Thermal Characteristics of Expandable Graphite–Wood Particle Composites
Abstract
:1. Introduction
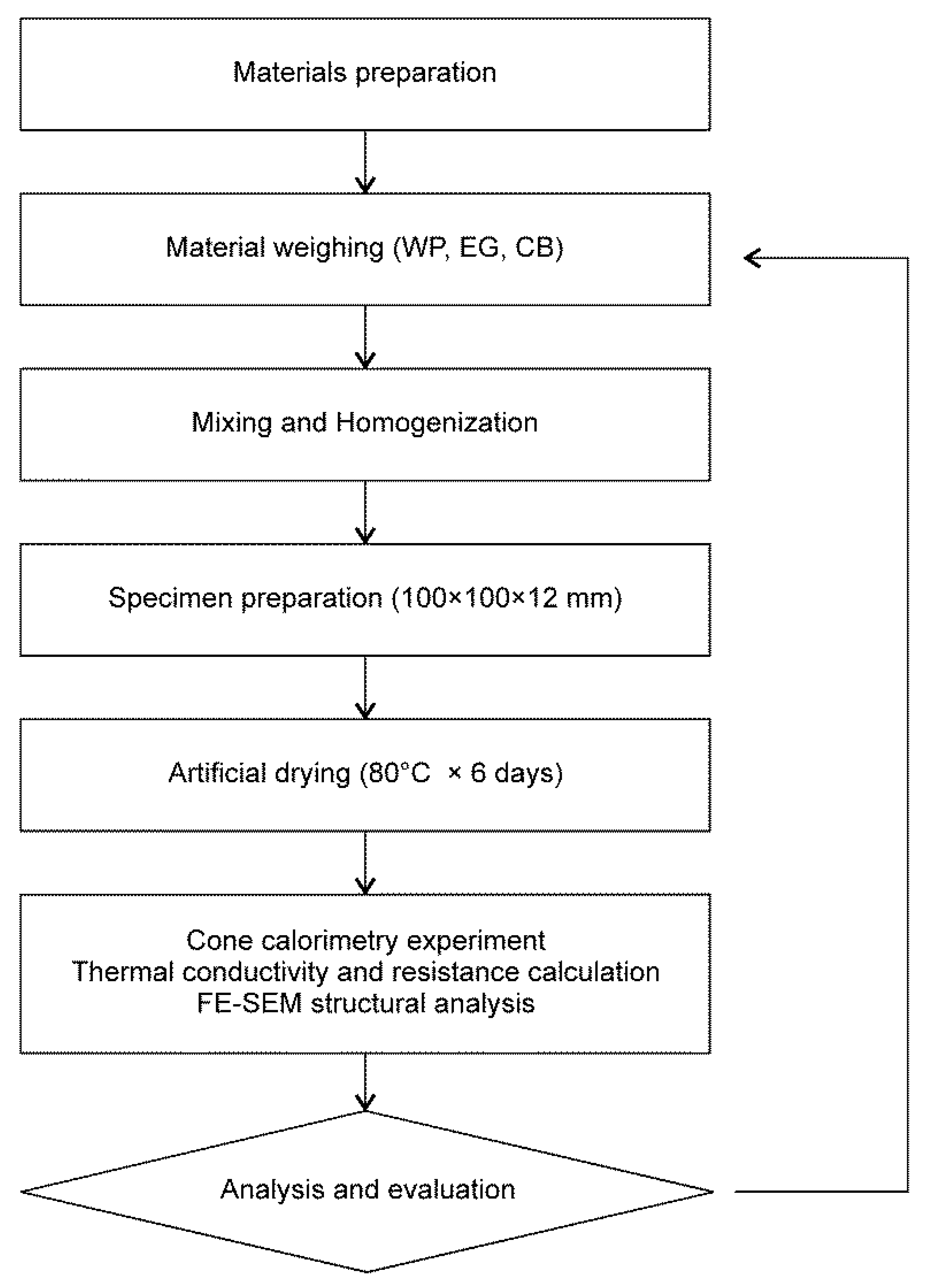
2. Materials and Methods
2.1. Material Selection and Composite Fabrication
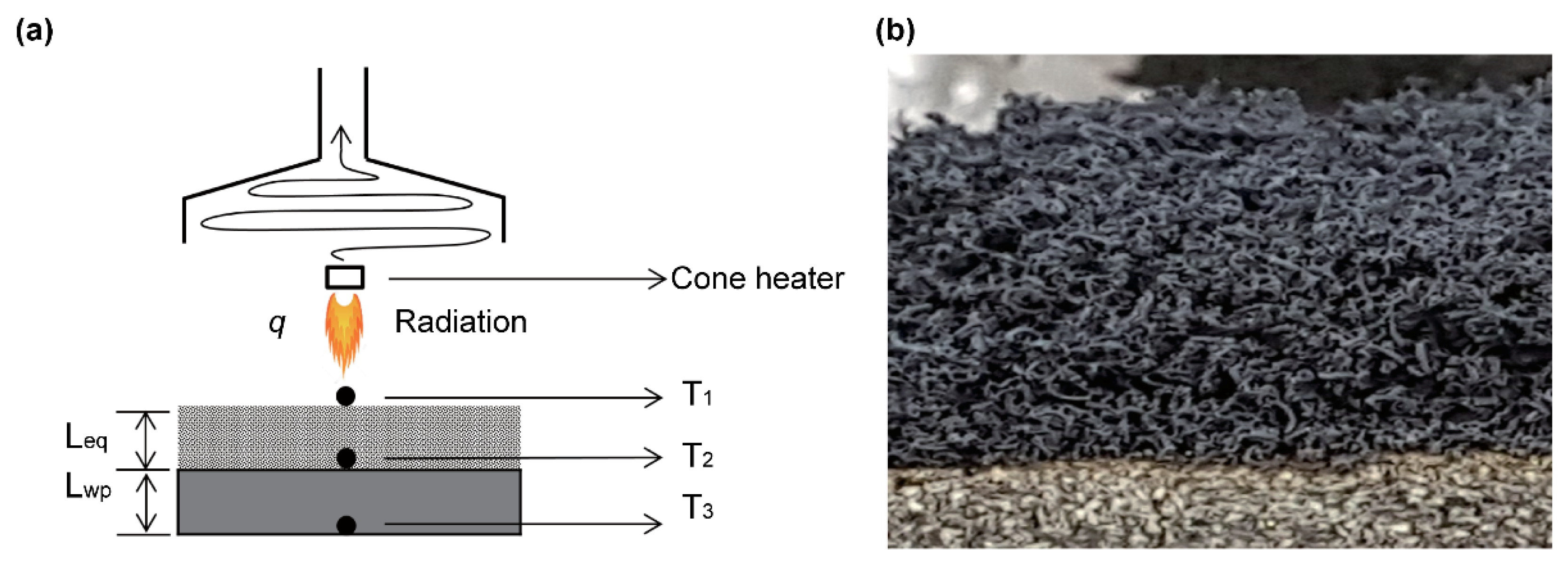
2.2. Cone Calorimetry Experiment
3. Results and Discussion
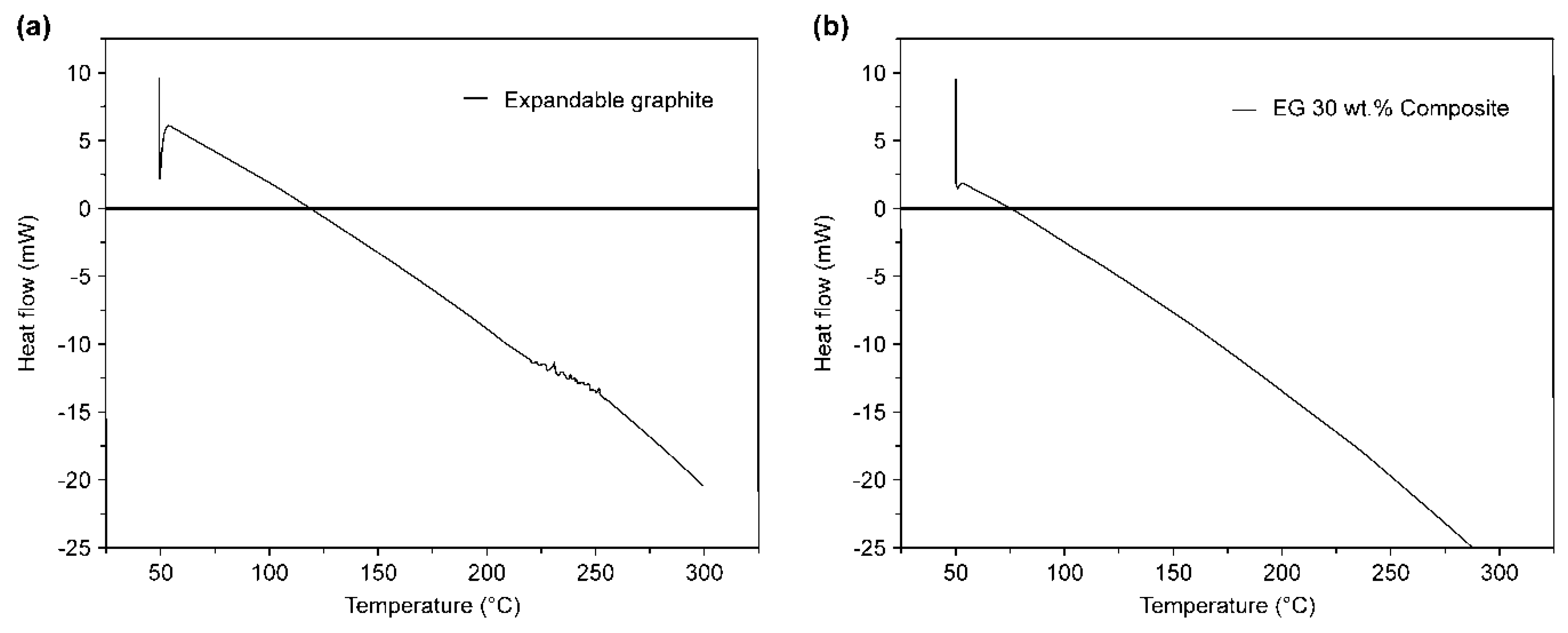
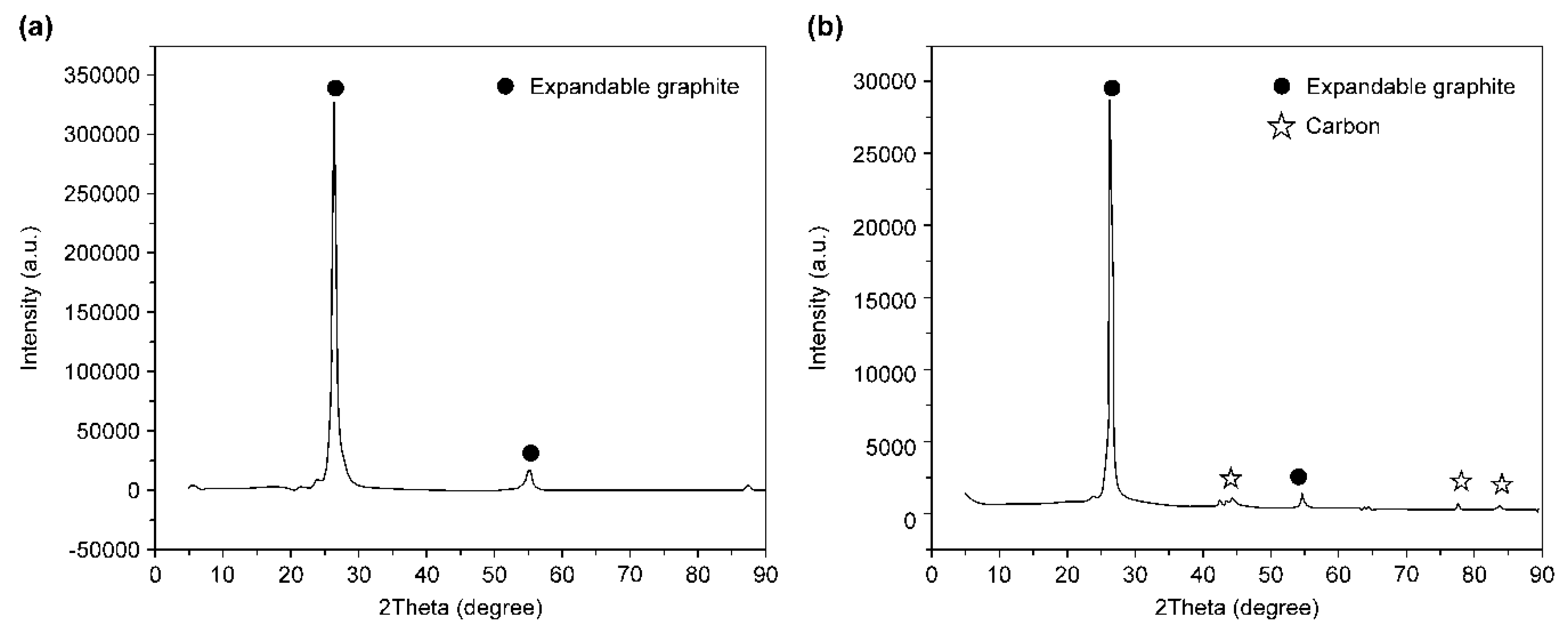
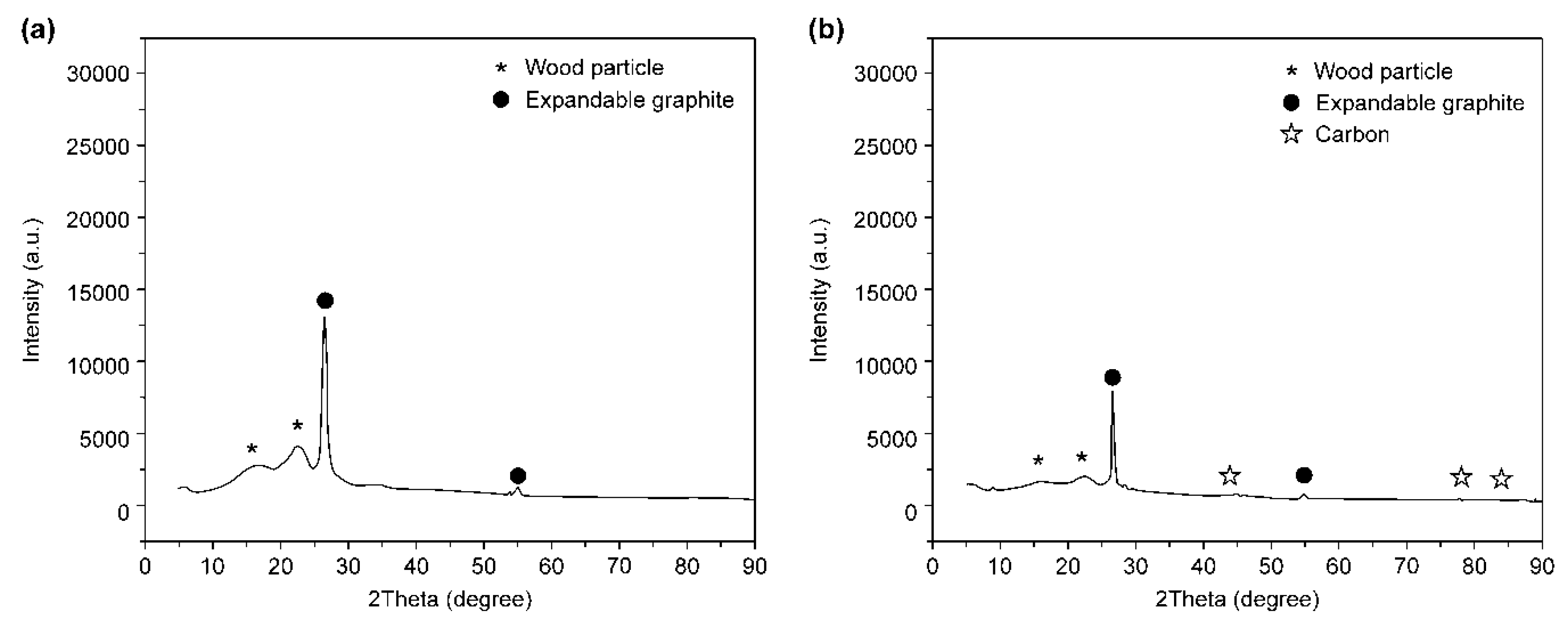
