Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization
Abstract
:1. Introduction
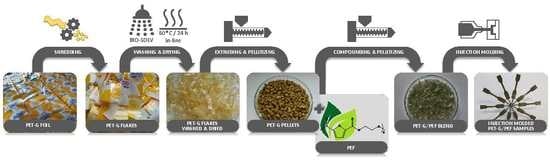
2. Materials and Methods
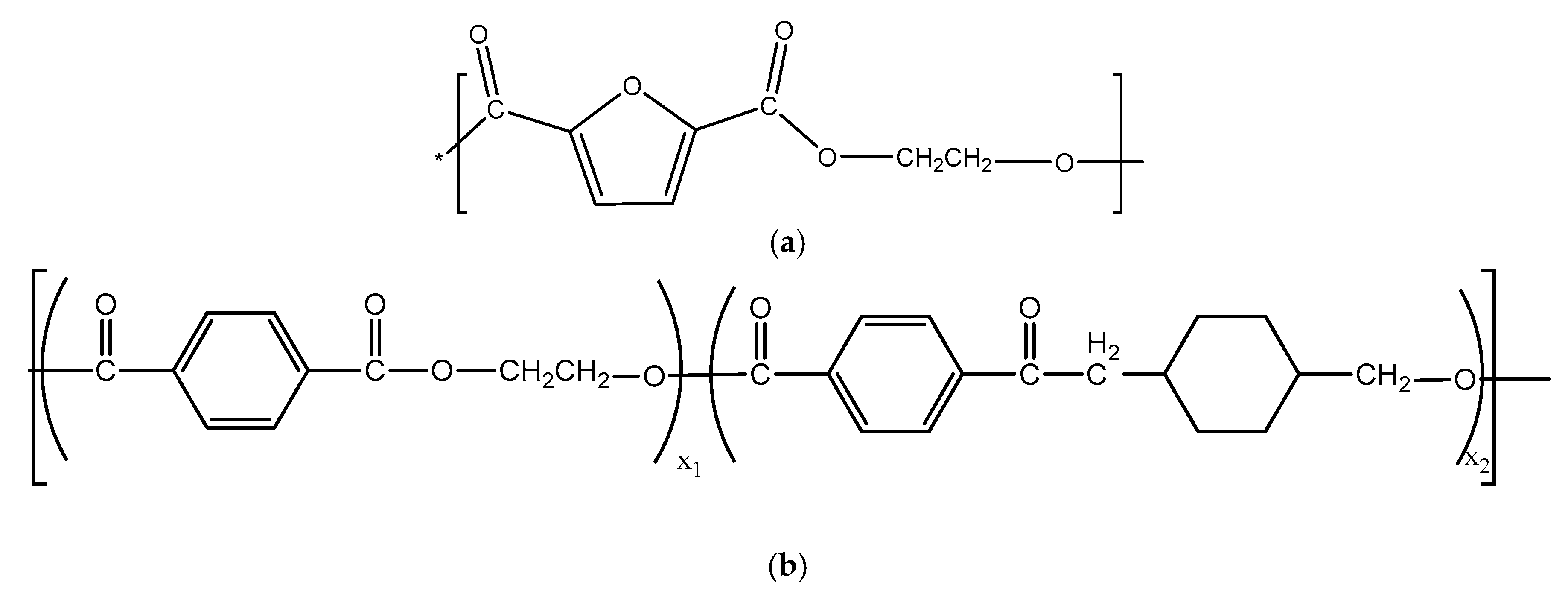
2.1. Preparation of Poly(Ethylene 2,5-Furanoate) (PEF)
2.2. Preparation of PET-G pellets
2.3. Preparation of PET-G/PEF Blends
2.4. Characterization of PET-G/PEF Blends
3. Results and Discussion
3.1. Solubility Assessment
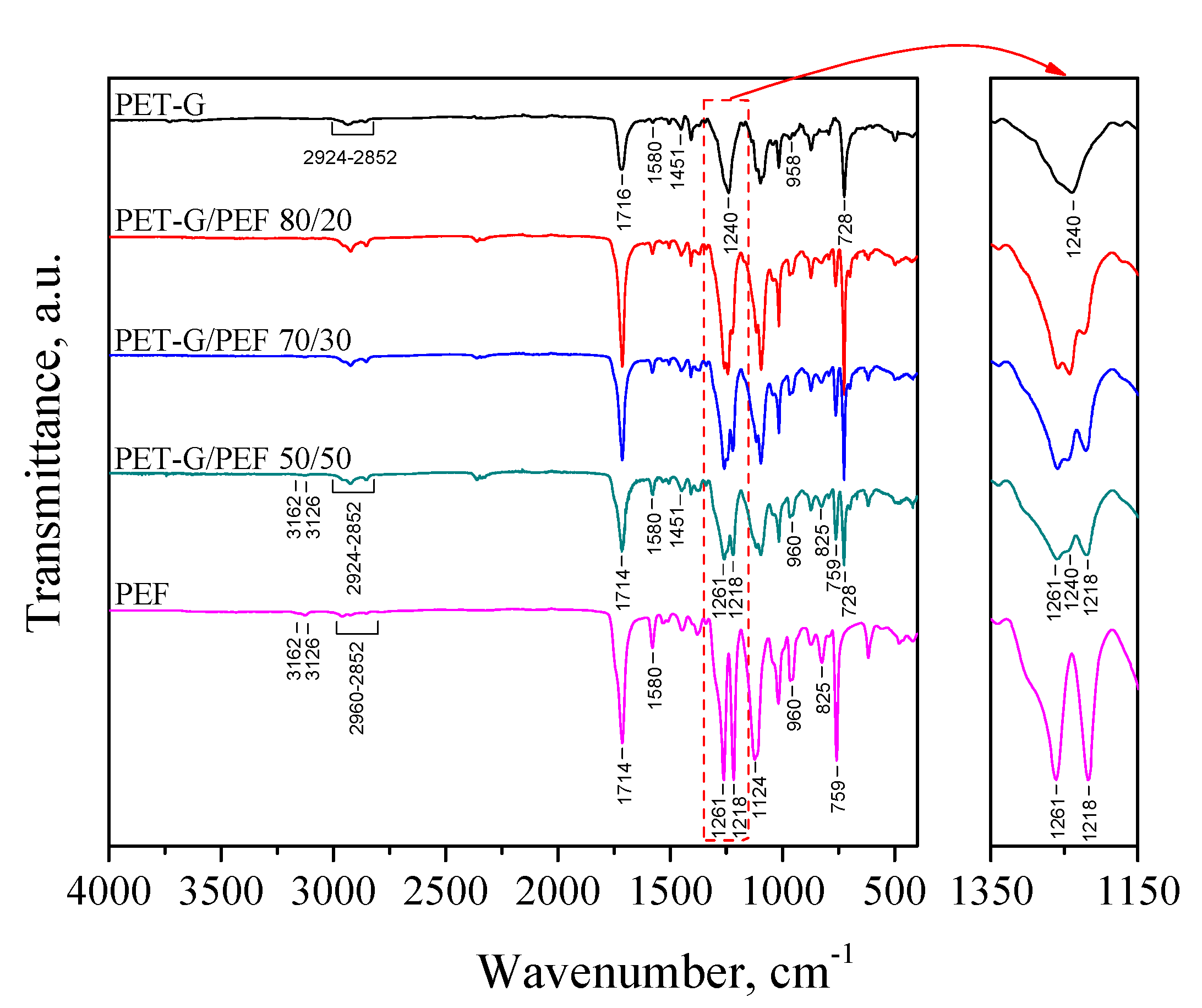
3.2. Analysis of Interfacial Interactions
3.3. Morphology of PET-G/PEF Blends
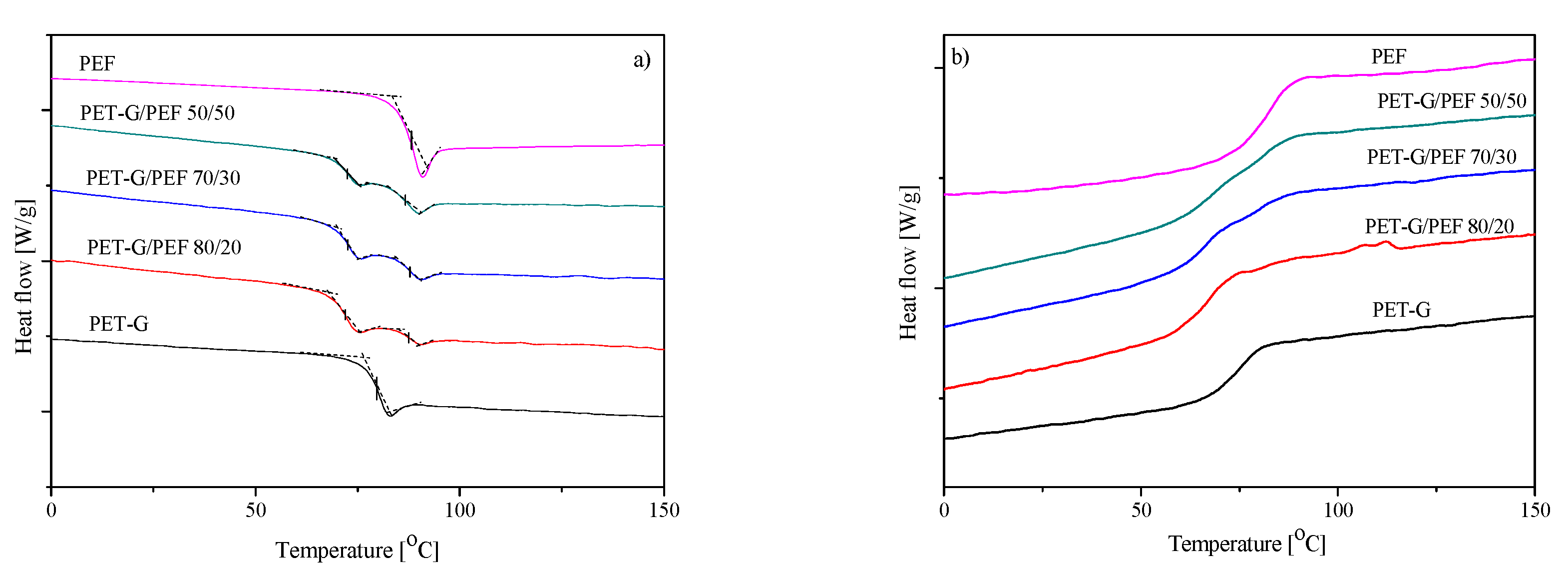
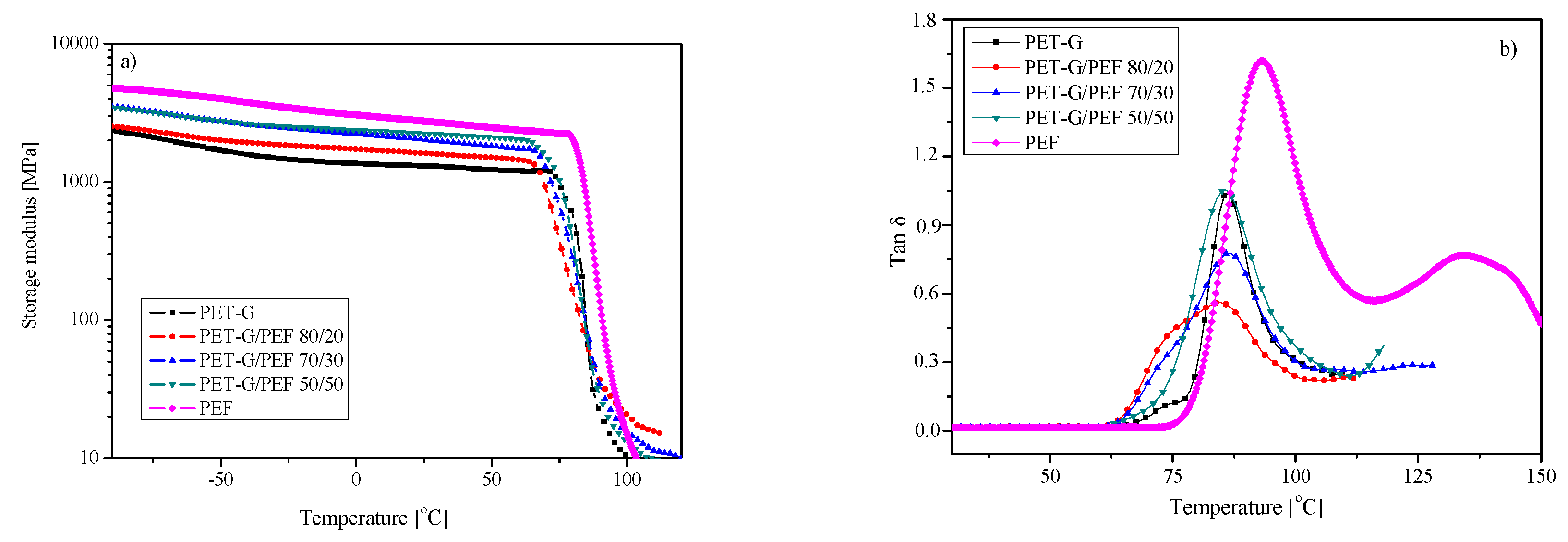
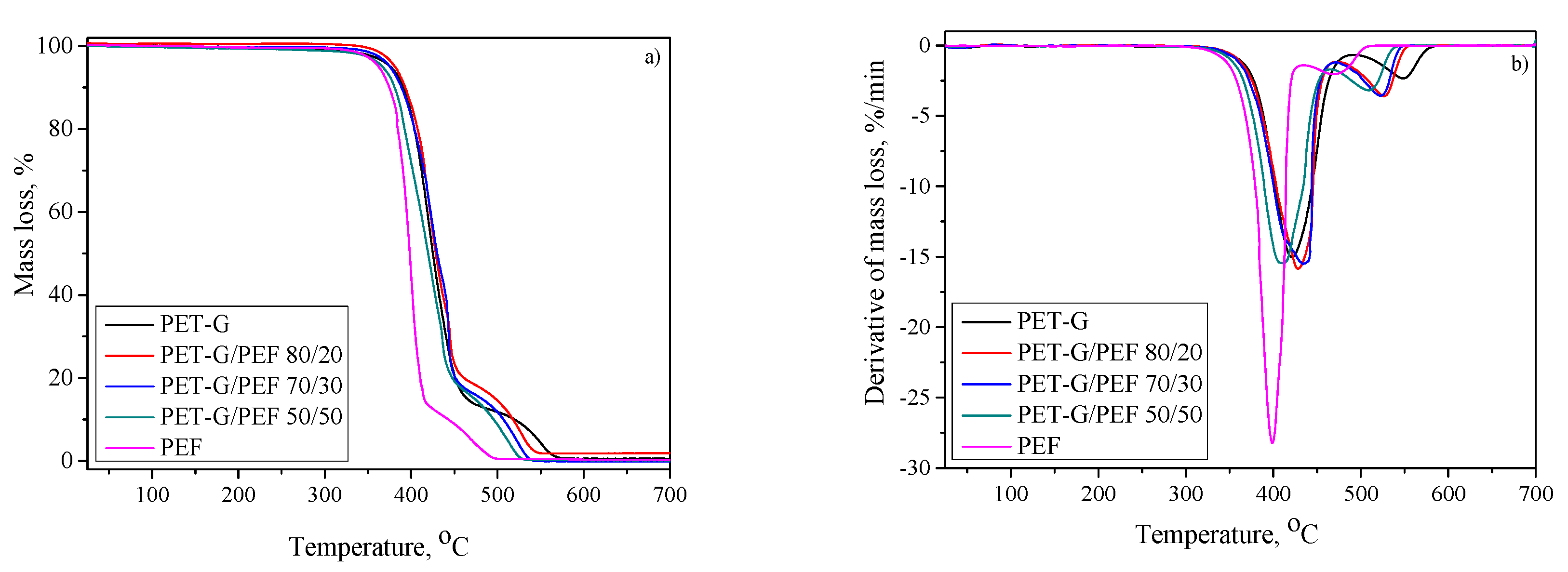
3.4. Structural and Thermal Properties
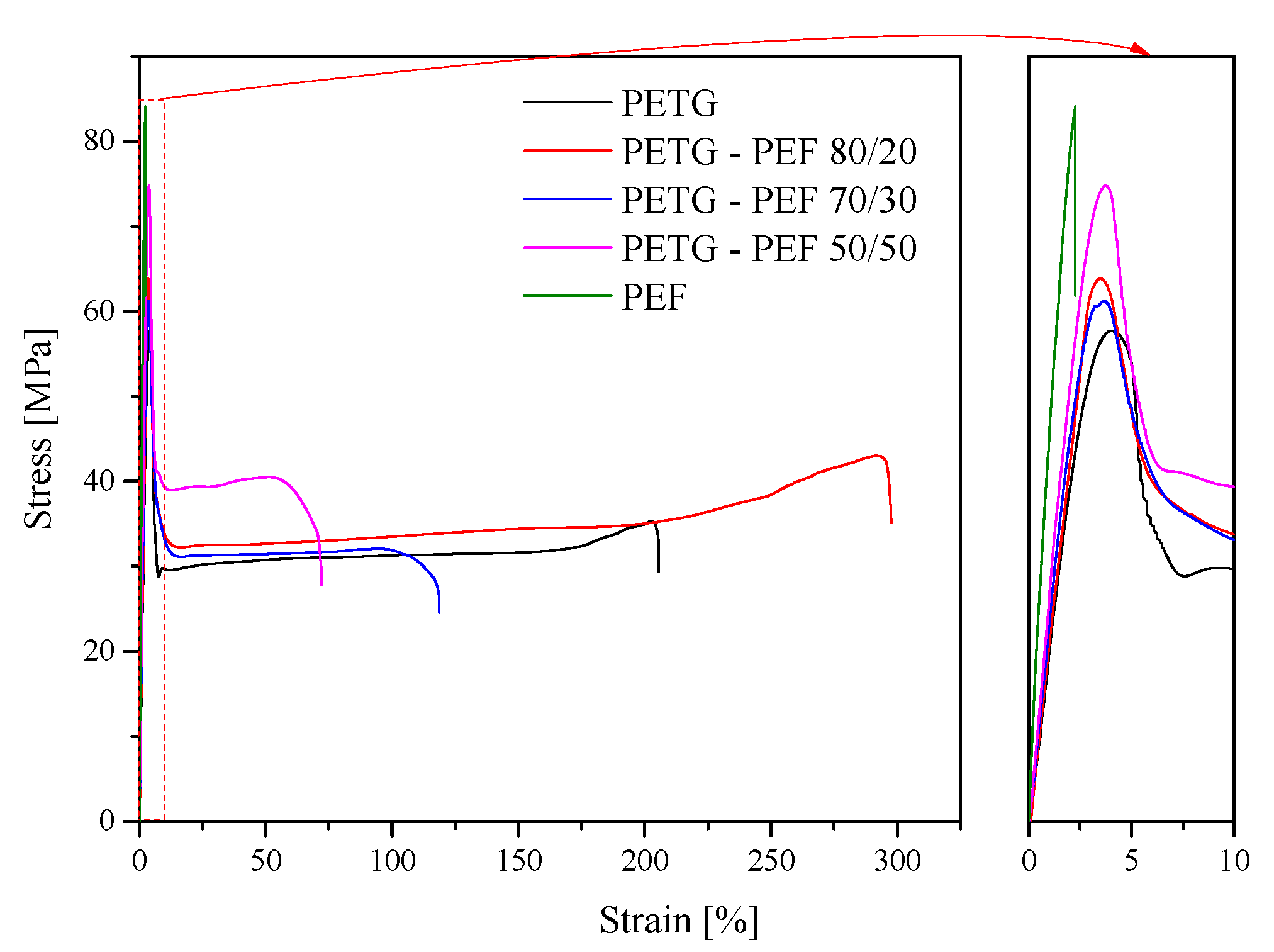
3.5. Mechanical Properties
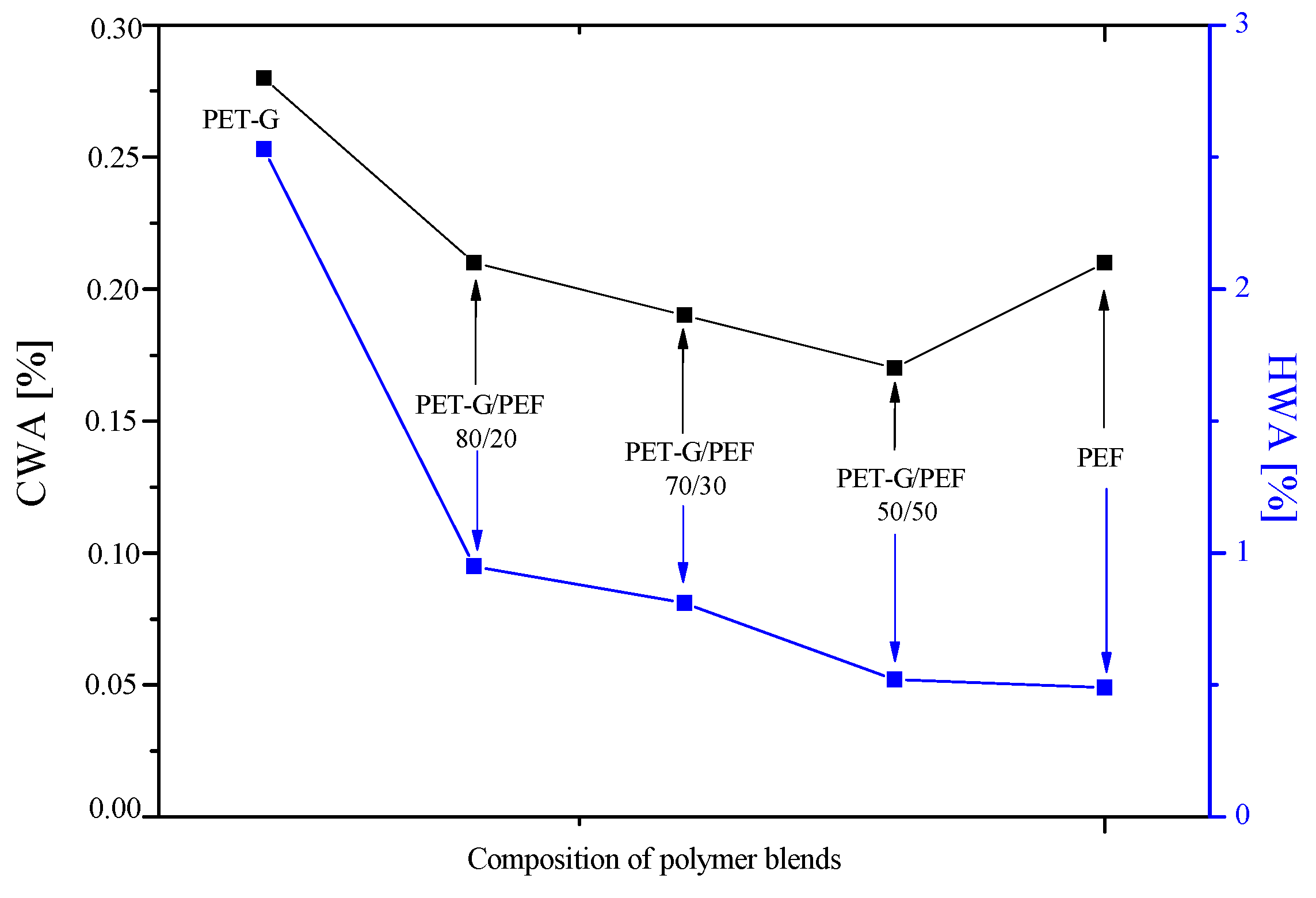
3.6. Water Absorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paul, D.R. Polymer Blends; Academic Press: London, UK, 1978. [Google Scholar]
- Utracki, L.A. Polymer Blends and Alloys; Hanser: Munich, Germany, 1989. [Google Scholar]
- Cicala, G.; Tosto, C.; Latteri, A.; La Rosa, A.D.; Blanco, I.; Elsabbagh, A.; Russo, P.; Ziegmann, G. Green composites based on blends of polypropylene with liquid wood reinforced with hemp fibers: Thermomechanical properties and the effect of recycling cycle. Materials 2017, 10, 998. [Google Scholar] [CrossRef] [Green Version]
- Van Krevelen, D.W. Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780080548197. [Google Scholar]
- Available online: https://www.ecowatch.com/22-facts-about-plastic-pollution-and-10-things-we-can-do-about-it-1881885971.html (accessed on 10 April 2020).
- Long, T.E. Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0471498564. [Google Scholar]
- Turner, S.R. Development of amorphous copolyesters based on 1,4-cyclohexanedimethanol. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5847–5852. [Google Scholar] [CrossRef]
- Tsai, Y.; Facn, C.-H.; Hung, C.-Y.; Fuu-Jen, T. Poly(ethylene terephthalate) copolymers that contain 5-tert-butylisophthalic acid and 1-3/1-4-cyclohexanedimethanol: Synthesis, characterization, and properties. J. Appl. Polym. Sci. 2008, 109, 2598–2604. [Google Scholar] [CrossRef]
- Latko-Durałek, P.; Dydek, K.; Boczkowska, A. Thermal, Rheological and Mechanical Properties of PETG/rPETG Blends. J. Polym. Environ. 2019, 27, 2600–2606. [Google Scholar] [CrossRef] [Green Version]
