1. Introduction
Over the centuries, silk threads have been fabricated conventionally into braided, knitted and non-woven matrices. Recently, it discovered that the material made from silk performed attractive properties such as biocompatibility, biodegradation, non-toxicity, and adsorption properties, which are important for medical applications [
1,
2]. Therefore, versatile processabilities from dissolved fibroin fibers to fabricate various morphologies of silk such as sponges, hydrogels, films, mats, micro-particles, and microneedles were made [
2,
3] for various medical applications such as tissue engineering, drug delivery [
4,
5,
6,
7], and osteotomy fixation [
8,
9]. On the other hand, after being dissolved, the fibroin structure has been found to be severely degraded in the peptide-strand structure of silk fibroin [
10]. The correlations of the degraded phenomena on the experiment processability will be clarified in this paper.
Over the past two decades, significant research has focused on producing bio-resins (e.g., silk fibroin) via various methods as an environmentally friendly alternative for a wide range of accessories made from synthetic materials. These materials not only offer mechanical properties equal to the conventional plastics but are also biodegradable, biocompatible, and non-toxic, which are suitable for biomedical applications [
11]. This is the reason why this silk-based resin is also a strong potential candidate to apply as biodegradable polymeric materials in biomedical fields, which is also mentioned in [
12,
13,
14]. The goal is to attain mechanical properties similar to those of polyether ether ketone (PEEK) resin [
15], which is one of the super engineering plastics that has been obtained. To reach this goal, the present research focuses on improving the mechanical properties of silk resins.
The conventional method to produce silk resin is by hot-pressing silk powder. Silk resin is produced by hot-pressing silk powder with 27% water between stainless plates under a pressure of 44 MPa and at a temperature of 160 °C for 1 h [
16]. Silk resin may also be produced by hot-pressing laminated silk fibroin sheets [
17]. However, the water absorbance of the laminated sheets significantly exceeds that of turtle shell. The hardness is equivalent to that of tortoiseshell, which can serve as a substitute material for turtle shells in applications such as eyeglass frames and ornaments. However, the bending strength of silk resin is 70 MPa, which is much less than that of tortoiseshell (225–333 MPa).
In previous work, we fabricated silk resin with a bending strength of 100 MPa and a bending Young’s modulus of 4.5 GPa [
18] by hot-pressing commercial silk powder obtained by the dissolution of Bombyx mori (B. mori) silk thread after degumming in an aqueous solution of a neutral salt, desalination, and then freeze-drying [
19]. We used commercial silk powder with 20 wt% of water added, heated to 150–170 °C using spark plasma sintering followed by cold-pressing at 20–30 MPa for a short time. The resulting silk resin has a thermal conductivity of 0.44 W/(mK) and a glass transition temperature of 180 °C.
Recently, we introduced a more straightforward method to prepare silk resin that involves hot-pressing silk powders directly by milling raw silk fibers (without degumming) from B. mori and Eri silk fibers at 170 °C and 31.2 MPa [
20]. The maximum three-point bending strength and the Young’s modulus of the resulting silk resin are 122 MPa and 8.7 GPa, respectively, for B. mori silk resin and 100 MPa and 8.5 GPa, respectively, for Eri silk resin. Analysis by attenuated total reflectance Fourier-transform infrared (ATR-FTIR) spectroscopy shows that, after resinification and drying, the β-sheet content in B. mori silk increases and the random coil (RC) structure decreases. In contrast, after resinification, the secondary structure in Eri silk resin goes from a random coil structure to a β-strand structure, which converts to a β-sheet structure after drying.
Another approach is to use hot-rolling to fabricate silk resin sheets from silk powder, which allows hot-rolling equipment to be used for continuous production [
21]. Silk powder mixed with 20 wt% of water is wrapped in a pulp sheet and processed by hot-rolling at 130 °C. A curing area of 90% is attained with a rolling reduction ratio of 60%. Because the bending strength decreases significantly upon increasing the rolling reduction ratio, the optimum three-point bending strength of the silk resin sheet is 100 MPa for a 40% rolling reduction ratio. According to previous X-ray diffraction (XRD) analyses [
18,
20,
21,
22], the silk resin sheets have the silk II crystal structure, which is the β-sheet crystal. Casting from an aqueous solution of silk fibroin produces a β-rich silk film that depends on the casting conditions, such as drying temperature, drying rate, type of substrate, and the initial concentration of fibroin [
23,
24,
25]. Another method of producing the β-sheet form is by using temperature-controlled water vapor annealing [
26,
27,
28] or by soaking in a polar solvent such as ethanol [
29,
30,
31,
32,
33,
34,
35,
36,
37].
