Revisiting the Charge-Transfer States at Pentacene/C60 Interfaces with the GW/Bethe–Salpeter Equation Approach
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Energy Levels at the Interface
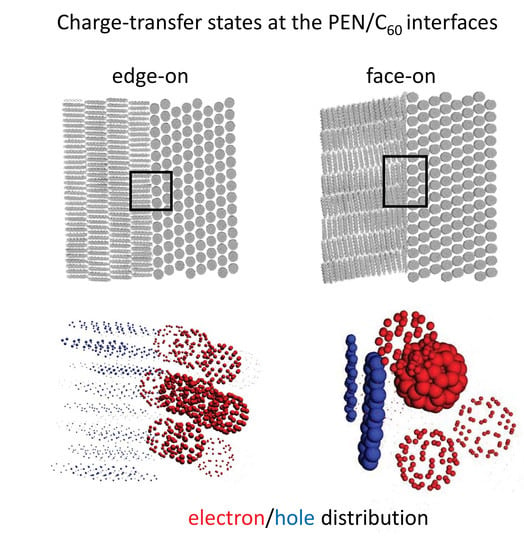
3.2. Interfacial CT States
3.3. Estimation of Charge Transfer Exciton Binding Energy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BSE | Bethe-Salpeter equation |
| COHSEX | Coulomb-hole plus screened exchange |
| DFT | density functional theory |
| EQE | external quantum efficiency |
| FMO | fragment molecular orbital |
| TDDFT | time-dependent density functional theory |
Appendix A



References
- Clarke, T.M.; Durrant, J.R. Charge Photogeneration in Organic Solar Cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef] [PubMed]
- Vandewal, K. Interfacial charge transfer states in condensed phase systems. Annu. Rev. Phys. Chem. 2016, 67, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rand, B.P.; Forrest, S.R. Engineering Charge-Transfer States for Efficient, Low-Energy-Loss Organic Photovoltaics. Trends Chem. 2019, 1, 815–829. [Google Scholar] [CrossRef]
- Vandewal, K.; Tvingstedt, K.; Gadisa, A.; Inganäs, O.; Manca, J.V. Relating the Open-Circuit Voltage to Interface Molecular Properties of Donor: Acceptor Bulk Heterojunction Solar Cells. Phys. Rev. B 2010, 81, 125204. [Google Scholar] [CrossRef] [Green Version]
- Menke, S.M.; Ran, N.A.; Bazan, G.C.; Friend, R.H. Understanding Energy Loss in Organic Solar Cells: Toward a New Efficiency Regime. Joule 2018, 2, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Benduhn, J.; Tvingstedt, K.; Piersimoni, F.; Ullbrich, S.; Fan, Y.; Tropiano, M.; McGarry, K.A.; Zeika, O.; Riede, M.K.; Douglas, C.J.; et al. Intrinsic Non-Radiative Voltage Losses in Fullerene-Based Organic Solar Cells. Nat. Energy 2017, 2, 17053. [Google Scholar] [CrossRef]
- Körzdörfer, T.; Brèdas, J.L. Organic electronic materials: Recent advances in the DFT description of the ground and excited states using tuned range-separated hybrid functionals. Acc. Chem. Res. 2014, 47, 3284–3291. [Google Scholar]
- Coropceanu, V.; Chen, X.K.; Wang, T.; Zheng, Z.; Brédas, J.L. Charge-transfer electronic states in organic solar cells. Nat. Rev. Mater. 2019, 4, 689–707. [Google Scholar] [CrossRef]
- Ma, H.; Troisi, A. Direct Optical Generation of Long-Range Charge-Transfer States in Organic Photovoltaics. Adv. Mater. 2014, 26, 6163–6167. [Google Scholar] [CrossRef]
- Yang, B.; Yi, Y.; Zhang, C.R.; Aziz, S.G.; Coropceanu, V.; Brédas, J.L. Impact of Electron Delocalization on the Nature of the Charge-Transfer States in Model Pentacene/C60 Interfaces: A Density Functional Theory Study. J. Phys. Chem. C 2014, 118, 27648–27656. [Google Scholar] [CrossRef]
- Zheng, Z.; Brédas, J.L.; Coropceanu, V. Description of the charge transfer states at the pentacene/C60 interface: Combining range-separated hybrid functionals with the polarizable continuum model. J. Phys. Chem. Lett. 2016, 7, 2616–2621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Tummala, N.R.; Fu, Y.T.; Coropceanu, V.; Brédas, J.L. Charge-Transfer States in Organic Solar Cells: Understanding the Impact of Polarization, Delocalization, and Disorder. ACS Appl. Mater. Inter. 2017, 9, 18095–18102. [Google Scholar] [CrossRef] [PubMed]