- Ignatyev, I.A.; Thielemans, W.; Vander Beke, B. Recycling of Polymers: A Review. ChemSusChem 2014, 7, 1579–1593. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Pipertzis, A.; Kasmi, N.; Bikiaris, D.N.; Papageorgiou, D.G.; Floudas, G.; Papageorgiou, G.Z. Green polymeric materials: On the dynamic homogeneity and miscibility of furan-based polyester blends. Polymer (Guildf). 2019, 174, 187–199. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, Z.; Hu, X.; Li, Y.; Zhang, Q.; Li, Y.; Wang, R.; Zhang, L. Bio-based polyesters based on 2,5-furandicarboxylic acid as 3D-printing materials: Design, preparation and performances. Eur. Polym. J. 2019, 114, 476–484. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Irska, I.; Pawlikowska, D.; Piesowicz, E. Synthesis and characterization of new reactive polymer blends based on post-consumer glycol-modified poly(ethylene terephthalate) foils and poly(tetramethylene oxide). Polimery 2018, 63, 45–48. [Google Scholar] [CrossRef]
- Vannini, M.; Marchese, P.; Celli, A.; Lorenzetti, C. Fully biobased poly(propylene 2,5-furandicarboxylate) for packaging applications: Excellent barrier properties as a function of crystallinity. Green Chem. 2015, 17, 4162–4166. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Liu, S.; Wang, G. Biobased copolyesters: Synthesis, structure, thermal and mechanical properties of poly(ethylene 2,5-furandicarboxylate-co-ethylene 1,4-cyclohexanedicarboxylate). Polym. Degrad. Stab. 2018, 154, 96–102. [Google Scholar] [CrossRef]
- Cicala, G.; Giordano, D.; Tosto, C.; Filippone, G.; Recca, A.; Blanco, I. Polylactide (PLA) filaments a biobased solution for additive manufacturing: Correlating rheology and thermomechanical properties with printing quality. Materials 2018, 11, 1191. [Google Scholar] [CrossRef] [Green Version]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates - The US Department of Energy’s “top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Campisi, S.; Veith, G.M.; Prati, L. Pd-modified Au on carbon as an effective and durable catalyst for the direct oxidation of HMF to 2,5-furandicarboxylic acid. ChemSusChem 2013, 6, 609–612. [Google Scholar] [CrossRef]
- Gomes, M.; Gandini, A.; Silvestre, A.J.D.; Reis, B. Synthesis and characterization of poly(2,5-furan dicarboxylate)s based on a variety of diols. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3759–3768. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Physical aging in amorphous poly(ethylene furanoate): Enthalpic recovery, density, and oxygen transport considerations. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 389–399. [Google Scholar] [CrossRef]