3.1. DSC and XRD Analyses
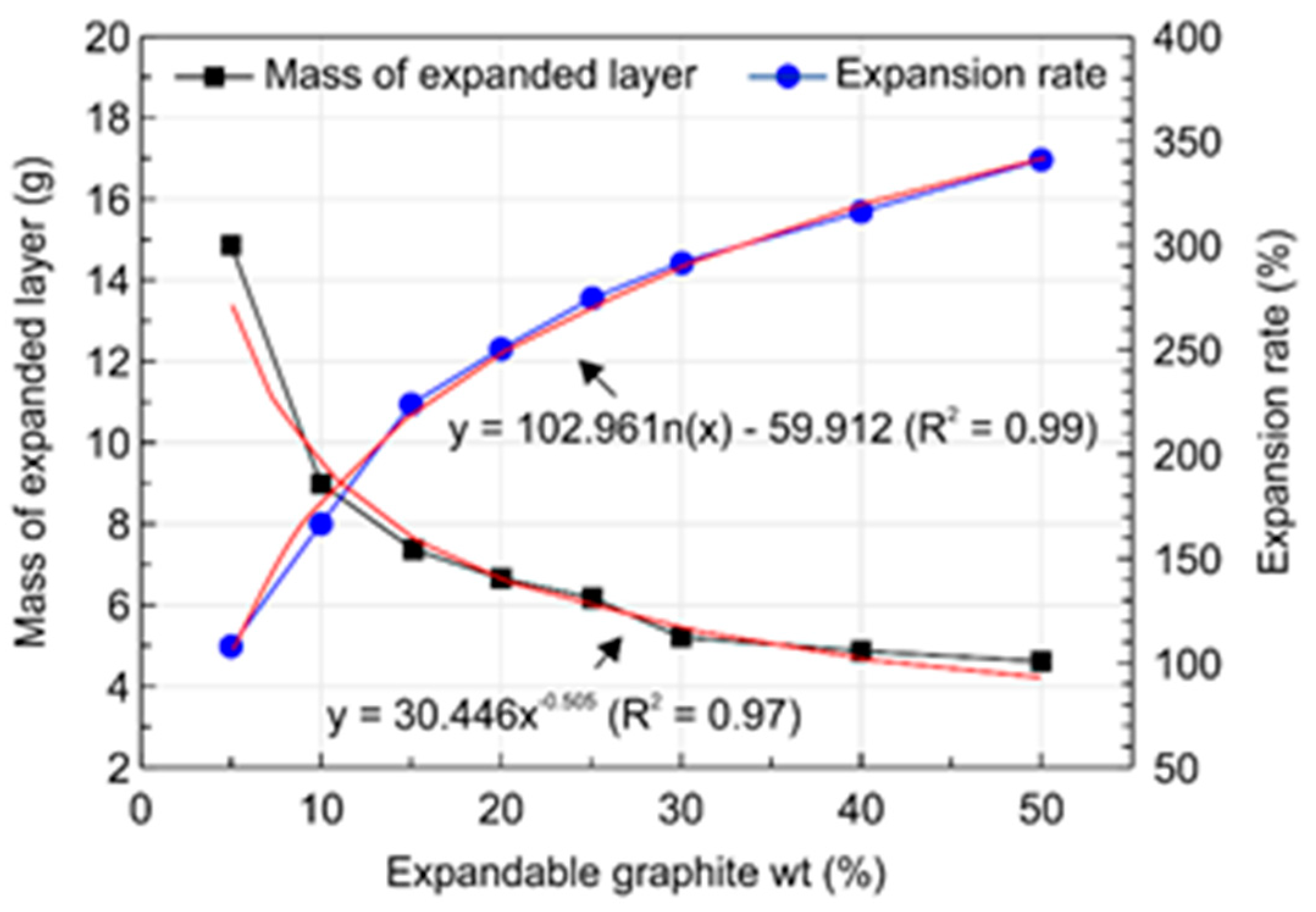
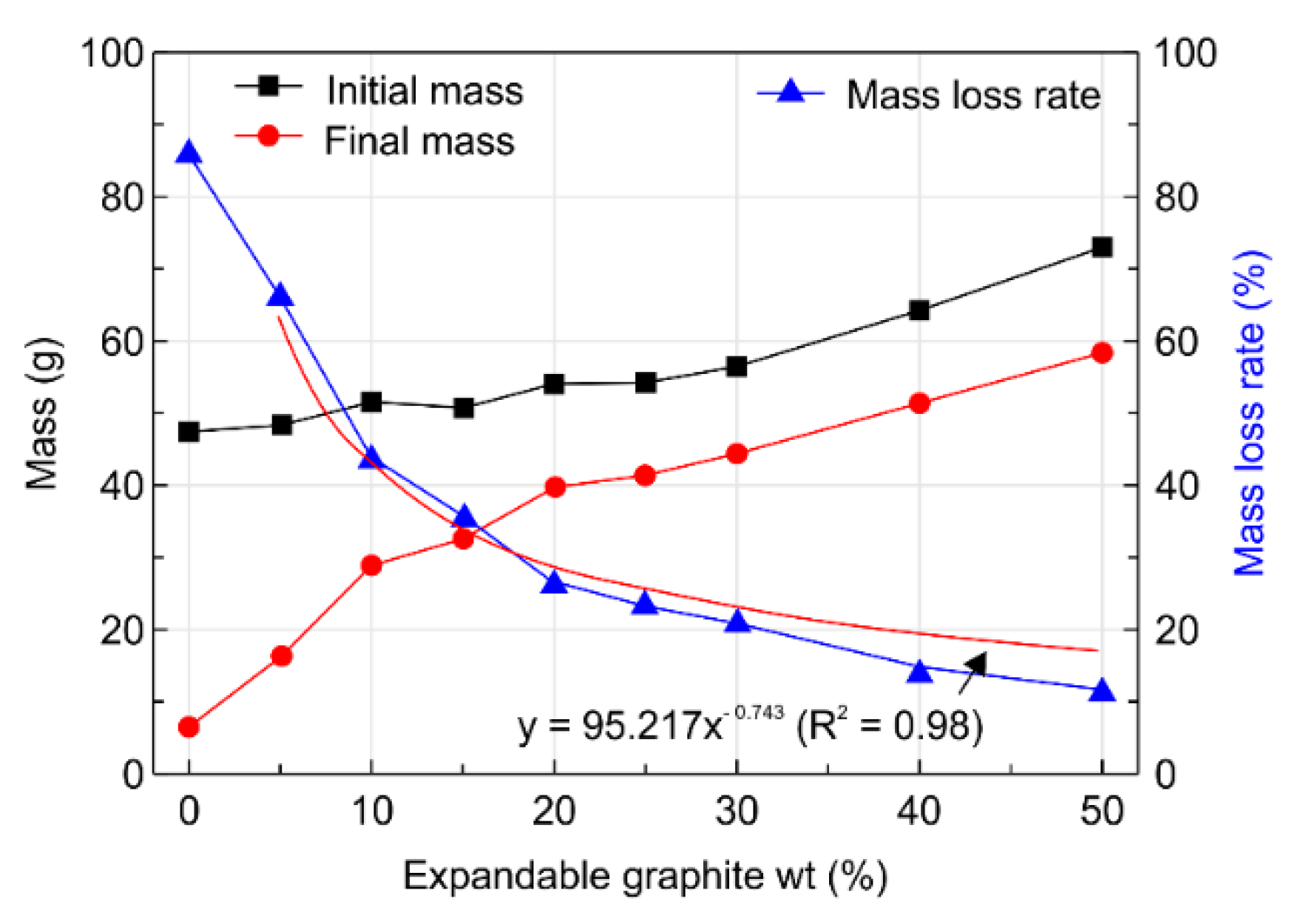
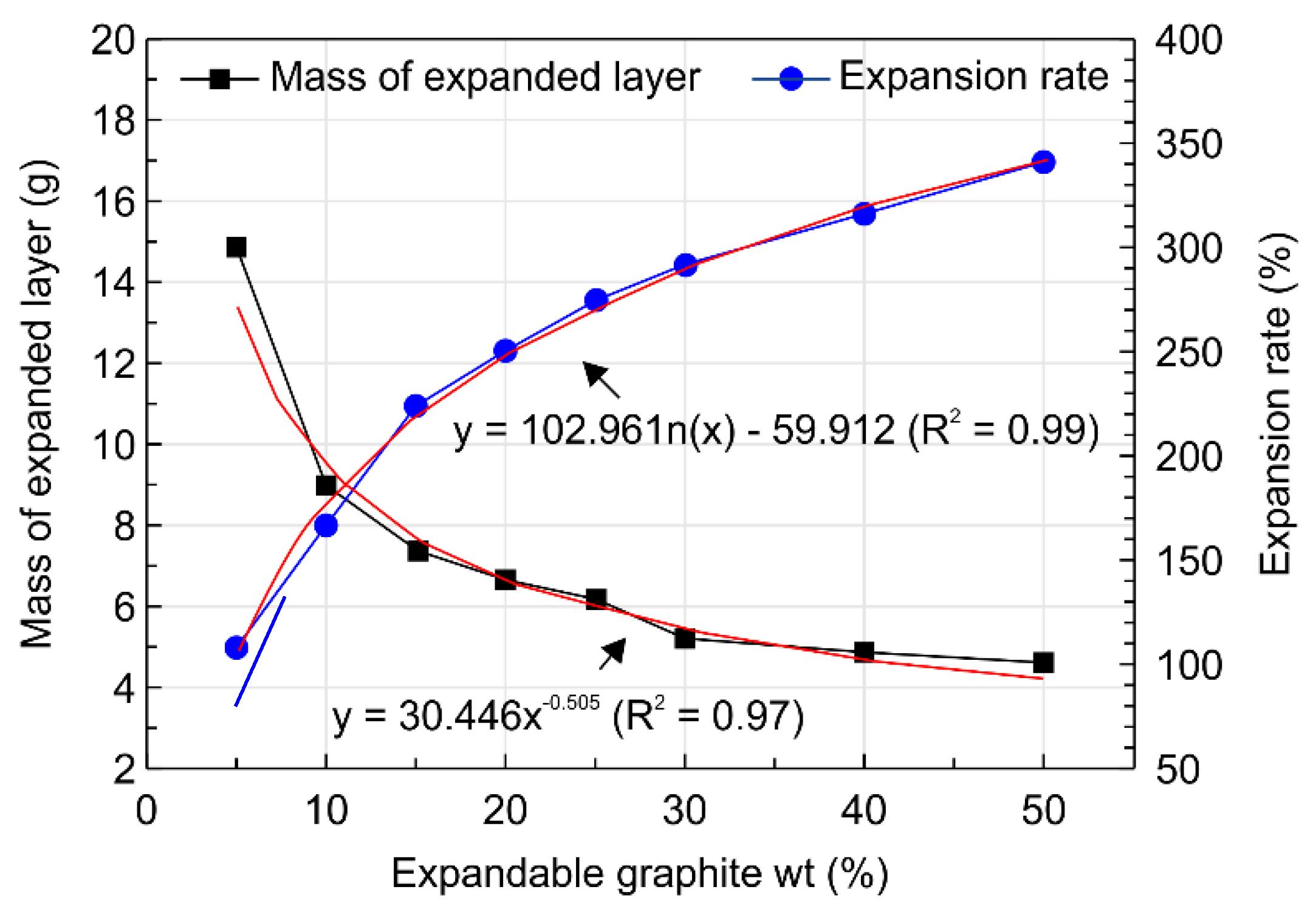
3.2. Physical Characteristics of the Expandable Graphite Composites
3.3. Thermal Characteristics of Expandable Graphite Composites
3.4. Changes in the Microstructure of Expandable Graphite
4. Conclusions
- As a result of the DSC analysis, a faster heat absorption rate compared that of the pure expandable graphite was observed when the expandable graphite was added to the wood particles. This was due to the absorption of a large amount of heat for the carbonization of the wood particles in the specimen. In the XRD analysis after combustion, the diffraction peak level of the wood particles decreased, and carbon was detected by the combustion of the wood particles and thermal decomposition of expandable graphite.