- Neaton, J.B.; Hybertsen, M.S.; Louie, S.G. Renormalization of Molecular Electronic Levels at Metal-Molecule Interfaces. Phys. Rev. Lett. 2006, 97, 216405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; D’Avino, G.; Duchemin, I.; Beljonne, D.; Blase, X. Accurate description of charged excitations in molecular solids from embedded many-body perturbation theory. Phys. Rev. B 2018, 97, 035108. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T.; Noguchi, Y. Development of the Fragment-Based COHSEX Method for Large and Complex Molecular Systems. Phys. Rev. B 2018, 98, 205140. [Google Scholar] [CrossRef]
- Tiago, M.L.; Northrup, J.E.; Louie, S.G. Ab initio calculation of the electronic and optical properties of solid pentacene. Phys. Rev. B 2003, 67, 115212. [Google Scholar] [CrossRef]
- Sharifzadeh, S.; Biller, A.; Kronik, L.; Neaton, J.B. Quasiparticle and Optical Spectroscopy of the Organic Semiconductors Pentacene and PTCDA from First Principles. Phys. Rev. B 2012, 85, 125307. [Google Scholar] [CrossRef] [Green Version]
- Cudazzo, P.; Sottile, F.; Rubio, A.; Gatti, M. Exciton dispersion in molecular solids. J. Phys. Condens. Matter 2015, 27, 113204. [Google Scholar] [CrossRef] [Green Version]
- Duchemin, I.; Jacquemin, D.; Blase, X. Combining the GW Formalism with the Polarizable Continuum Model: A State-Specific Non-Equilibrium Approach. J. Chem. Phys. 2016, 144, 164106. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, Y.; Sugino, O. High-Lying Triplet Excitons of Thermally Activated Delayed Fluorescence Molecules. J. Phys. Chem. C 2017, 121, 20687–20695. [Google Scholar] [CrossRef]
- Fujita, T.; Noguchi, Y.; Hoshi, T. Charge-transfer excited states in the donor/acceptor interface from large-scale GW calculations. J. Chem. Phys. 2019, 151, 114109. [Google Scholar] [CrossRef]
- Hedin, L. New Method for Calculating the One-Particle Green’s Function with Application to the Electron-Gas Problem. Phys. Rev. 1965, 139, A796. [Google Scholar] [CrossRef]
- Rohlfing, M.; Louie, S.G. Electron-Hole Excitations and Optical Spectra from First Principles. Phys. Rev. B 2000, 62, 4927–4944. [Google Scholar] [CrossRef]
- Blase, X.; Attaccalite, C. Charge-Transfer Excitations in Molecular Donor-Acceptor Complexes within the Many-Body Bethe-Salpeter Approach. Appl. Phys. Lett. 2011, 99, 171909. [Google Scholar] [CrossRef]
- Bakulin, A.; Rao, A.; Pavelyev, V.G.; van Loosdrecht, P.H.M.; Pshenichnikov, M.S.; Niedzialek, D.; Cornil, J.; Beljonne, D.; Friend, R.H. The role of driving energy and delocalized States for charge separation in organic semiconductors. Science 2012, 335, 1340. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Burghardt, I. Ultrafast Charge Separation in Organic Photovoltaics Enhanced by Charge Delocalization and Vibronically Hot Exciton Dissociation. J. Am. Chem. Soc. 2013, 135, 16364–16367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gélinas, S.; Rao, A.; Kumar, A.; Smith, S.L.; Chin, A.W.; Clark, J.; van der Poll, T.S.; Bazan, G.C.; Friend, R.H. Ultrafast Long-Range Charge Separation in Organic Semiconductor Photovoltaic Diodes. Science 2014, 343, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Tamai, Y. Delocalization boosts charge separation in organic solar cells. Polym. J. 2020, 1–10. [Google Scholar] [CrossRef]
- Salzmann, I.; Duhm, S.; Opitz, R.; Johnson, R.L.; Rabe, J.P.; Koch, N. Structural and Electronic Properties of Pentacene-Fullerene Heterojunctions. J. Appl. Phys. 2008, 104, 114518. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Nakayama, Y.; Uragami, Y.; Kinjo, H.; Mizuno, Y.; Mase, K.; Koswattage, K.R.; Ishii, H. Electronic Structures of a Well-Defined Organic Hetero-Interface: C60 on Pentacene Single Crystal. E-J. Surf. Sci. Nanotechnol. 2015, 13, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Nishi, T.; Kanno, M.; Kuribayashi, M.; Nishida, Y.; Hattori, S.; Kobayashi, H.; von Wrochem, F.; Rodin, V.; Nelles, G.; Tomiya, S. Impact of Molecular Orientation on Energy Level Alignment at C60/Pentacene Interfaces. Appl. Phys. Lett. 2018, 113, 163302. [Google Scholar] [CrossRef]
- Brigeman, A.N.; Fusella, M.A.; Yan, Y.; Purdum, G.E.; Loo, Y.L.; Rand, B.P.; Giebink, N.C. Revealing the Full Charge Transfer State Absorption Spectrum of Organic Solar Cells. Adv. Energy Mater. 2016, 6, 1601001. [Google Scholar] [CrossRef]
- Lin, Y.L.; Zhang, F.; Kerner, R.A.; Yang, T.C.J.; Kahn, A.; Rand, B.P. Variable Charge Transfer State Energies at Nanostructured Pentacene/C60 Interfaces. Appl. Phys. Lett. 2018, 112, 213302. [Google Scholar] [CrossRef]
- Minami, T.; Nakano, M.; Castet, F. Nonempirically Tuned Long-Range Corrected Density Functional Theory Study on Local and Charge-Transfer Excitation Energies in a Pentacene/C60 Model Complex. J. Phys. Chem. Lett. 2011, 2, 1725–1730. [Google Scholar] [CrossRef]
- Verlaak, S.; Beljonne, D.; Cheyns, D.; Rolin, C.; Linares, M.; Castet, F.; Cornil, J.; Heremans, P. Electronic Structure and Geminate Pair Energetics at Organic-Organic Interfaces: The Case of Pentacene/C60 Heterojunctions. Adv. Funct. Mater. 2009, 19, 3809–3814. [Google Scholar] [CrossRef]
- Fedorov, D.G.; Kitaura, K. (Eds.) The Fragment Molecular Orbital Method: Practical Applications to Large Molecular Systems; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tanaka, S.; Mochizuki, Y.; Komeiji, Y.; Okiyama, Y.; Fukuzawa, K. Electron-Correlated Fragment-Molecular- Orbital Calculations for Biomolecular and Nano Systems. Phys. Chem. Chem. Phys. 2014, 16, 10310–10344. [Google Scholar] [CrossRef]
- Tsuneyuki, S.; Kobori, T.; Akagi, K.; Sodeyama, K.; Terakura, K.; Fukuyama, H. Molecular Orbital Calculation of Biomolecules with Fragment Molecular Orbitals. Chem. Phys. Lett. 2009, 476, 104–108. [Google Scholar] [CrossRef]
- Fedorov, D.G.; Kitaura, K. Many-body expansion of the Fock matrix in the fragment molecular orbital method. J. Chem. Phys. 2017, 147, 104106. [Google Scholar] [CrossRef]