The goal of the present work is to prepare high-strength silk resin sheets by hot-rolling and to investigate the processability of these sheets with and without a pretreatment consisting of soaking in ethanol or boiling water. We also evaluate the mechanical, thermal, and structural properties of the silk resin sheets. This approach is motivated by the hypothesis that preparing silk resin sheets by hot-pressing and subsequent hot-rolling will improve the mechanical properties of the sheets because the hot-pressing process increases the silk II crystal structure, which is then aligned by hot-rolling the silk resin sheets. To optimize the hot-rolling process, the original resins underwent several pretreatments, in particular, soaking in ethanol or boiling water. A previous study reported that the presence of water reduces the strength of the crystalline structure by reducing the number of hydrogen bonds, the hydrogen bond lifetime, and the peak rupture force while increasing the specific interaction energy [
37]. The same research also confirmed that this effect of moisture and the position-dependent mechanical response of silk II crystallites should help enhance the mechanical properties of silk fibroin [
38].
2. Materials and Experiment
2.1. Materials
This study used commercially available silk powder (KB Seiren, Ltd., Shiga, Japan) derived from B. mori silk produced by dissolving the waste of spinning-silk thread in an aqueous neutral salt solution, followed by desalination, solidification, precipitation, dehydration, drying, and finally pulverization [
19]. The result is called “regenerated silk” (RS) powder. Pulverized powders of B. mori silk thread or Eri silk thread without degumming were prepared by milling as previously reported [
20]. Ethanol (Kanto Chemical. Co., Inc., Tokyo, Japan, 99.5%) and distilled water were used for the pretreatment.
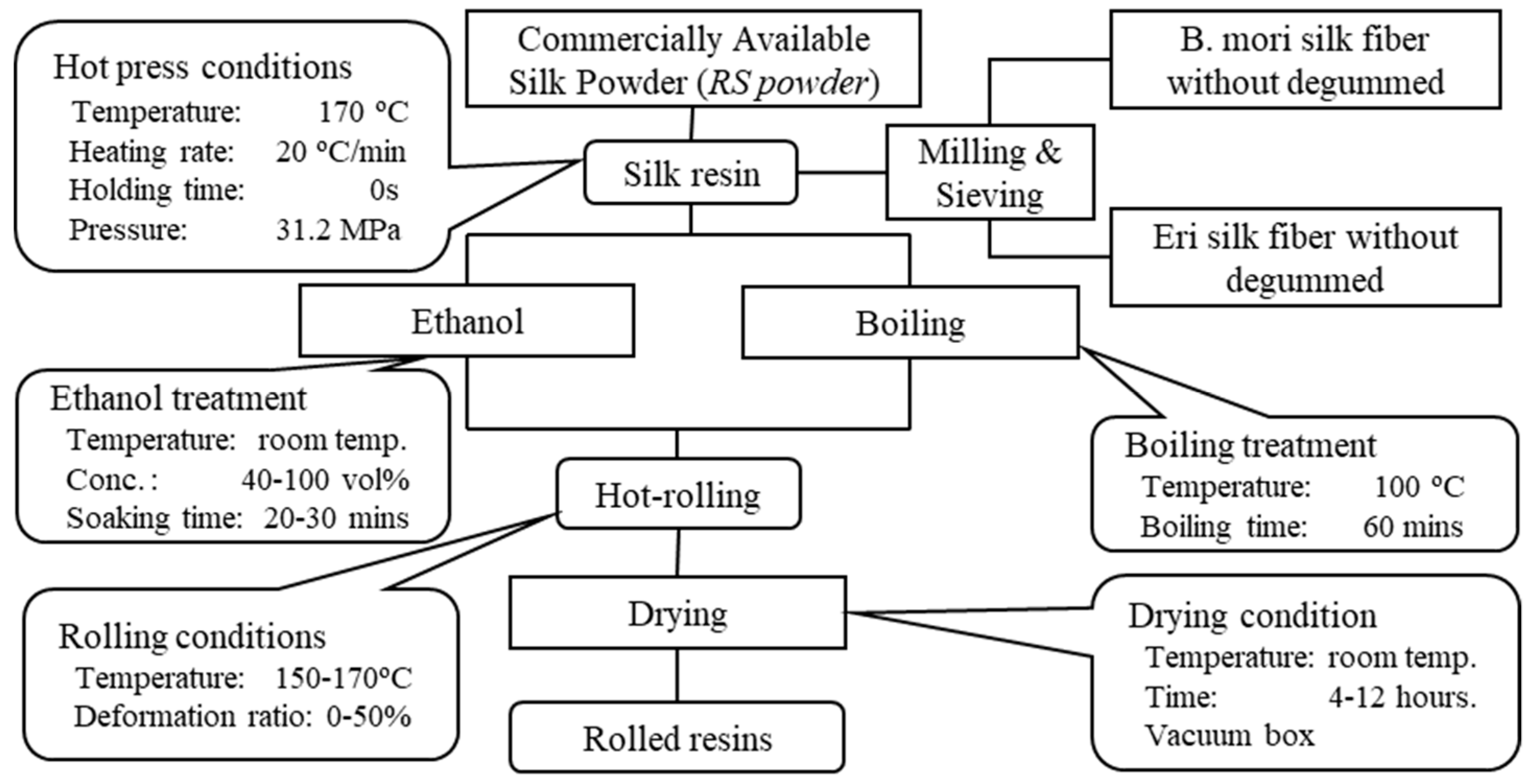
Figure 1 shows a flow chart describing how to prepare the silk resin.
2.2. Amino Acid Composition
