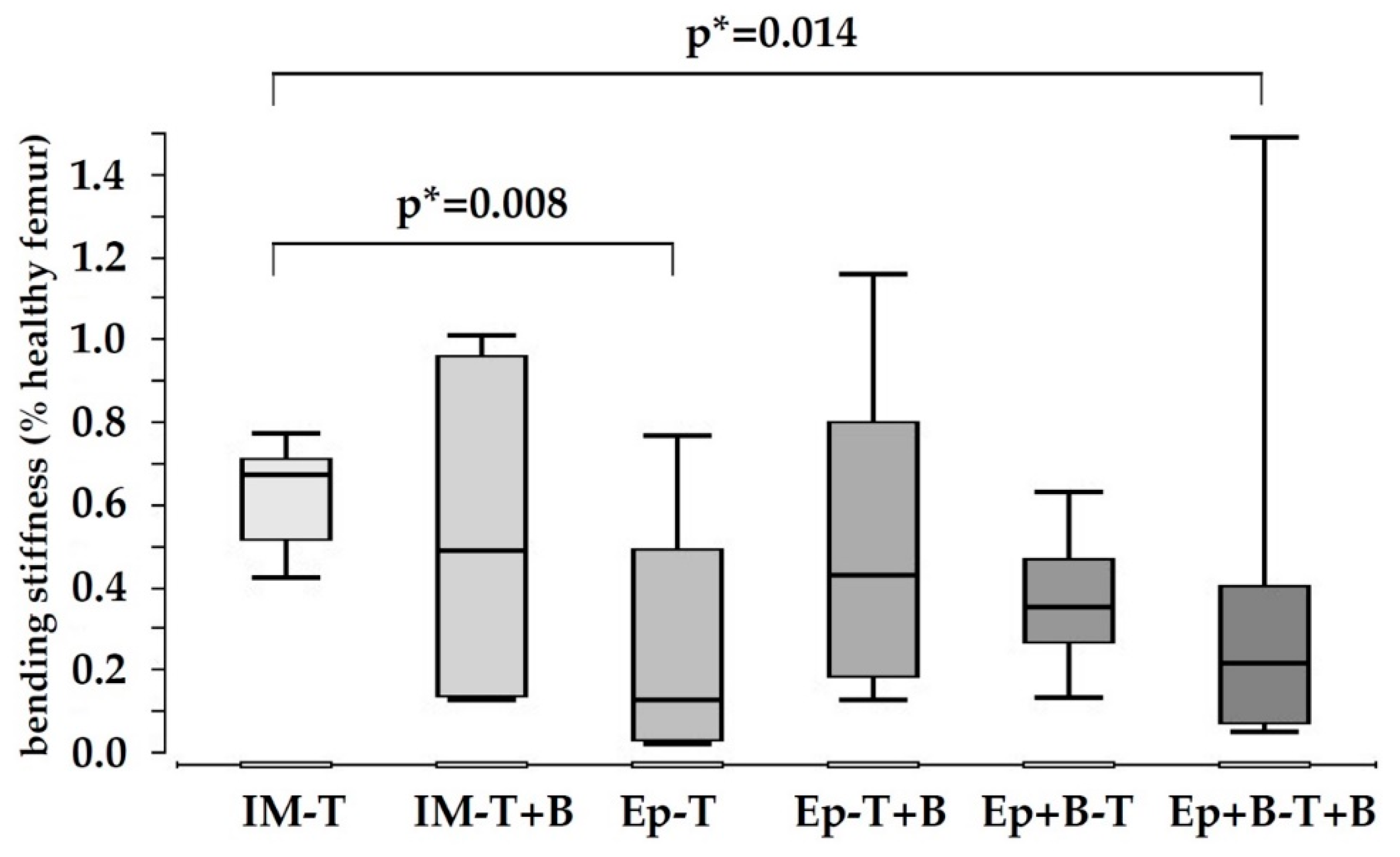
3.2. Low Biomechanical Properties in All Treatment Groups
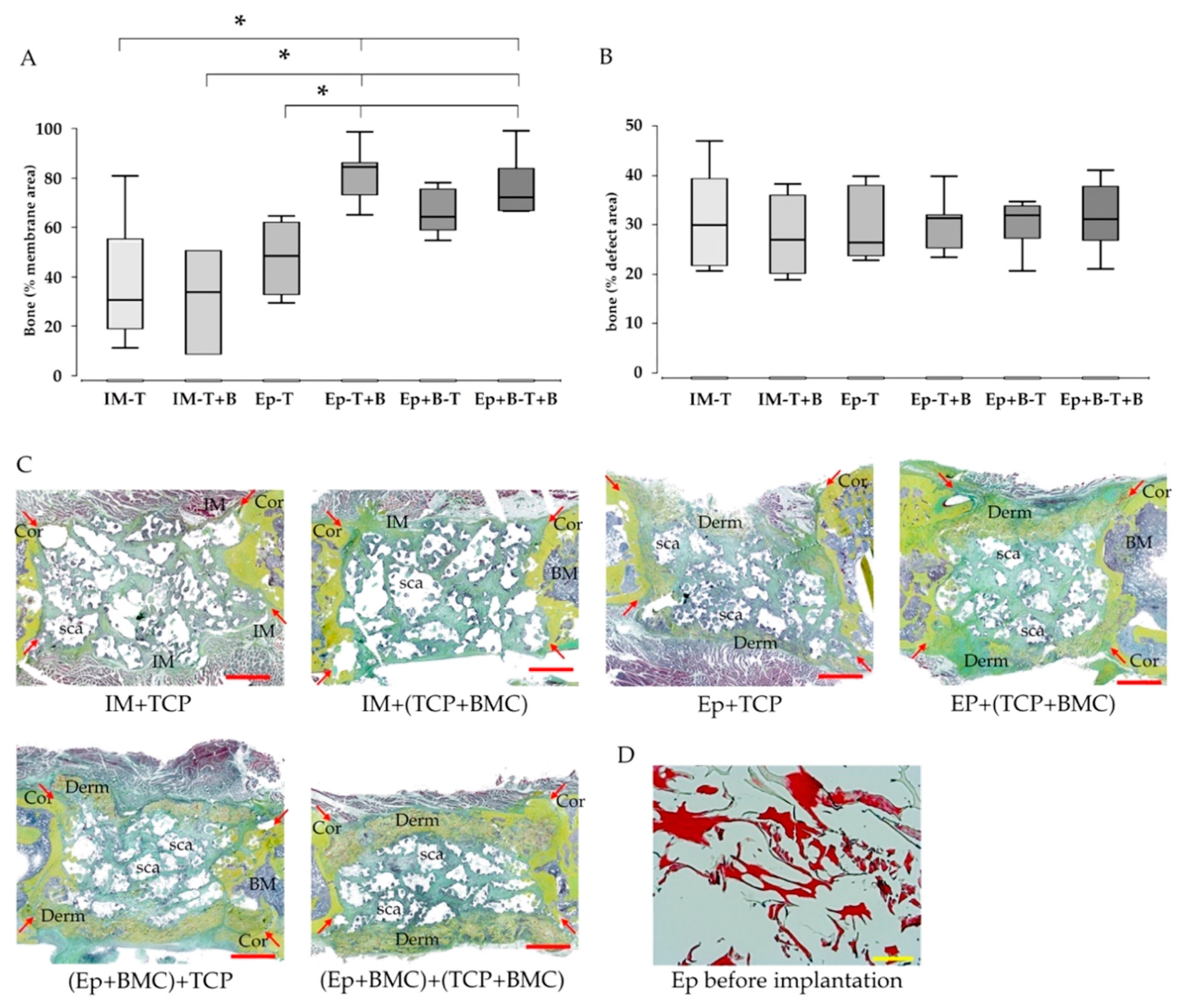
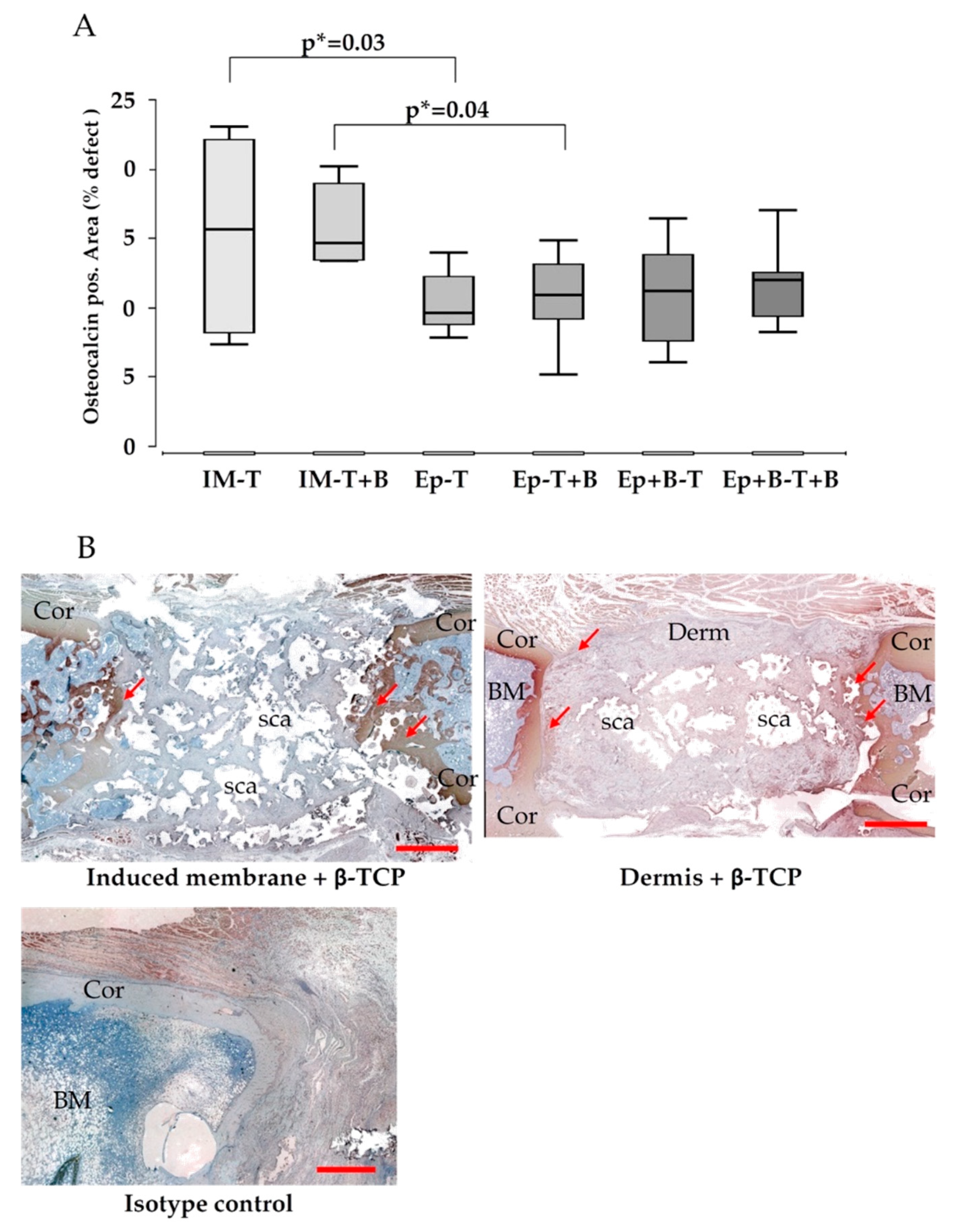
3.3. Increased Bone Formation in Epiflex®-Groups with BMC
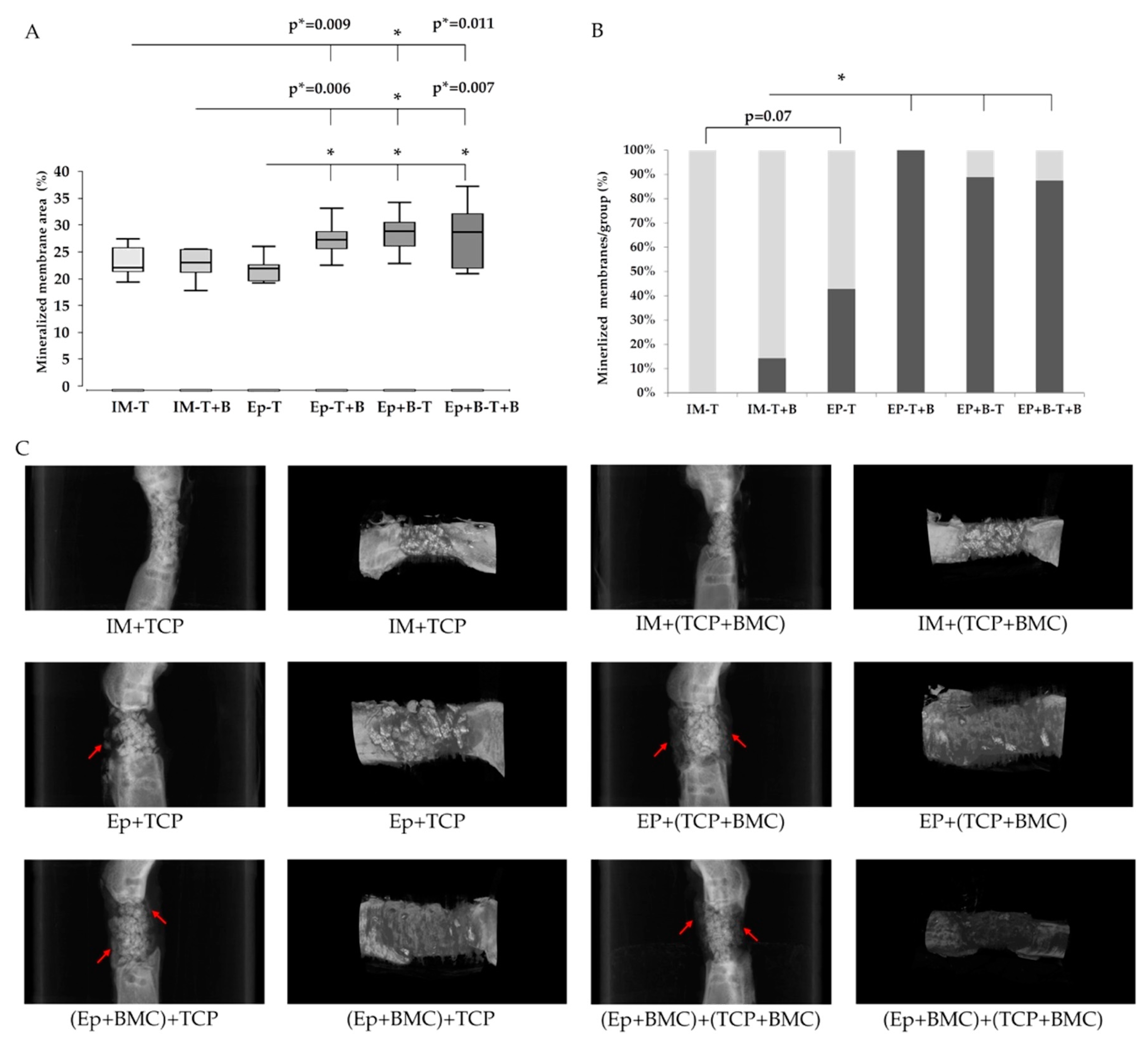
3.4. Callus Maturation
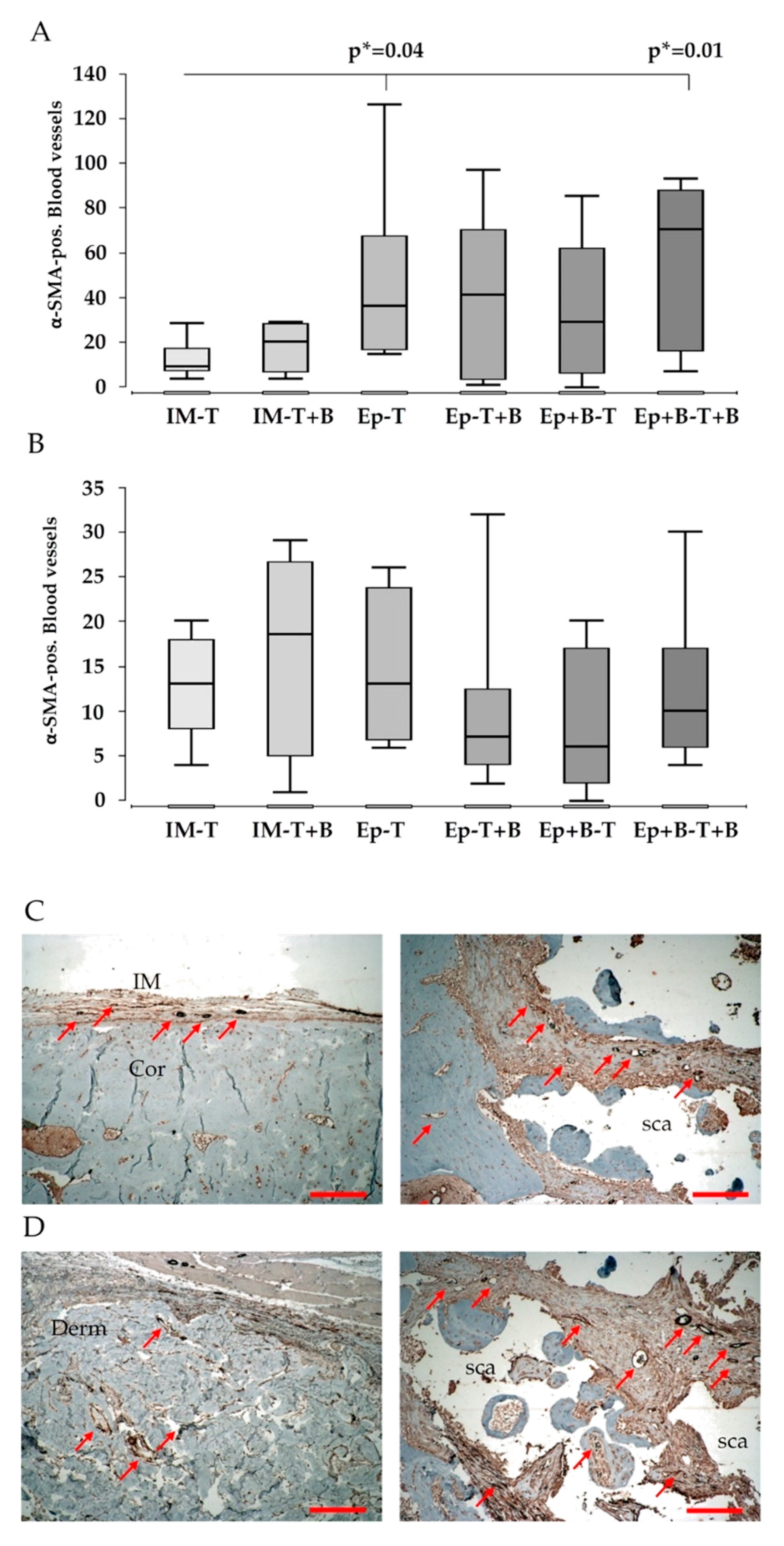
3.5. Vascularization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schemitsch, E.H. Size Matters: Defining Critical in Bone Defect Size! J. Orthop. Trauma. 2017, 31 (Suppl. 5), 20–22. [Google Scholar] [CrossRef]
- Keating, J.F.; Simpson, A.H.R.W.; Robinson, C.M. The management of fractures with bone loss. J. Bone Jt. Surg. Br. 2005, 87, 142–150. [Google Scholar] [CrossRef]
- Hannouche, D.; Petite, H.; Sedel, L. Current trends in the enhancement of fracture healing. J. Bone Jt. Surg. Br. 2001, 83, 157–164. [Google Scholar] [CrossRef]
- Masquelet, A.C.; Fitoussi, F.; Begue, T.; Muller, G.P. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann. Chir. Plast. Esthétique 2000, 45, 346–353. [Google Scholar]
- Keramaris, N.C.; Kaptanis, S.; Moss, H.L.; Loppini, M.; Pneumaticos, S.; Maffulli, N. Endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) in bone healing. Curr. Stem Cell Res. Ther. 2012, 7, 293–301. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Nau, C.; Basan, S.; Relja, B.; Wilhelm, K.; Schaible, A.; Frank, J.; Barker, J.; Marzi, I. Establishment and characterization of the Masquelet induced membrane technique in a rat femur critical-sized defect model. J. Tissue Eng. Regen. Med. 2016, 10, E382–E396. [Google Scholar] [CrossRef]