- Fujita, T.; Mochizuki, Y. Development of the Fragment Molecular Orbital Method for Calculating Nonlocal Excitations in Large Molecular Systems. J. Phys. Chem. A 2018, 122, 3886–3898. [Google Scholar] [CrossRef]
- Fujita, T.; Alam, M.K.; Hoshi, T. Thousand-Atom Ab Initio Calculations of Excited States at Organic/Organic Interfaces: Toward First-Principles Investigations of Charge Photogeneration. Phys. Chem. Chem. Phys. 2018, 20, 26443–26452. [Google Scholar] [CrossRef]
- Nakano, T.; Kaminuma, T.; Sato, T.; Akiyama, Y.; Uebayasi, M.; Kitaura, K. Fragment molecular orbital method: Use of approximate electrostatic potential. Chem. Phys. Lett. 2002, 351, 475–480. [Google Scholar] [CrossRef]
- Mochizuki, Y.; Koikegami, S.; Amari, S.; Segawa, K.; Kitaura, K.; Nakano, T. Configuration Interaction Singles Method with Multilayer Fragment Molecular Orbital Scheme. Chem. Phys. Lett. 2005, 406, 283–288. [Google Scholar] [CrossRef]
- D’Avino, G.; Muccioli, L.; Castet, F.; Poelking, C.; Andrienko, D.; Soos, Z.G.; Cornil, J.; Beljonne, D. Electrostatic phenomena in organic semiconductors: Fundamentals and implications for photovoltaics. J. Phys. Condens. Matter 2016, 28, 433002. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K. Advanced understanding on electronic structure of molecular semiconductors and their interfaces. Jpn. J. Appl. Phys. 2018, 57, 03EA03. [Google Scholar] [CrossRef]
- Yamada, K.; Yanagisawa, S.; Koganezawa, T.; Mase, K.; Sato, N.; Yoshida, H. Impact of the Molecular Quadrupole Moment on Ionization Energy and Electron Affinity of Organic Thin Films: Experimental Determination of Electrostatic Potential and Electronic Polarization Energies. Phys. Rev. B 2018, 97, 245206. [Google Scholar] [CrossRef]
- D’Avino, G.; Mothy, S.; Muccioli, L.; Zannoni, C.; Wang, L.; Cornil, J.; Beljonne, D.; Castet, F. Energetics of Electron—Hole Separation at P3HT/PCBM Heterojunctions. J. Phys. Chem. C 2013, 117, 12981–12990. [Google Scholar] [CrossRef]
- Kitoh-Nishioka, H.; Ando, K. Charge-Transfer Matrix Elements by FMO-LCMO Approach: Hole Transfer in DNA with Parameter Tuned Range-Separated DFT. Chem. Phys. Lett. 2015, 621, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T.; Haketa, Y.; Maeda, H.; Yamamoto, T. Relating stacking structures and charge transport in crystal polymorphs of the pyrrole-based π-conjugated molecule. Org. Electron. 2017, 49, 53–63. [Google Scholar] [CrossRef]
- Chiba, M.; Fedorov, D.G.; Kitaura, K. Time-dependent density functional theory with the multilayer fragment molecular orbital method. Chem. Phys. Lett. 2007, 444, 346–350. [Google Scholar]
- Chiba, M.; Koido, T. Electronic excitation energy calculation by the fragment molecular orbital method with three-body effects. J. Chem. Phys. 2010, 133, 044113. [Google Scholar] [CrossRef]
- Taguchi, N.; Mochizuki, Y.; Nakano, T. Fragment molecular orbital calculations for excitation energies of blue- and yellow-fluorescent proteins. Chem. Phys. Lett. 2011, 504, 76–82. [Google Scholar]