The amino acid content in the fibroin samples was determined using a High-Speed Amino AL-8800 instrument (Hitachi, Japan), which hydrolyzes the peptide bonds of the silk-fibroin powder and resins in a 4 mol/L methanesulfonic acid solution, allowing automated amino acid analysis (Wako Pure Chemical, Osaka, Japan) with 0.2 wt% 3-(2-aminoethyl) indole used as acid catalyst at 110 °C for 24 h.
2.3. Resinification
Silk powder, after being filled into a 20-mm-diameter cylindrical stainless-steel die, was pressed at 31.2 MPa while being heated from room temperature to 170 °C using a hot-pressing apparatus (H300–05, AS One, Osaka, Japan) in order to sinter the silk resin. All the process mentioned above is called resinification. After the resinification process, the silk resin was removed immediately from the stainless-steel die and cooled to room temperature. The resinification method to obtain silk resin is described in detail elsewhere [
20].
2.4. Ethanol and Boiling-Water Pretreatments
The hot-pressed silk resin was next pretreated by immersion in 40–100 vol% ethanol for 20–30 min or by immersion in boiling deionized water (100 °C) for 60 min.
2.5. Hot-Rolling
The pretreated silk resin was calendered using manually operated hot-rolling equipment (IMC-1989, Imoto Machinery Co. Ltd., Kyoto, Japan) with roller dimensions of φ60 mm × 132 mm and operated at 150–170 °C.
The rolling reduction ratio R ranged from 0% to 50%, where R is the percent change in thickness due to rolling per unit initial thickness:
In Equation (1),
h1 is the initial sample thickness before rolling, and
h2 is the final sample thickness after rolling, as shown in
Figure 2.
The goal of the hot-rolling process is to increase the content of crystalline structure (β sheets, aggregated strands) in silk fibroin and to improve the alignment, thereby increasing the strength of the resin. As mentioned above, water is an important factor in the hot-rolling process, which means that efficient pretreatments should produce a significant concentration of hydrophilic amino acids. Moreover, the highest-strength hot-rolled resin samples are those with minimal surface defects, such as wavy edges, zipper cracks, edge cracks, alligatoring, etc.