- Nau, C.; Simon, S.; Schaible, A.; Seebach, C.; Schröder, K.; Marzi, I.; Henrich, D. Influence of the induced membrane filled with syngeneic bone and regenerative cells on bone healing in a critical size defect model of the rat’s femur. Injury 2018, 49, 1721–1731. [Google Scholar] [CrossRef]
- Janko, M.; Pöllinger, S.; Schaible, A.; Bellen, M.; Schröder, K.; Heilani, M.; Fremdling, C.; Marzi, I.; Nau, C.; Henrich, D.; et al. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur. J. Trauma Emerg. Surg. 2020, 46, 265–276. [Google Scholar] [CrossRef]
- Jeon, O.; Song, S.J.; Bhang, S.H.; Choi, C.-Y.; Kim, M.J.; Kim, B.S. Additive effect of endothelial progenitor cell mobilization and bone marrow mononuclear cell transplantation on angiogenesis in mouse ischemic limbs. J. Biomed. Sci. 2007, 14, 323–330. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Schaible, A.; Relja, B.; Jugold, M.; Bönig, H.; Marzi, I. Cell-based therapy by implanted human bone marrow-derived mononuclear cells improved bone healing of large bone defects in rats. Tissue Eng. Part A 2015, 21, 1565–1578. [Google Scholar] [CrossRef]
- Nau, C.; Henrich, D.; Seebach, C.; Schröder, K.; Fitzsimmons, S.J.; Hankel, S.; Barker, J.H.; Marzi, I.; Frank, J. Treatment of Large Bone Defects with a Vascularized Periosteal Flap in Combination with Biodegradable Scaffold Seeded with Bone Marrow-Derived Mononuclear Cells: An Experimental Study in Rats. Tissue