- Burgess, S.K.; Leisen, J.E.; Kraftschik, B.E.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Chain mobility, thermal, and mechanical properties of poly(ethylene furanoate) compared to poly(ethylene terephthalate). Macromolecules 2014, 47, 1383–1391. [Google Scholar] [CrossRef]
- Burgess, S.K.; Mikkilineni, D.S.; Yu, D.B.; Kim, D.J.; Mubarak, C.R.; Kriegel, R.M.; Koros, W.J. Water sorption in poly(ethylene furanoate) compared to poly(ethylene terephthalate). Part 2: Kinetic sorption. Polymer (Guildf). 2014, 55, 6870–6882. [Google Scholar] [CrossRef]
- Burgess, S.K.; Wenz, G.B.; Kriegel, R.M.; Koros, W.J. Penetrant transport in semicrystalline poly(ethylene furanoate). Polymer (Guildf). 2016, 98, 305–310. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Smyrnioti, D.; Nikolaidis, G.N.; Tsitsimaka, I.; Christodoulou, E.; Bikiaris, D.N.; Charitopoulou, M.A.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Sustainable plastics from biomass: Blends of polyesters based on 2,5-furandicarboxylic acid. Polymers 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, S.K.; Kriegel, R.M.; Koros, W.J. Carbon dioxide sorption and transport in amorphous poly(ethylene furanoate). Macromolecules 2015, 48, 2184–2193. [Google Scholar] [CrossRef]
- Mao, Y.; Kriegel, R.M.; Bucknall, D.G. The crystal structure of poly(ethylene furanoate). Polymer (Guildf). 2016, 102, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, C.P.; Kalfoglou, N.K. Compatibility behaviour of blends of poly(ethylene terephthalate) with an amorphous copolyester. Polymer (Guildf). 1997, 38, 631–637. [Google Scholar] [CrossRef]
- Szostak, M. Właściwości mechaniczne mieszanin PET/PETG wykonywanych technologią wtryskiwania z wykorzystaniem mieszalnika dynamicznego. Mech. Czas. Tech. 2009, 3, 331–336. [Google Scholar]
- Nabi Saheb, D.; Jog, J.P. Crystallization and equilibrium melting behavior of PBT/PETG blends. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2439–2444. [Google Scholar] [CrossRef]
- Jheng, L.C.; Yang, C.Y.; Leu, M.T.; Hsu, K.H.; Wu, J.H.; Ruan, J.; Shih, K.C. Novel impacts of glycol-modified poly(ethylene terephthalate)(PETG) to crystallization behavior of polyethylene naphthalate (PEN) within stretched miscible blends. Polymer (Guildf). 2012, 53, 2758–2768. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.L.; Li, H.Y.; Li, B.; Wang, K.; Zhang, Q.; Fu, Q. Superior tensile extensibility of PETG/PC amorphous blends induced via uniaxial stretching. Chinese J. Polym. Sci. 2011, 29, 125–132. [Google Scholar] [CrossRef]