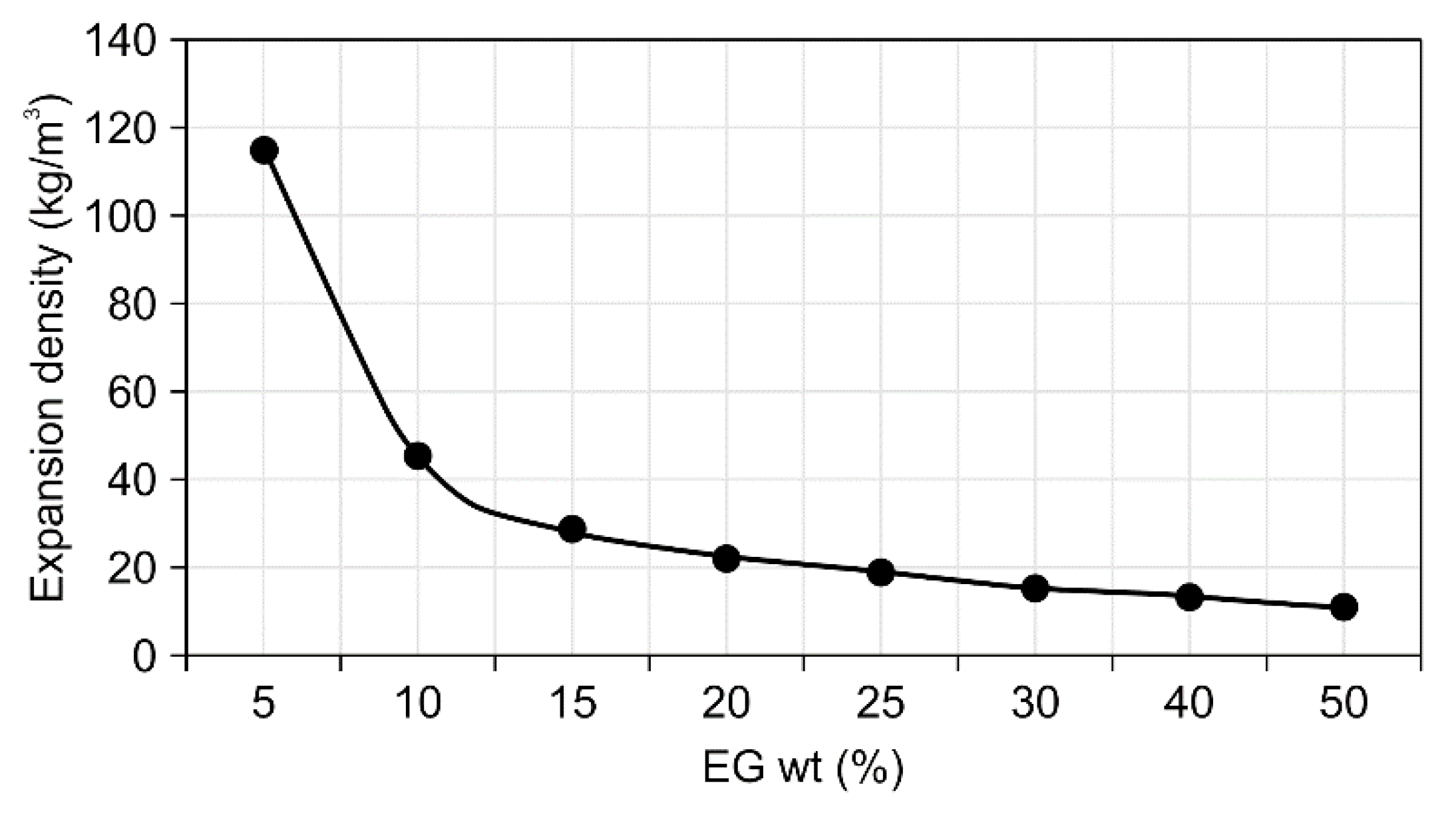
- Analysis of the physical properties of the expandable graphite–wood particle composites revealed that increasing the expandable graphite content from 0 to 50 wt.% increased the expansion rate from 0% to 341.7%. Given that expandable graphite is a mixture of sulfuric acid and an oxidant and is produced from processes that involve oxidation, washing, drying, heating, and expansion, such an observation was shown to result from the wide expandability layer that arises when expandable graphite expands upon exposure to heat and produces gas.
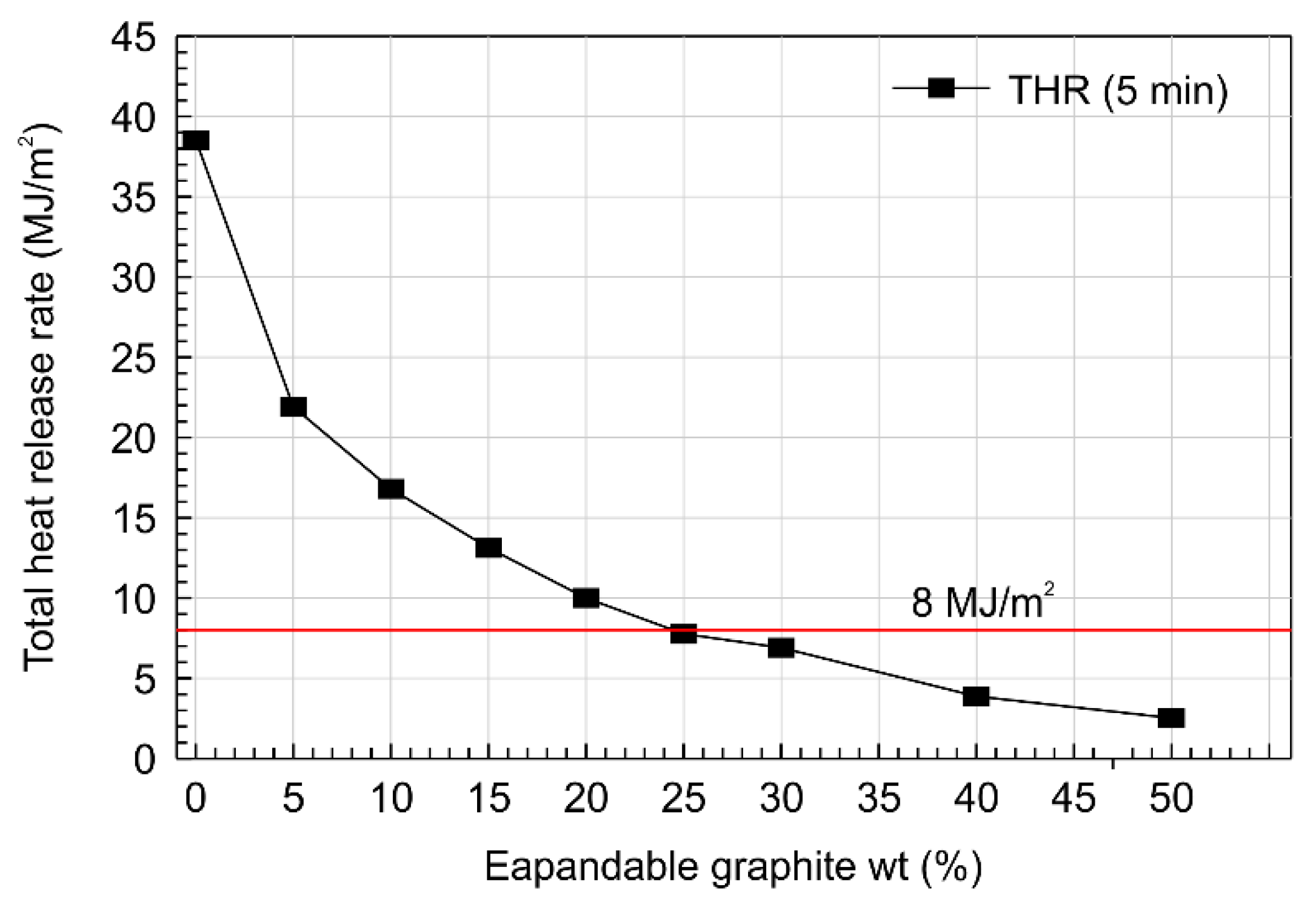
- Analysis of the thermal characteristics of the expanded graphite layer (Lwp) composites with added expandable graphite showed that increasing the expandable graphite content from 0 to 50 wt.% decreased the total heat released from 38.63 to 2.50 MJ/m2, and when expandable graphite contained more than 30%, it showed a stable fire retarding effect. It was concluded that this resulted from the delay in ignition time and decrease in the total heat released with increasing expandable graphite content.