Eng. Part A 2016, 22, 133–141. [Google Scholar] [CrossRef]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bönig, H.; Marzi, I.; et al. Characterization of bone marrow mononuclear cells on biomaterials for bone tissue engineering in vitro. BioMed Res. Int. 2015, 2015, 762407–762412. [Google Scholar]
- Vitacolonna, M.; Belharazem, D.; Hohenberger, P.; Roessner, E.D. In-vivo quantification of the revascularization of a human acellular dermis seeded with EPCs and MSCs in co-culture with fibroblasts and pericytes in the dorsal chamber model in pre-irradiated tissue. Cell Tissue Bank. 2017, 18, 27–43. [Google Scholar] [CrossRef]
- Feng, X.; Tan, J.; Pan, Y.; Wu, Q.; Ruan, S.; Shen, R.; Chen, X.; Du, Y. Control of hypertrophic scar from inception by using xenogenic (porcine) acellular dermal matrix (ADM) to cover deep second degree burn. Burns 2006, 32, 293–298. [Google Scholar] [CrossRef]
- Sín, P.; Brychta, P. Cutometrical measurement confirms the efficacy of the composite skin grafting using allogeneic acellular dermis in burns. Acta Chir. Plast. 2006, 48, 59–64. [Google Scholar]
- Espinosa-de-los-Monteros, A.; de la Torre, J.I.; Marrero, I.; Andrades, P.; Davis, M.R.; Vásconez, L.O. Utilization of human cadaveric acellular dermis for abdominal hernia reconstruction. Ann. Plast. Surg. 2007, 58, 264–267. [Google Scholar] [CrossRef]
- Baillie, D.R.; Stawicki, S.P.; Eustance, N.; Warsaw, D.; Desai, D. Use of human and porcine dermal-derived bioprostheses in complex abdominal wall reconstructions: A literature review and case report. Ostomy Wound Manag. 2007, 53, 30–37. [Google Scholar]