- Lacroix, C.; Bousmina, M.; Carreau, P.J.; Favis, B.D.; Michel, A. Properties of PETG/EVA blends: 1. Viscoelastic, morphological and interfacial properties. Polymer (Guildf). 1996, 37, 2939–2947. [Google Scholar] [CrossRef]
- Lacroix, C.; Bousmina, M.; Carreau, P.J.; Llauro, M.F.; Pétiaud, R.; Michel, A. Properties of PETG/EVA blends: 2. Study of reactive compatibilization by n.m.r. spectroscopy and linear viscoelastic properties. Polymer (Guildf). 1996, 37, 2949–2956. [Google Scholar] [CrossRef]
- Zhang, X.; Li, B.; Wang, K.; Zhang, Q.; Fu, Q. The effect of interfacial adhesion on the impact strength of immiscible PP/PETG blends compatibilized with triblock copolymers. Polymer (Guildf). 2009, 50, 4737–4744. [Google Scholar] [CrossRef]
- Blanco, I.; Rapisarda, M.; Portuesi, S.; Ognibene, G.; Cicala, G. Thermal behavior of PEI/PETG blends for the application in fused deposition modelling (FDM). AIP Conf. Proc. 2018, 1981, 020181. [Google Scholar]
- Paszkiewicz, S.; Irska, I.; Piesowicz, E. Modification of substandard EPDM with amorphous thermoplastic polyesters (PETG and PEF): Microstructure and physical properties. Polish J. Chem. Technol. 2018, 20, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Jiang, M.; Wang, G.; Zhou, G. Novel biobased high toughness PBAT/PEF blends: Morphology, thermal properties, crystal structures and mechanical properties. New J. Chem. 2020, 44, 3112–3121. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Kasmi, N.; Siampani, M.; Terzopoulou, Z.N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Exploring next-generation engineering bioplastics: Poly(alkylene furanoate)/poly(alkylene terephthalate) (PAF/PAT) blends. Polymers 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszkiewicz, S.; Taraghi, I.; Pawlikowska, D.; Szymczyk, A.; Irska, I.; Stanik, R.; Linares, A.; Ezquerra, T.A.; Piesowicz, E. Influence of hybrid system of nanofillers on the functional properties of postconsumer PET-G–based nanocomposites. Polym. Adv. Technol. 2019, 30, 2983–2992. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Irska, I.; Piesowicz, E.; Jotko, M.; Lisiecki, S.; Bartkowiak, A.; Sieradzka, M.; Fryczkowski, R.; et al. Improvement of barrier properties of glycol modified poly(ethylene terephthalate) based nanocomposites containing graphene derivatives forms. Polimery 2017, 62, 868–874. [Google Scholar] [CrossRef]
- Holden, G. Thermoplastic Elastomers. In Rubber Technology; Morton, M., Ed.; Springer: Boston, MA, USA, 1987; Volume 53, ISBN 9788578110796. [Google Scholar]