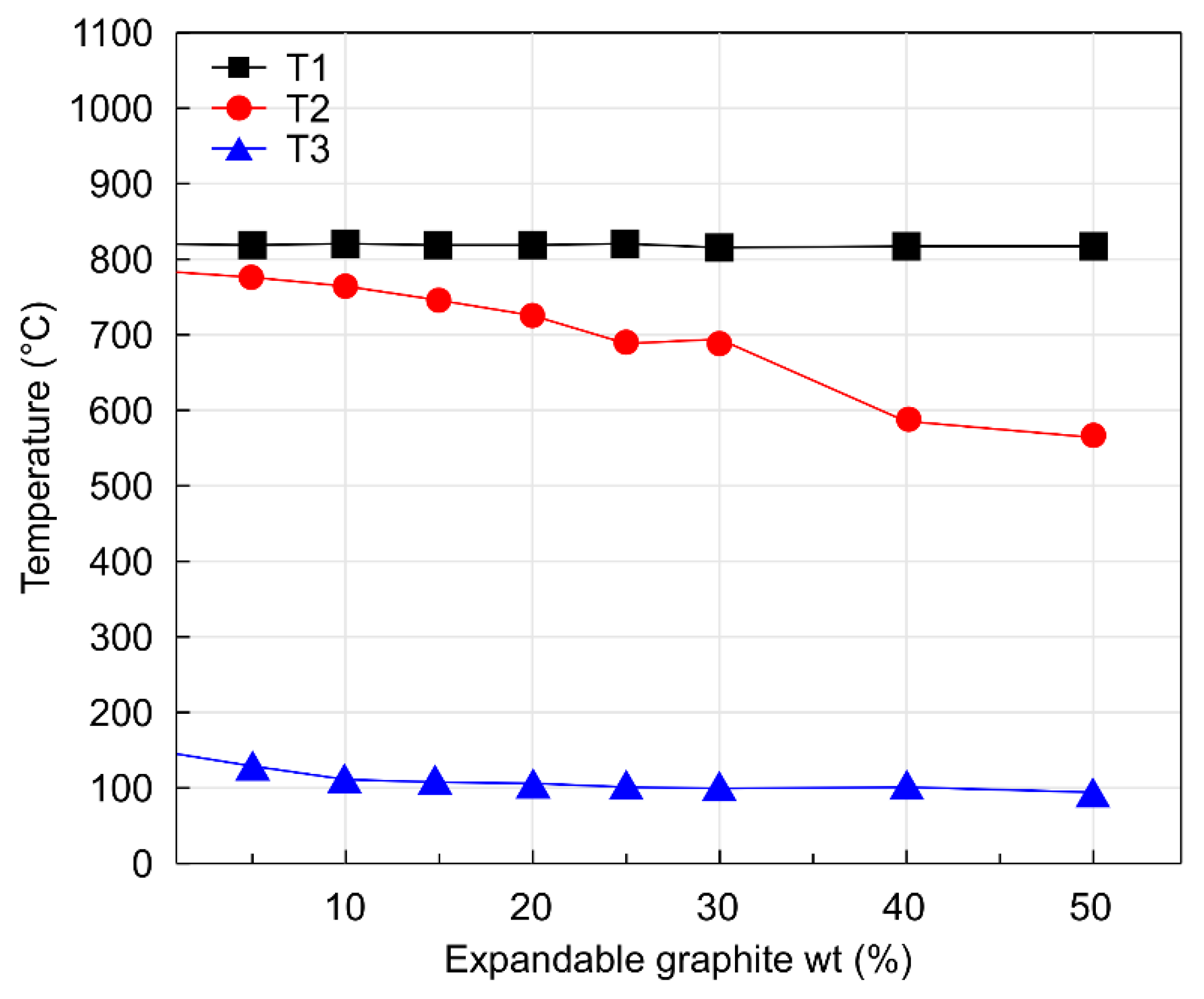
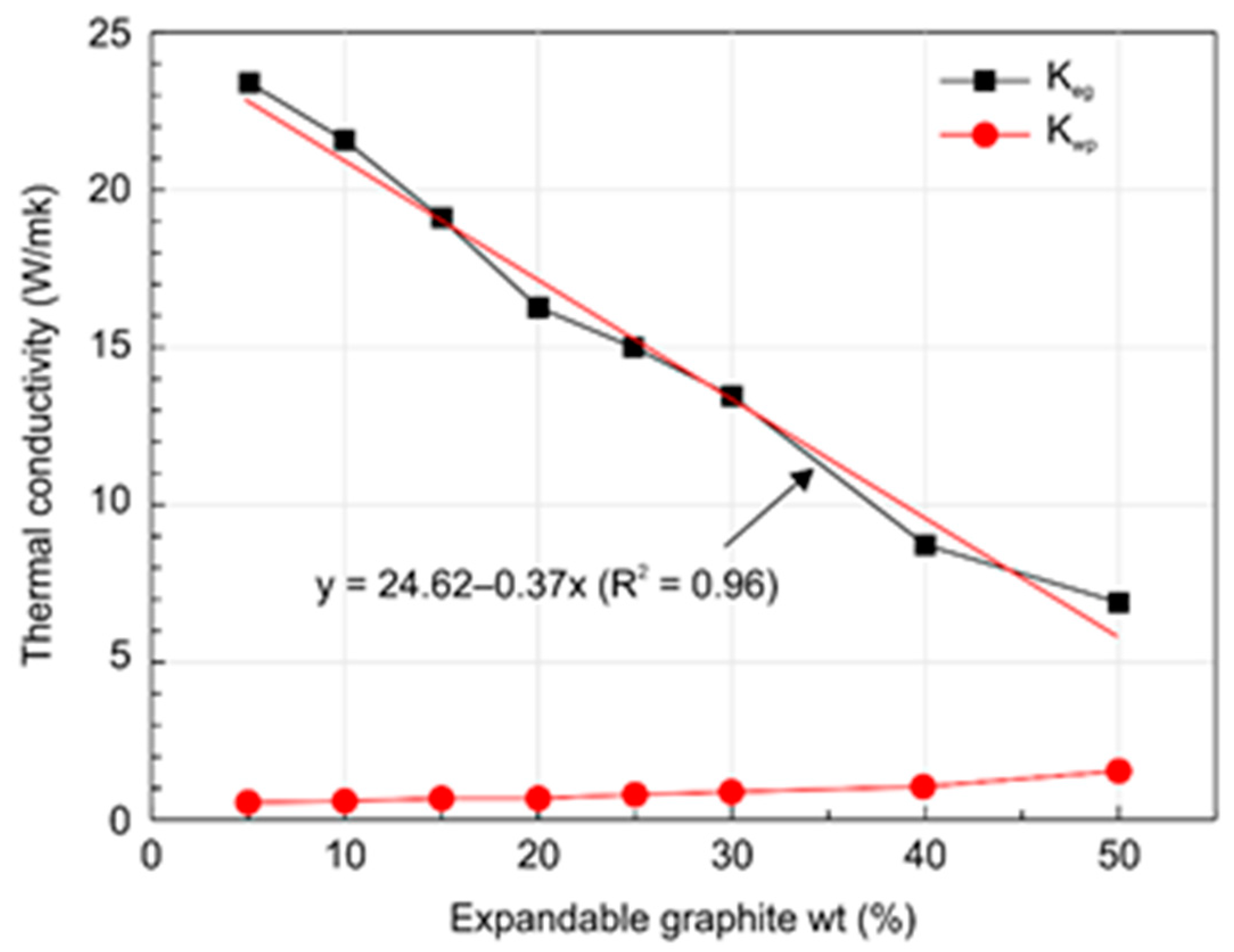
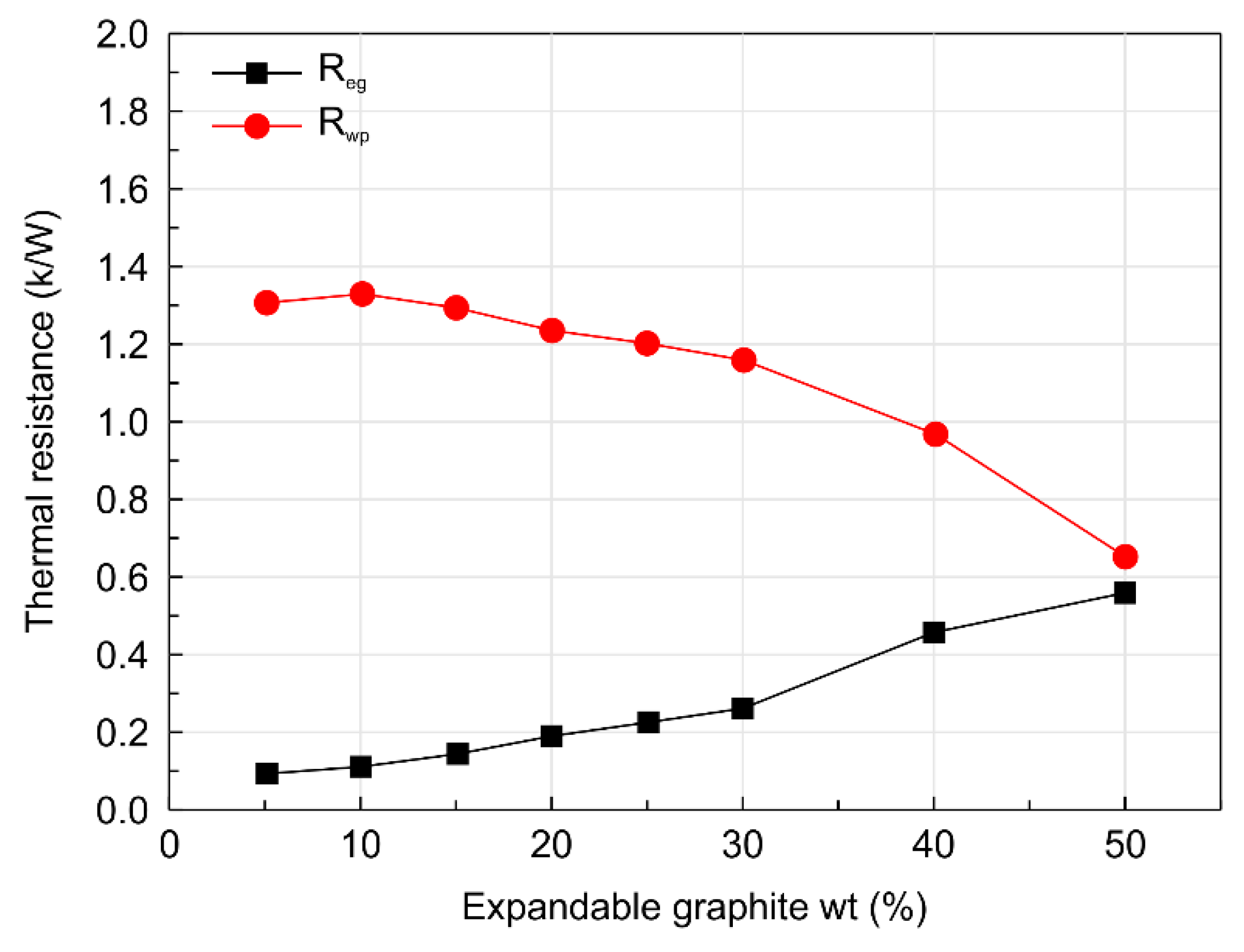
- Increasing the expandable graphite content from 0 to 50 wt.% decreased the thermal conductivity of the expanded graphite layer from 24.62 to 7.83 W/m·K. In general, the thermal conductivity of expandable graphite tends to increase when it exists in the unburnt state. However, when expandable graphite undergoes combustion upon exposure to heat, both the expandability of graphite and the thickness of the expanded layerincrease, which in turn tends to decrease the thermal conductivity in the expanded layer due to the hindered transport of heat caused by the heat transfer shielding effect.