- Yoshida, H.; Yamada, K.; Tsutsumi, J.; Sato, N. Complete Description of Ionization Energy and Electron Affinity in Organic Solids: Determining Contributions from Electronic Polarization, Energy Band Dispersion, and Molecular Orientation. Phys. Rev. B 2015, 92, 075145. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, M.; Schellhammer, K.S.; Ortstein, K.; Benduhn, J.; Gaul, C.; Hinderhofer, A.; Toro, L.P.; Scholz, R.; Kublitski, J.; Roland, S.; et al. Impact of molecular quadrupole moments on the energy levels at organic heterojunctions. Nat. Commun. 2019, 10, 2466. [Google Scholar] [CrossRef] [PubMed]
- Thouless, D. Electrons in disordered systems and the theory of localization. Phys. Rep. 1974, 13, 93–142. [Google Scholar] [CrossRef]
- Hoshi, T.; Imachi, H.; Kuwata, A.; Kakuda, K.; Fujita, T.; Matsui, H. Numerical aspect of large-scale electronic state calculation for flexible device material. Jpn. J. Ind. Appl. Math. 2019, 36, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hill, I.; Kahn, A.; Soos, Z.; Pascal, R., Jr. Charge-separation energy in films of π-conjugated organic molecules. Chem. Phys. Lett. 2000, 327, 181–188. [Google Scholar] [CrossRef]
- Yoshida, H. Low-Energy Inverse Photoemission Study on the Electron Affinities of Fullerene Derivatives for Organic Photovoltaic Cells. J. Phys. Chem. C 2014, 118, 24377–24382. [Google Scholar] [CrossRef]







| PEN | C60 | ||
|---|---|---|---|
| gas (a) | LUMO | −0.74 | −1.84 |
| HOMO | −5.56 | −6.74 | |
| Edge-on (E) | LUMO | −0.28 | −2.12 |
| HOMO | −4.78 | −6.69 | |
| Edge-on (E+I) | LUMO | −0.87 | −2.75 |
| HOMO | −4.26 | −6.12 | |
| Face-on (E) | LUMO | −0.63 | −1.64 |
| HOMO | −5.13 | −6.22 | |
| Face-on (E+I) | LUMO | −1.30 | −2.14 |
| HOMO | −4.53 | −5.76 |
| U | ||||
|---|---|---|---|---|
| Edge-on | gas (a) | 1.92 | 3.18 | 1.27 |
| E | 1.70 | 2.96 | 1.26 | |
| E+I | 0.91 | 1.58 | 0.68 | |
| Face-on | gas (a) | 1.50 | 3.11 | 1.61 |
| E | 1.72 | 3.33 | 1.61 | |
| E+I | 1.19 | 1.99 | 0.80 |
| U | Reh (nm) | IPR Elec | IPR Hole | OS | ||||
|---|---|---|---|---|---|---|---|---|
| Edge-on | Lowest | 0.70 | 1.41 | 0.71 | 1.35 | 1.50 | 2.55 | 8.9 × 10−5 |
| Brightest (a) | 0.99 | 1.57 | 0.58 | 1.53 | 9.92 | 25.73 | 2.3 × 10−3 | |
| Face-on | Lowest | 1.15 | 1.96 | 0.81 | 0.84 | 1.09 | 1.14 | 0.03 |
| Brightest (a) | 1.40 | 2.30 | 0.90 | 0.76 | 1.98 | 1.32 | 0.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, T.; Noguchi, Y.; Hoshi, T. Revisiting the Charge-Transfer States at Pentacene/C60 Interfaces with the GW/Bethe–Salpeter Equation Approach. Materials 2020, 13, 2728. https://doi.org/10.3390/ma13122728
Fujita T, Noguchi Y, Hoshi T. Revisiting the Charge-Transfer States at Pentacene/C60 Interfaces with the GW/Bethe–Salpeter Equation Approach. Materials. 2020; 13(12):2728. https://doi.org/10.3390/ma13122728
Chicago/Turabian StyleFujita, Takatoshi, Yoshifumi Noguchi, and Takeo Hoshi. 2020. "Revisiting the Charge-Transfer States at Pentacene/C60 Interfaces with the GW/Bethe–Salpeter Equation Approach" Materials 13, no. 12: 2728. https://doi.org/10.3390/ma13122728