- Hendrickx, B.; Vranckx, J.J.; Luttun, A. Cell-based vascularization strategies for skin tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 13–24. [Google Scholar] [CrossRef]
- Janko, M.; Dietz, K.; Rachor, J.; Sahm, J.; Schröder, K.; Schaible, A.; Nau, C.; Seebach, C.; Marzi, I.; Henrich, D. Improvement of Bone Healing by Neutralization of microRNA-335-5p, but not by Neutralization of microRNA-92A in Bone Marrow Mononuclear Cells Transplanted into a Large Femur Defect of the Rat. Tissue Eng. Part A 2019, 25, 55–68. [Google Scholar] [CrossRef]
- Nau, C.; Seebach, C.; Trumm, A.; Schaible, A.; Kontradowitz, K.; Meier, S.; Buechner, H.; Marzi, I.; Henrich, D. Alteration of Masquelet“s induced membrane characteristics by different kinds of antibiotic enriched bone cement in a critical size defect model in the rat’s femur. Injury 2016, 47, 325–334. [Google Scholar] [CrossRef]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bonig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Meier, S.; Nau, C.; Bönig, H.; Marzi, I. Safety and feasibility of cell-based therapy of autologous bone marrow-derived mononuclear cells in plate-stabilized proximal humeral fractures in humans. J. Transl. Med. 2016, 14, 314. [Google Scholar] [CrossRef]