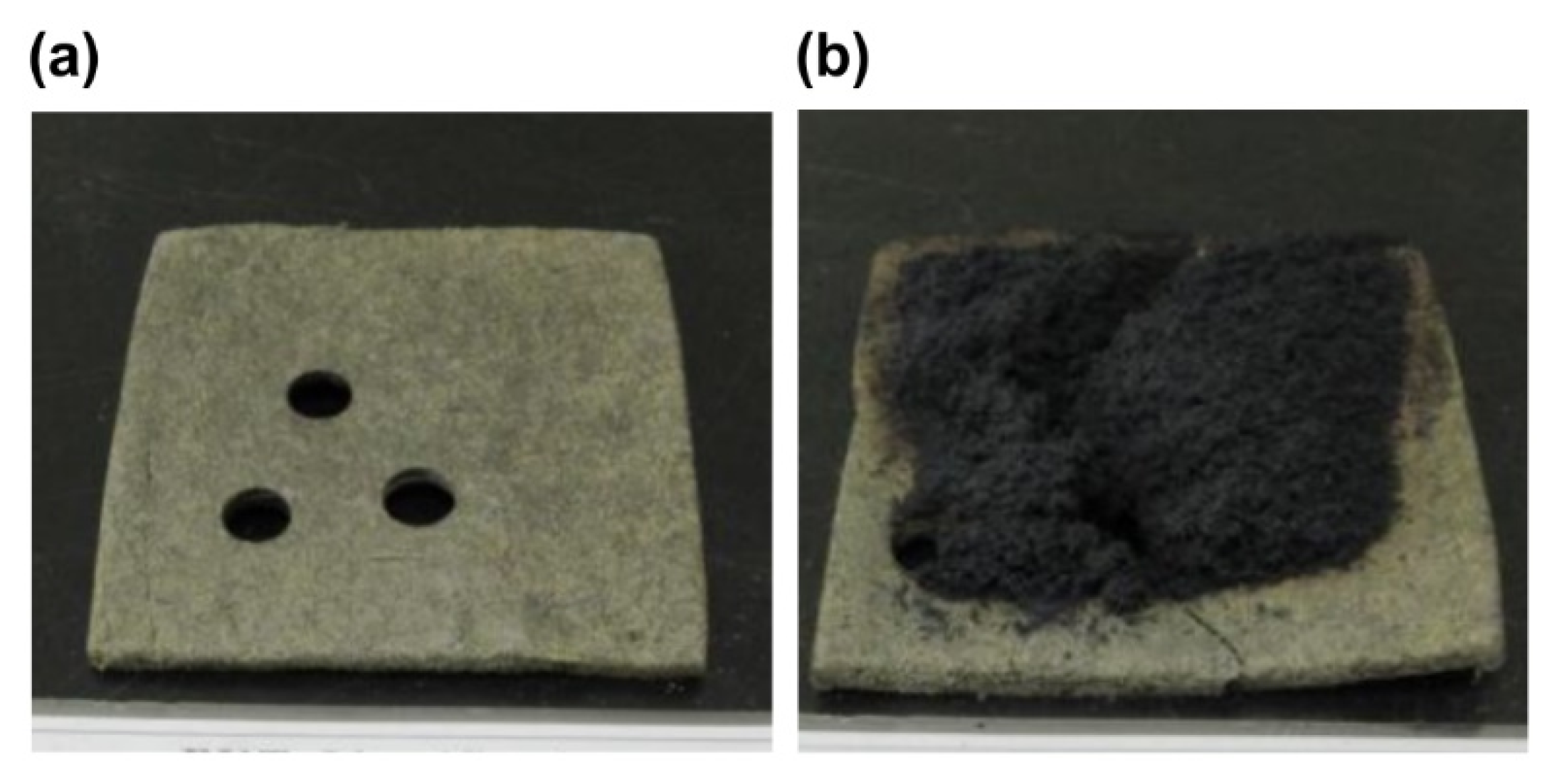
- Smoke toxicity tests with the 30 wt.% expandable graphite specimen showed that the time to inactivity of the white mouse was 14.9 min on average, which meets the lethal toxic potency standard (time to inactivity > 9 min) of a fire retardant. In the case of wood particles, the time to inactivity was 5.8 min, which does not meet the standard. In the case of the expandable graphite 30 wt.% composite material, the time to inactivity appears to have increased because of the smaller burned mass as compared to that in wood particles, thus reducing the generated amount of harmful gases such as CO and CO2.