- Fakirov, S. Handbook of Condensation Thermoplastic Elastomers; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–619. [Google Scholar]
- Hoy, K.L. New values of the solubility parameters from vapor pressure data. J. Paint Technol. 1970, 42, 76–118. [Google Scholar]
- Chen, T.; Zhang, W.; Zhang, J. Alkali resistance of poly(ethylene terephthalate) (PET) and poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) (PETG) copolyesters: The role of composition. Polym. Degrad. Stab. 2015, 120, 232–243. [Google Scholar] [CrossRef]
- Paszkiewicz, S.; Szymczyk, A.; Pawlikowska, D.; Irska, I.; Taraghi, I.; Pilawka, R.; Gu, Y.; Li, X.; Tu, Y.; Piesowicz, E. Synthesis and characterization of poly(ethylene terephthalate-co-1,4-cyclohexanedimethylene terephtlatate)-block-poly(tetramethylene oxide) copolymers. RSC Adv. 2017, 7, 41745. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Jiang, G.; Li, G.; Wu, Z.; Zhang, J. Poly(ethylene glycol-co-1,4-cyclohexanedimethanol terephthalate) random copolymers: Effect of copolymer composition and microstructure on the thermal properties and crystallization behavior. RSC Adv. 2015, 5, 60570–60580. [Google Scholar] [CrossRef]
- Kwiatkowska, M.; Kowalczyk, I.; Kwiatkowski, K.; Szymczyk, A.; Rosłaniec, Z. Fully biobased multiblock copolymers of furan-aromatic polyester and dimerized fatty acid: Synthesis and characterization. Polymer (Guildf). 2016, 99, 503–512. [Google Scholar] [CrossRef]
- Sousa, A.F.; Coelho, J.F.J.; Silvestre, A.J.D. Renewable-based poly((ether)ester)s from 2,5-furandicarboxylic acid. Polymer (Guildf). 2016, 98, 129–135. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda Van Ekenstein, G.O.R.; Loos, K. A biocatalytic approach towards sustainable furanic-aliphatic polyesters. Polym. Chem. 2015, 6, 5198–5211. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, K.; Evstatiev, M.; Fakirov, S.; Evstatiev, O.; Ishii, M.; Harrass, M. Microfibrillar reinforced composites from PET/PP blends: Processing, morphology and mechanical properties. Compos. Sci. Technol. 2005, 65, 107–116. [Google Scholar] [CrossRef]
- Pospiech, D.; Häußler, L.; Korwitz, A.; Fischer, O.; Starke, S.; Jehnichen, D.; Köppl, T.; Altstädt, V. The miscibility of poly(butylene terephthalate) (PBT) with phosphorus polyester flame retardants. High. Perform. Polym. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Calderón, B.A.; Sobkowicz, M.J. Evidence of compatibility and thermal stability improvement of poly(propylene carbonate) and polyoxymethylene blends. J. Appl. Polym. Sci. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Gandini, A.; Armando, J.D.; Silvestre, C.P.N.; Souza, A.F.; Gomes, M. The Furan Counterpart of Poly(ethylene terephthalate): An Alternative Material Based on Renewable Resources. J. Polym. Sci. Part A Polym. Chem. Polym. Sci. Part. A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Gubbels, E.; Jasinska-Walc, L.; Koning, C.E. Synthesis and characterization of novel renewable polyesters based on 2,5-furandicarboxylic acid and 2,3-butanediol. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 890–898. [Google Scholar] [CrossRef]
- Knoop, R.J.I.; Vogelzang, W.; Van Haveren, J.; Van Es, D.S. High molecular weight poly(ethylene-2,5-furanoate); Critical aspects in synthesis and mechanical property determination. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4191–4199. [Google Scholar] [CrossRef]
- Momanyi, J.; Herzog, M.; Muchiri, P. Analysis of thermomechanical properties of selected class of recycled thermoplastic materials based on their applications. Recycling 2019, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Codou, A.; Moncel, M.; Van Berkel, J.G.; Guigo, N.; Sbirrazzuoli, N. Glass transition dynamics and cooperativity length of poly(ethylene 2,5-furandicarboxylate) compared to poly(ethylene terephthalate). Phys. Chem. Chem. Phys. 2016, 18, 16647–16658. [Google Scholar] [CrossRef] [PubMed]
- Thirtha, V.; Lehman, R.; Nosker, T. Glass transition effects in immiscible polymer blends. Annu. Tech. Conf. - ANTEC, Conf. Proc. 2005, 6, 303–307. [Google Scholar] [CrossRef] [Green Version]
- Thirtha, V.; Lehman, R.; Nosker, T. Glass transition phenomena in melt-processed polystyrene/polypropylene blends. Polym. Eng. Sci. 2005, 45, 1187–1193. [Google Scholar] [CrossRef]
- Irska, I.; Paszkiewicz, S.; Gorący, K.; Linares, A.; Ezquerra, T.A.; Jędrzejewski, R.; Rosłaniec, Z.; Piesowicz, E. Poly(Butylene terephthalate)/polylactic acid based copolyesters and blends: Miscibility-structure-property relationship. Express Polym. Lett. 2020, 14, 26–47. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Li, Q.; Li, Y.; Zhang, H.; Yu, J.; Cao, A. Thermal degradation and their kinetics of biodegradable poly(butylene succinate-co-butylene terephthate)s under nitrogen and air atmospheres. Polym. Degrad. Stab. 2006, 91, 1685–1693. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal properties of thermoplastic starch/synthetic polymer blends with potential biomedical applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- Ciro, E.; Parra, J.; Zapata, M.; Murillo, E.A. Effect of the Recycled Rubber on the Properties of Recycled Rubber/Recycled Polypropylene Blends. Ingeniería y Ciencia 2015, 11, 173–188. [Google Scholar] [CrossRef]