- Vitacolonna, M.; Belharazem, D.; Hohenberger, P.; Roessner, E.D. Effect of dynamic seeding methods on the distribution of fibroblasts within human acellular dermis. Cell Tissue Bank. 2015, 16, 605–614. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Kähling, C.; Scherzed, A.; Wilhelm, K.; Tewksbury, R.; Powerski, M.; Marzi, I. Simultaneous cultivation of human endothelial-like differentiated precursor cells and human marrow stromal cells on beta-tricalcium phosphate. Tissue Eng. Part C Methods 2009, 15, 551–560. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schröder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2017, 106, 3009. [Google Scholar] [CrossRef]
- Garvey, W.; Fathi, A.; Bigelow, F.; Carpenter, B.; Jimenez, C. Improved Movat pentachrome stain. Stain. Technol. 1986, 61, 60–62. [Google Scholar] [CrossRef]
- Henrich, D.; Seebach, C.; Verboket, R.; Schaible, A.; Marzi, I.; Bonig, H. The osteo-inductive activity of bone-marrow-derived mononuclear cells resides within the CD14+ population and is independent of the CD34+ population. Eur. Cells Mater. 2018, 35, 165–177. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblasts-a diverse population at the center of it all. Int. Rev. Cell Mol. Biol. 2009, 276, 161–214. [Google Scholar]
- Holton, L.H.; Haerian, H.; Silverman, R.P.; Chung, T.; Elisseeff, J.H.; Goldberg, N.H.; Slezak, S. Improving long-term projection in nipple reconstruction using human acellular dermal matrix: An animal model. Ann. Plast. Surg. 2005, 55, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Gaio, N.; Martino, A.; Toth, Z.; Watson, J.T.; Nicolaou, D.; McBride-Gagyi, S. Masquelet technique: The effect of altering implant material and topography on membrane matrix composition, mechanical and barrier properties in a rat defect model. J. Biomech. 