2.6. Scanning Electron Microscope
Scanning electron micrographs of the surface morphology were obtained by first carbon coating the samples with a CC-40F carbon coater (Meiwafosis Co. Ltd. Shinjuku, Japan) and then imaging using a JSM-6510 scanning electron microscope (SEM; Jeol, Japan) at magnifications of ×200 and ×500.
2.7. Mechanical Properties
Three-point bending tests were implemented using an Autograph AGS-X series universal tensile testing machine (Shimadzu, Tokyo, Japan) with the silk resin cut into 3 × 18 × 2.5 mm3 samples. The tensile strength of all samples was measured both parallel to the rolling direction and normal to the surface of the resin sheet (i.e., orthogonal to the rolling direction) on a support interval of 14 mm and at a stretching speed of 0.5 mm/min−1. The results reported are the average of a minimum of five samples produced under identical hot-rolling conditions. The bending elastic modulus was calculated from the stress-strain curve. Depending on the type of pretreatment (ethanol or water boiling), RS resins were dried prior to testing at 100 °C in a vacuum-type oven for 4 to 12 h to vary the water content and thereby determine how water content affects the mechanical properties of RS resins. The water content of the silk samples was measured using Karl Fischer titration (860 KF Thermoprep, Metrohm AG, Herisau, Switzerland).
2.8. Thermogravimetric Analysis TGA/DTG
Thermogravimetric analysis and difference thermo gravimetry (TGA and DTG) were implemented using a TG/DTA7300 instrument (Hitachi, Tokyo, Japan) to determine the thermal behavior of RS silk powders, the original RS resins, and RS hot-rolled resins by measuring the percent weight loss and decomposition peaks from the TGA and DTG curves. The measurements were made from 30 to 600 °C at a heating rate of 10 °C/min under a nitrogen atmosphere (50 mL/min). The test samples weighed 8–10 mg.
2.9. Structural Analyses
The RS resins were cut into 10 × 10 mm2 samples for structural analysis using ATR-FTIR (FT/IR-6600, Jasco, Tokyo, Japan) over the range 700–4000 cm−1 and with a resolution of 4 cm−1. Second derivatives and multipeak Gaussian fits were used to determine the secondary structural composition of amide I. In addition, the crystallinity was determined using XRD (Ultimate IV Protectus, Rigaku, Tokyo, Japan) with Cu Kα radiation (40 kV and 40 A) at a scan speed of 0.2 deg/min and with a sidestep 0.01 deg over the 2θ range from 5° to 35°.
4. Conclusions
The mechanical properties of silk resins increase upon hot-rolling the resins. It is easier to hot-roll RS resin than Bombyx mori silk resin or Eri silk resin because of the inferior mechanical properties of the former. To support the hot-rolling process, we subjected the resin samples to an ethanol pretreatment or a boiling-water pretreatment to degrade the mechanical properties of the resins before hot-rolling. The results show that the RS hot-rolled resin with boiling-water pretreatment has the best mechanical properties (229 MPa three-point bending strength and 12.5 GPa Young’s modulus).
The mechanical properties of RS hot-rolled resin depend on the water content. Lower water content in the resin corresponds to better mechanical properties. The best mechanical properties are attained with 2.64% water content. However, the mechanical properties decline dramatically when the water content decreases below 2.64%, which we attribute to non-freezing bound water, which plays a role in cross-linking. Removing this water breaks the polymeric structure of the resin.
Compared with the other research [
15], the highest bending strength of RS hot-rolled resin (229 MPa) is stronger than those of PEEK (~170 MPa), which is one of the super engineering plastics obtained. Moreover, Young’s modulus of RS hot-rolled resin (12.5 GPa) is four times greater than Young’s modulus of PEEK (~3 GPa) [
15]. The pretreatment and hot-rolling process not only improved the mechanical properties of RS resin but also enhanced the water stability and the stability during the in vitro degradation test of the silk materials because of the increase of the silk fibroin crystal conformation [
5]. According to this, the functions of silk resins have been further extended based on the advantageous properties of silk materials (biocompatibility, biodegradation, non-toxicity, high strength, etc.). It is predicted that the silk bio-resin will be used as a biodegradable polymeric material in plentiful applications that play an important role for biomedical applications (e.g., biodegradation orthopedic implants).