- Gu, L.; Luo, K.; Huang, B.; Song, Y. Effects of fillers on the transfer properties of UV waterless offset ink. Appl. Mech. Mater. 2013, 262, 510–513. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Liu, T.; Cao, X.; Zhu, H. Preparation and characterization of PEG/SiO2 composites as shape-stabilized phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2013, 118, 48–53. [Google Scholar] [CrossRef]
- Makhlouf, A.; Satha, H.; Frihi, D.; Gherib, S.; Segula, R. Optimization of the crystallinity of polypropylene / submicronic-talc composites: The role of filler ratio and cooling rate. eXPRESS Polym. Lett. 2016, 10, 237–247. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E.; Narkis, M. Sliding wear, viscoelasticity, and brittleness of polymers. J. Mater. Res. 2006, 21, 2422–2428. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Hagg Lobland, H.E.; Khoja, S. Brittleness and toughness of polymers and other materials. Mater. Lett. 2015, 159, 478–480. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, K.; Li, B.; Chen, F.; Fu, Q. Brittle-ductile transition in the PETG/PC blends by adding PTW elastomer. Polym. Adv. Technol. 2010, 21, 401–407. [Google Scholar] [CrossRef]
- Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-state polymerization of poly(ethylene furanoate) biobased polyester, I: Effect of catalyst type on molecular weight increase. Polymers 2017, 9, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Min, K.D.; Nam, B.U.; Park, O.O. High molecular weight bio furan-based co-polyesters for food packaging applications: Synthesis, characterization and solid-state polymerization. Green Chem. 2016, 18, 5142–5150. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A.; Fambri, L.; Lonardi, C.; Šlouf, M.; Kolařik, J. Linear low density polyethylene/cycloolefin copolymer blends. Express Polym. Lett. 2011, 5, 23–37. [Google Scholar] [CrossRef]
- Shen, J.; Wang, M.; Li, J.; Guo, S.; Xu, S.; Zhang, Y.; Li, T.; Wen, M. Simulation of mechanical properties of multilayered propylene-ethylene copolymer/ethylene 1-octene copolymer composites by equivalent box model and its experimental verification. Eur. Polym. J. 2009, 45, 3269–3281. [Google Scholar] [CrossRef]
- Karmaker, A.C. Effect of water absorption on dimensional stability and impact energy of jute fibre reinforced polypropylene. J. Mater. Sci. Lett. 1997, 16, 462–464. [Google Scholar] [CrossRef]