2018, 72, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zuo, R.; Long, H.; Wang, Y.; Zhang, Y.; Sun, C.; Luo, G.; Zhang, Y.; Li, C.; Zhou, Y.; et al. Advances in the Masquelet technique: Myeloid-derived suppressor cells promote angiogenesis in PMMA-induced membranes. Acta Biomater. 2020, 108, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Gindraux, F.; Loisel, F.; Bourgeois, M.; Oudina, K.; Melin, M.; de Billy, B.; Sergent, P.; Leclerc, G.; Petite, H.; Auber, F.; et al. Induced membrane maintains its osteogenic properties even when the second stage of Masquelet’s technique is performed later. Eur. J. Trauma Emerg. Surg. 2020, 46, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Aho, O.M.; Lehenkari, P.; Ristiniemi, J.; Lehtonen, S.; Risteli, J.; Leskelä, H.V. The mechanism of action of induced membranes in bone repair. J. Bone Jt. Surg. Am. 2013, 95, 597–604. [Google Scholar] [CrossRef]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schröder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef]
- Liu, K.; Wang, Y.; Sun, Y.; Qi, X.; Tian, L.; Zhao, Y.; Xu, Y.; Liu, X. [Masquelet technique combined with artificial dermis for the treatment of bone and soft tissue defects in rabbits]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2019, 33, 578–585. [Google Scholar]