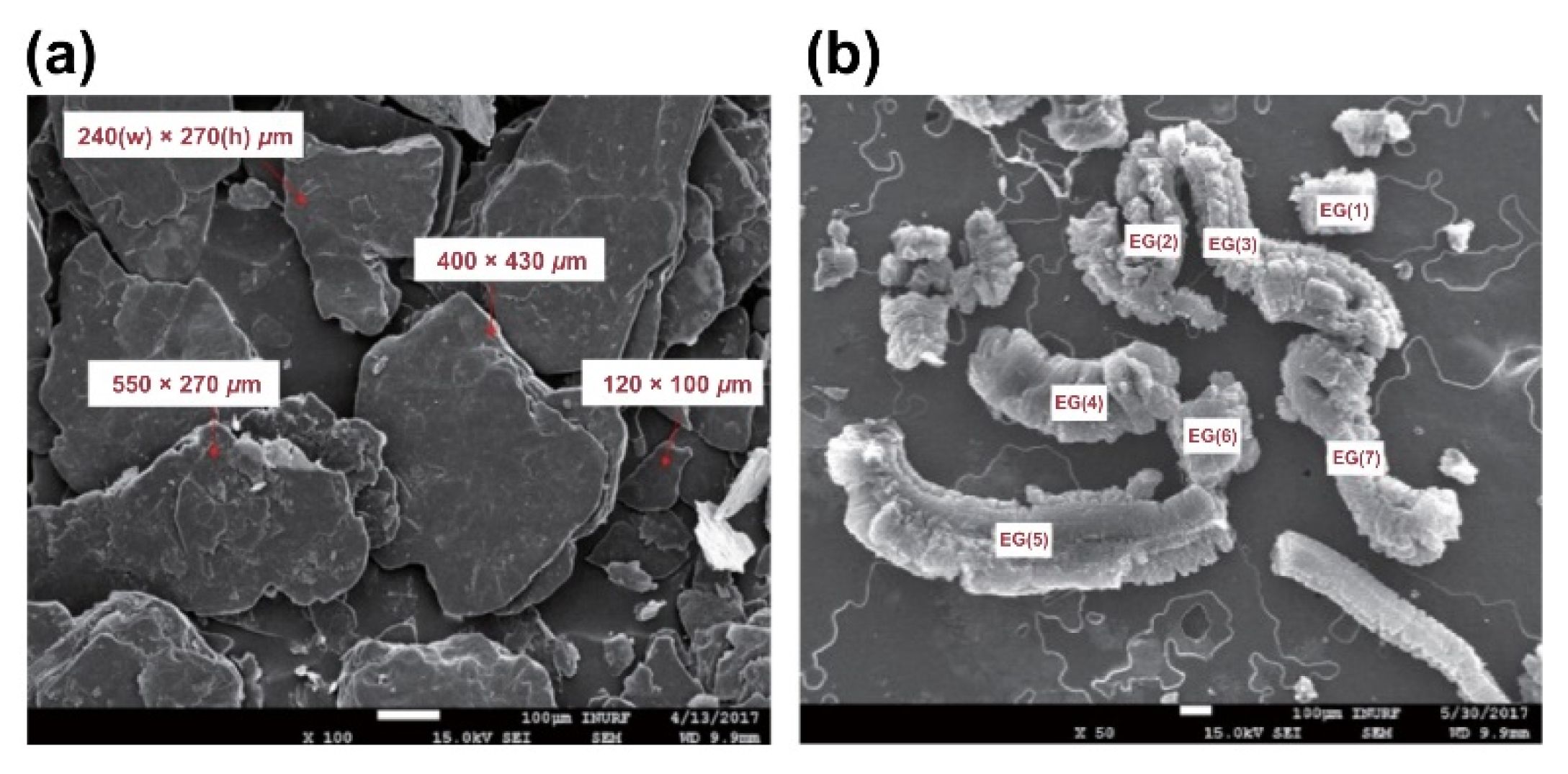
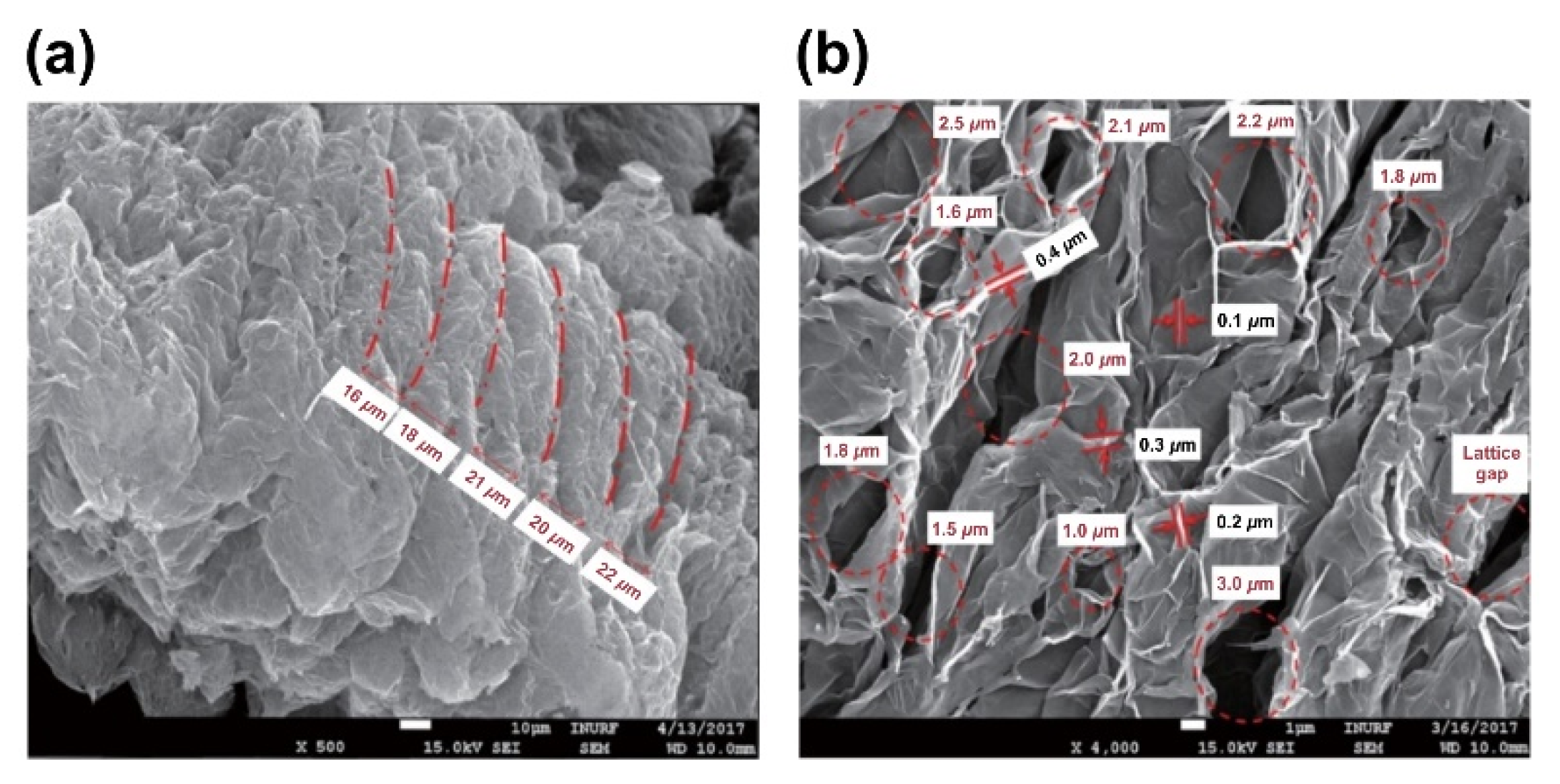
- The structure of expandable graphite before combustion was characterized by a laminated layer structure 120–550 μm in width, 100–430 μm in length, and approximately 10 μm in thickness. The microstructure of the expanded graphite was revealed to be a worm shape, and the amount of expanded graphite was observed to increase in proportion with increase in the expandable graphite content. Additionally, the microstructure of expanded graphite was characterized by a fine lattice layer with a 16–22-μm interlayer spacing, repeating at a thickness of 0.1–0.4 μm and an interval of 1.0–2.5 μm, and the enhanced thermal resistance of the composite was due to the decrease in thermal conductivity.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lowden, L.A.; Hull, T.R. Flammability behavior of wood and a review of the methods for its reduction. Fire Sci. Rev. 2013, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Hashim, R.; Sulaiman, O.; Kumar, R.N.; Tamyez, P.F.; Murphy, R.J.; Ali, Z. Physical and mechanical properties of flame retardant urea formaldehyde medium density fiberboard. J. Mater. Process. Technol. 2009, 209, 635–640. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, C.; Hu, Y.; Yang, S.; Qing, Y.; Wu, Q. Flame retardancy and thermal degradation behavior of red gum wood treated with hydrate magnesium chloride. J. Ind. Eng. Chem. 2014, 20, 3536–3542. [Google Scholar] [CrossRef]
- Cha, J.M.; Hyun, S.H.; Kim, I.B.; Yoon, M.O. A study on the flame retardant performance of MDF wood according to flame retardant treatment method. J. Korean Inst. Fire Sci. 2011, 25, 146–155. [Google Scholar]
- Yu, Y.; Hou, J.; Dong, Z.; Wang, C.; Lu, F.; Song, P. Evaluating the flammability performance of Portland cement-bonded particleboards with different cement-wood ratios using a Cone calorimeter. J. Fire Sci. 2016, 34, 199–211. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, B.W.; Kwon, S.P.; Lee, B.H.; Kim, H.S.; Kim, H.J. Burning behavior of flooring materials in the cone calorimeter and evaluation of toxic smoke. Mokchae Konghak 2008, 36, 45–53. [Google Scholar]
- Khalili, P.; Tshai, K.Y.; Kong, I. Natural fiber reinforced expandable graphite filled composites: Evaluation of the flame retardancy, thermal and mechanical performances. Compos. Part A Appl. Sci. 2017, 100, 194–205. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, K.S.; Lee, J.R. Thermal and mechanical interfacial properties of expanded graphite/epoxy composites. J. Korean Ind. Eng. Chem. 2004, 15, 493–498. [Google Scholar]
- Laachachi, A.; Burger, N.; Apaydin, K.; Sonnier, R.; Ferriol, M. Is expanded graphite acting as flame retardant in epoxy resin? Polym. Degrad. Stab. 2015, 117, 22–29. [Google Scholar] [CrossRef]
- Krupa, I.; Nógellová, Z.; Špitalský, Z.; Malíková, M.; Sobolčiak, P.; Abdelrazeq, H.W.; Ouederni, M.; Karkri, M.; Janigová, I.; Al-Maadeed, M.A.S. Positive influence of expanded graphite on the physical behavior of phase composite phase change material based on linear low density polyethylene and paraffin wax. Thermochim. Acta 2015, 614, 218–225. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A.; Simioni, F.; Camino, G. Expandable graphite as an intumescent flame retardant in polyisocyanurate–polyurethane foams. Polym. Degrad. Stab. 2002, 77, 195–202. [Google Scholar] [CrossRef]
- Kruger, H.J.; Focke, W.W.; Mhike, W.; Taute, A.; Roberson, A.; Ofosu, O. Cone calorimeter study of polyethylene flame retarded with expandable graphite and intumescent fire-retardant additives. J. Fire Sci. 2014, 32, 498–517. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, L.; Liu, B. Prediction of thermal conductivity of composite polymer materials filled with expanded graphite sheet fillers. J. Thermoplast. Compos. Mater. 2016, 29, 1573–1586. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, X. Study on paraffin/expanded graphite composite phase change thermal energy storage material. Energy Convers. Manag. 2006, 47, 303–310. [Google Scholar] [CrossRef]
- Duan, Z.J.; Zhang, H.Z.; Sun, L.X.; Cao, Z.; Xu, F.; Zou, Y.J.; Chu, H.L.; Qiu, S.J.; Xiang, C.L.; Zhou, H.Y. CaCl2.6H2O/expanded graphite composite as form-stable phase change materials for thermal energy storage. J. Therm. Calorim. 2014, 115, 111–117. [Google Scholar] [CrossRef]