- Gaudichet-Maurin, E.; Thominette, F.; Verdu, J. Water sorption characteristics in moderately hydrophilic polymers, part 1: Effect of polar groups concentration and temperature in water sorption in aromatic polysulfones. J. Appl. Polym. Sci. 2008, 109, 3279–3285. [Google Scholar] [CrossRef]
- List of Products. Available online: www.coreychem.com (accessed on 5 May 2020).


| Solubility Parameters | PET-G [MPa1/2] | PEF [MPa1/2] |
|---|---|---|
| δtot. | 33.87 | 31.30 |
| δp | 13.88 | 16.95 |
| δh | 23.38 | 20.75 |
| δd | 20.20 | 16.19 |
| ΔδPET-G/PEF | 5.70 | |
| Sample | Tg [°C] | ΔCp [J/g∙°C] | TB [°C] | E’ at 25 °C [MPa] | Tα (tan δ) [°C] | Td,5% [°C] | Td,DTG1 [°C] | Td,DTG2 [°C] |
|---|---|---|---|---|---|---|---|---|
| PET-G | 80 | 0.49 | TB1=124 ± 2 TB2=140 ± 3 | 1307 | 86 | 376 | 421 | 549 |
| PET-G/PEF 80/20 | Tg1=72 Tg2=87 | ΔCp1=0.29 ΔCp2=0.14 | 127 ± 3 | 1607 | 85 | 381 | 429 | 528 |
| PET-G/PEF 70/30 | Tg1=72 Tg2=87 | ΔCp1=0.24 ΔCp2=0.18 | 137 ± 3 | 2020 | 86 | 375 | 434 | 522 |
| PET-G/PEF 50/50 | Tg1=72 Tg2=86 | ΔCp1=0.17 ΔCp2=0.23 | 174 ± 4 | 2247 | 86 | 367 | 410 | 509 |
| PEF | 87 | 0.49 | 197 ± 4 | 2755 | 93 | 362 | 399 | 470 |
| Sample | IV [dL/g] | E [MPa] | σy [MPa] | εy [%] | σB [MPa] | εB [%] | B (1012∙(%∙Pa)) |
|---|---|---|---|---|---|---|---|
| PET-G | 0.590 | 1.47 ± 0.02 | 57.69 ± 0.28 | 3.96 ± 0.23 | 35.32 ± 0.43 | 205.95 ± 2.87 | 3.7150 |
| PET-G/PEF 80/20 | 0.590 | 1.84 ± 0.14 | 63.83 ± 2.09 | 3.50 ± 0.09 | 43.01 ± 0.92 | 297.55 ± 30.18 | 2.0913 |
| PET-G/PEF 70/30 | 0.582 | 1.98 ± 0.08 | 60.67 ± 0.65 | 3.27 ± 0.24 | 32.36 ± 2.37 | 115.17 ± 5.43 | 4.2984 |
| PET-G/PEF 50/50 | 0.558 | 2.47 ± 0.03 | 74.78 ± 0.30 | 3.75 ± 0.19 | 37.83 ± 1.37 | 82.98 ± 7.47 | 5.3632 |
| PEF | 0.503 | 5.43 ± 0.03 | - | - | 84.07 ± 4.43 | 2.27 ± 0.14 | 159.9015 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz, S.; Irska, I.; Piesowicz, E. Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials 2020, 13, 2673. https://doi.org/10.3390/ma13122673
Paszkiewicz S, Irska I, Piesowicz E. Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials. 2020; 13(12):2673. https://doi.org/10.3390/ma13122673
Chicago/Turabian StylePaszkiewicz, Sandra, Izabela Irska, and Elzbieta Piesowicz. 2020. "Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization" Materials 13, no. 12: 2673. https://doi.org/10.3390/ma13122673
APA StylePaszkiewicz, S., Irska, I., & Piesowicz, E. (2020). Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials, 13(12), 2673. https://doi.org/10.3390/ma13122673