- DeBaun, M.R.; Stahl, A.M.; Daoud, A.I.; Pan, C.C.; Bishop, J.A.; Gardner, M.J.; Yang, Y.P. Preclinical induced membrane model to evaluate synthetic implants for healing critical bone defects without autograft. J. Orthop. Res. 2019, 37, 60–68. [Google Scholar] [CrossRef]
- Meinig, R.P.; Rahn, B.; Perren, S.M.; Gogolewski, S. Bone regeneration with resorbable polymeric membranes: Treatment of diaphyseal bone defects in the rabbit radius with poly(L-lactide) membrane. A pilot study. J. Orthop. Trauma 1996, 10, 178–190. [Google Scholar] [CrossRef]
- Gindraux, F.; Rondot, T.; de Billy, B.; Zwetyenga, N.; Fricain, J.C.; Pagnon, A.; Obert, L. Similarities between induced membrane and amniotic membrane: Novelty for bone repair. Placenta 2017, 59, 116–123. [Google Scholar] [CrossRef]
- Tarchala, M.; Engel, V.; Barralet, J.; Harvey, E.J. A pilot study: Alternative biomaterials in critical sized bone defect treatment. Injury 2018, 49, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Masquelet, A.C.; Begue, T. The concept of induced membrane for reconstruction of long bone defects. Orthop. Clin. N. Am. 2010, 41, 27–37. [Google Scholar] [CrossRef]
- Leiblein, M.; Koch, E.; Winkenbach, A.; Schaible, A.; Nau, C.; Büchner, H.; Schröder, K.; Marzi, I.; Henrich, D. Size matters: Effect of granule size of the bone graft substitute (Herafill®) on bone healing using Masquelet’s induced membrane in a critical size defect model in the rat’s femur. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 1469–1482. [Google Scholar] [CrossRef] [PubMed]