- Choi, B.K.; Choi, W.K.; Kuk, Y.S.; Kim, H.G.; Seo, M.K. A study on thermal behaviors of expanded graphite/erythritol composite. Appl. Chem. Eng. 2014, 25, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Karaipekli, A.; Sari, A.; Kaygusuz, K. Thermal conductivity improvement of stearic acid using expanded graphite and carbon fiber for energy storage applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Huang, Z.; Zhai, D.; Gao, X.; Xu, T.; Fang, Y.; Zhang, Z. Theoretical study on effective thermal conductivity of salt/expanded graphite composite material by using fractal method. Appl. Therm. Eng. 2015, 86, 309–317. [Google Scholar] [CrossRef]
- Afanasov, I.M.; Savchenko, D.V.; Ionov, S.G. Thermal conductivity and mechanical properties of expanded graphite. Inorg. Mater. 2009, 45, 486–490. [Google Scholar] [CrossRef]
- Kim, S.H.; Yu, S.G.; Seo, J.K.; Kim, S.M. Thermal Performance of Wooden Building Envelope by Thermal Conductivity of Structural Members. J. Korean Wood Sci. Technol. 2013, 41, 515–527. [Google Scholar] [CrossRef]
- Lee, S.H. The Theory and Practice of Fire Investigation; DongHwa Technology: Seoul, Korea, 2009; pp. 23–86. [Google Scholar]
- Kim, J.S.; Rie, D.H. Downward smoldering Fire Characteristics of Wood Chips and Wood Flour. J. Korean Soc. Hazard Mitig. 2009, 13, 269–274. [Google Scholar]
- Simpson, W.T. Drying and control of moisture content and dimensional changes. In Wood Handbook—Wood as an Engineering Material; Forest Product Laboratory U.S.D.A Forest Service Madison: Madison, WI, USA, 1987; pp. 1–21. [Google Scholar]
- Holman, J.P. Heat Transfer, 9th ed.; McGraw Hill: New York, NY, USA, 2002. [Google Scholar]
- Incropera, F.P.; DeWitt, D.P. Introduction to Heat Transfer, 4th ed.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Reaction-to-fire Tests-Heat Release, Smoke Production and Mass Loss Rate-Part 1: Heat Release Rate (Cone Calorimeter Method); KSA ISO 5660-1; Korean Agency for Technology and Standards: Seoul, Korea, 30 December 2008.
- Kim, N.K.; Kang, Y.; Rie, D.H. Study on the Necessity of Complex Hazard Assessment for Combustion Products of Wood-Based Building Materials. J. Korean Soc. Hazard Mitig. 2017, 17, 173–179. [Google Scholar] [CrossRef]



| Composition | Wood Particles | Expandable Graphite |
|---|---|---|
| Content (%) | Cellulose ≤ 50% Hemicellulose ≤ 25% Lignin ≤ 25% Chemical Content - Carbon: 55.61 wt.% - Oxygen: 44.39 wt.% | Fixed carbon: 93.8% Ash: 6.2% Moisture: 0.68% pH: 4.5 Expansion volume: 210 mL/g Expansion temperature: 180–200 °C |
| Size | 80–100 mesh | 80 mesh (82%) |
| Morphology |  |  |
| Specimen | Mass of Composite Materials (g) | ||||
|---|---|---|---|---|---|
| Case | EG wt.% | Expandable Graphite | Wood Particles | Common Binder | Sum |
| Case 1 | EG 0 wt.% | 0 | 100 | 300 | 400 |
| Case 2 | EG 5 wt.% | 21 | 100 | 300 | 421 |
| Case 3 | EG 10 wt.% | 45 | 100 | 300 | 445 |
| Case 4 | EG 15 wt.% | 71 | 100 | 300 | 471 |
| Case 5 | EG 20 wt.% | 100 | 100 | 300 | 500 |
| Case 6 | EG 25 wt.% | 134 | 100 | 300 | 534 |
| Case 7 | EG 30 wt.% | 172 | 100 | 300 | 572 |
| Case 8 | EG 40 wt.% | 267 | 100 | 300 | 667 |
| Case 9 | EG 50 wt.% | 400 | 100 | 300 | 800 |
| Item | Semi Noncombustible Material | Flame Retardant Material |
|---|---|---|
| THR (MJ/m2) | Below 8 MJ/m2 | Below 8 MJ/m2 |
| Time in which the heat release rate is exceeded 200 kW/m2 continuously | Below 10 s | Below 10 s |
| All core melt, penetrating cracks, slot change, etc., | No core melt, penetrating cracks, slot change | No core melt, penetrating cracks, slot change |
| Toxicity | Experimental mouse Activity over 9 min | Experimental mouse Activity over 9 min |
| Test time | 10 min (600 s) | 5 min (300 s) |
| Specimen | EG 0 wt.% (Wood Particles 100%) | EG 30 wt.% |
|---|---|---|
| No.1 | 5.5 | 14.8 |
| No.2 | 6.2 | 15.4 |
| No.3 | 5.7 | 14.5 |
| Average | 5.8 | 14.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun, K.; Kim, J.; Rie, D. Thermal Characteristics of Expandable Graphite–Wood Particle Composites. Materials 2020, 13, 2732. https://doi.org/10.3390/ma13122732
Chun K, Kim J, Rie D. Thermal Characteristics of Expandable Graphite–Wood Particle Composites. Materials. 2020; 13(12):2732. https://doi.org/10.3390/ma13122732
Chicago/Turabian StyleChun, Kwanok, Jeonggon Kim, and Dongho Rie. 2020. "Thermal Characteristics of Expandable Graphite–Wood Particle Composites" Materials 13, no. 12: 2732. https://doi.org/10.3390/ma13122732