| Group | Treatment | Number of Animals µCT/Biomechanics | Number of Animals for Histology | Donor-Animals for Syngenic BMC |
|---|---|---|---|---|
| 1 | IM + β-TCP | 6 | 10 | - |
| 2 | IM + (β-TCP + BMC) | 6 | 10 | 4 |
| 3 | Ep + β-TCP | 6 | 10 | - |
| 4 | Ep + (β-TCP + BMC) | 6 | 10 | 4 |
| 5 | (Ep + BMC) + β-TCP | 6 | 10 | 4 |
| 6 | (Ep + BMC) + (β-TCP + BMC) | 6 | 10 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiblein, M.; Kolb, T.; Christian, L.; Schröder, K.; Yaman, C.; Schaible, A.; Marzi, I.; Henrich, D.; Janko, M. Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat. Materials 2020, 13, 2629. https://doi.org/10.3390/ma13112629
Leiblein M, Kolb T, Christian L, Schröder K, Yaman C, Schaible A, Marzi I, Henrich D, Janko M. Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat. Materials. 2020; 13(11):2629. https://doi.org/10.3390/ma13112629
Chicago/Turabian StyleLeiblein, Maximilian, Tobias Kolb, Lion Christian, Katrin Schröder, Ceyhan Yaman, Alexander Schaible, Ingo Marzi, Dirk Henrich, and Maren Janko. 2020. "Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat" Materials 13, no. 11: 2629. https://doi.org/10.3390/ma13112629
APA StyleLeiblein, M., Kolb, T., Christian, L., Schröder, K., Yaman, C., Schaible, A., Marzi, I., Henrich, D., & Janko, M. (2020). Introduction of a New Surgical Method to Improve Bone Healing in a Large Bone Defect by Replacement of the Induced Membrane by a Human Decellularized Dermis Repopulated with Bone Marrow Mononuclear Cells in Rat. Materials, 13(11), 2629. https://doi.org/10.3390/ma13112629






