Microstructural Evolution of Cold-Rolled AA7075 Sheet during Solution Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
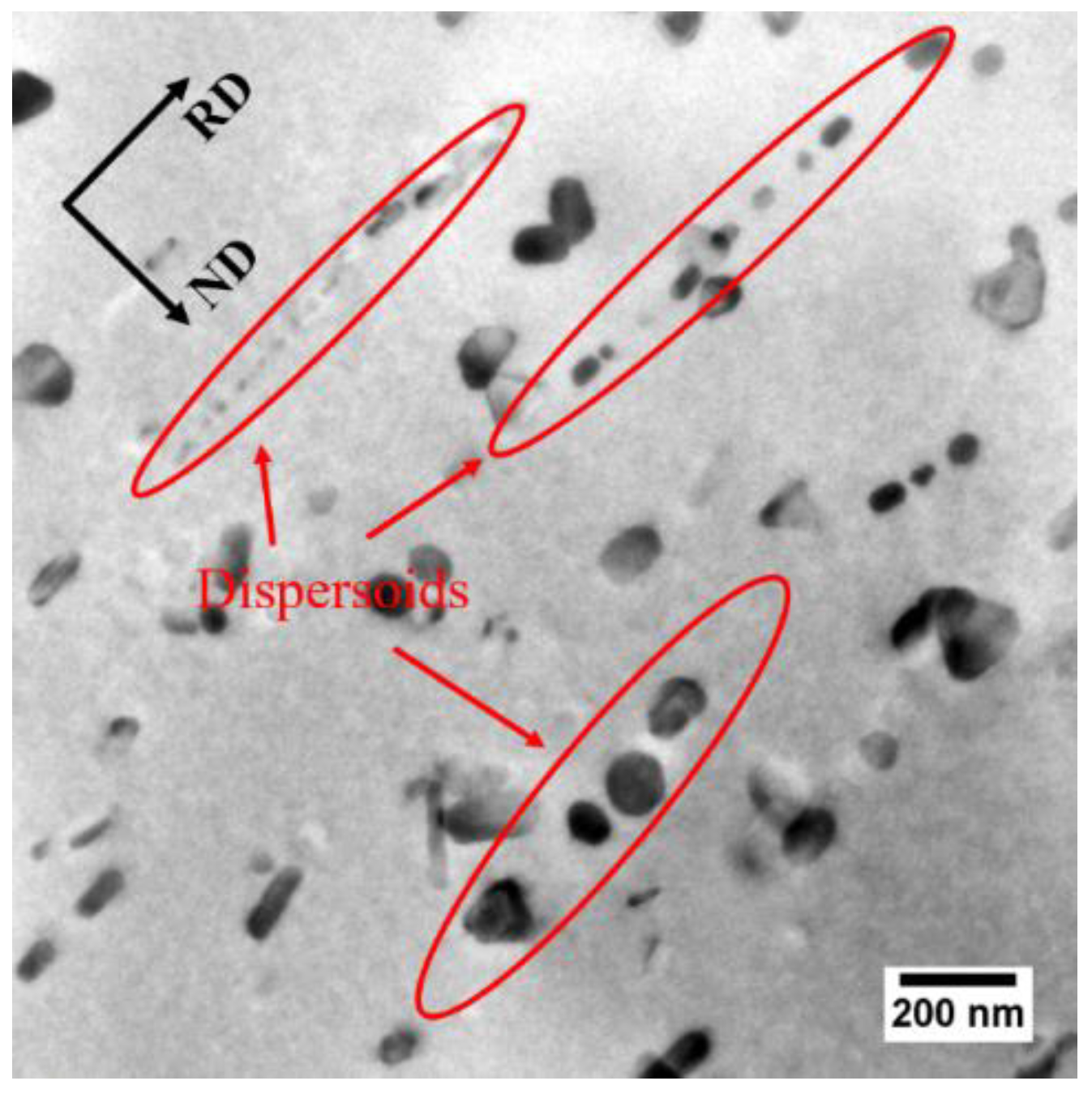
3.1. Microstructures in the Cold-Rolled Sheet
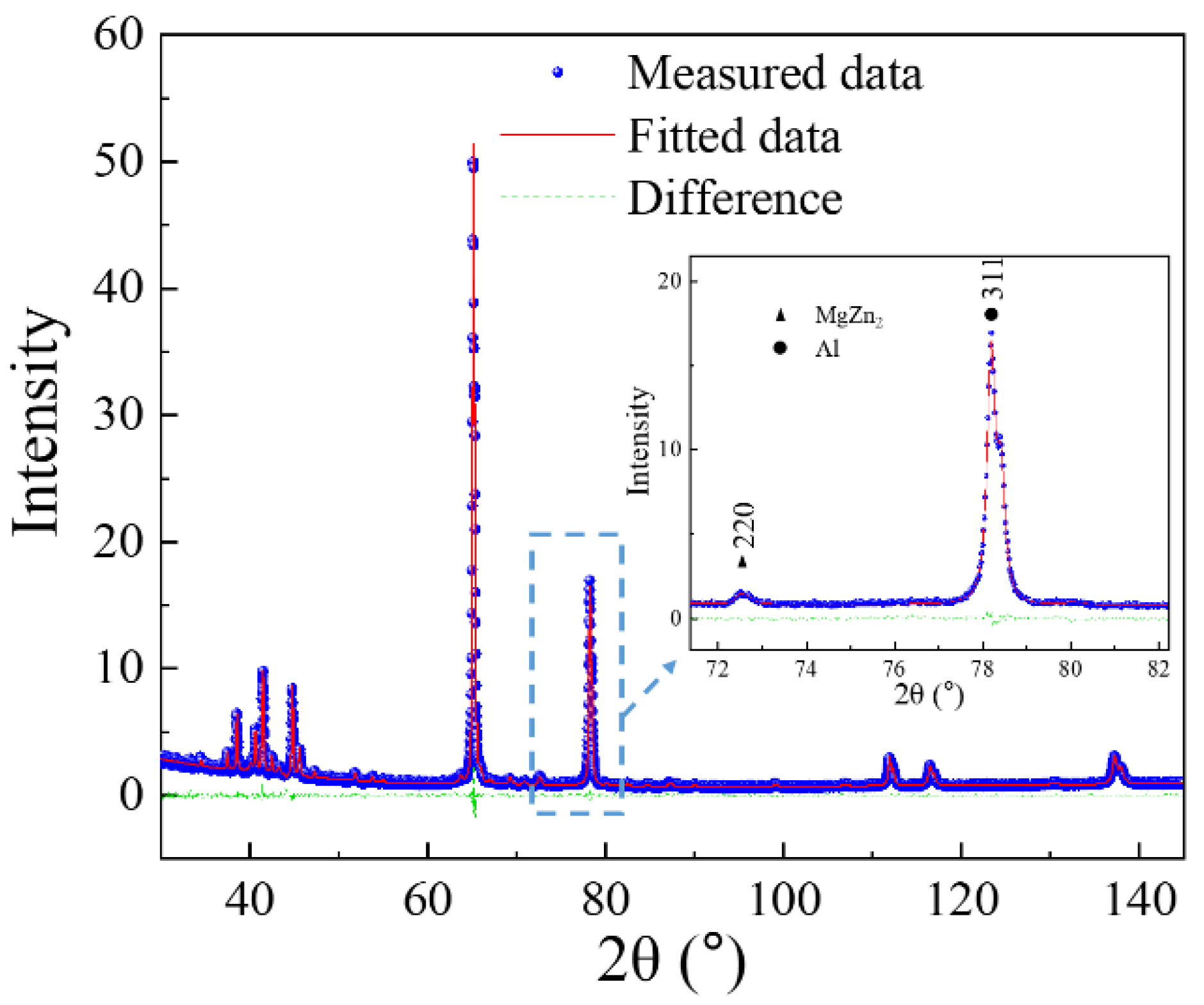
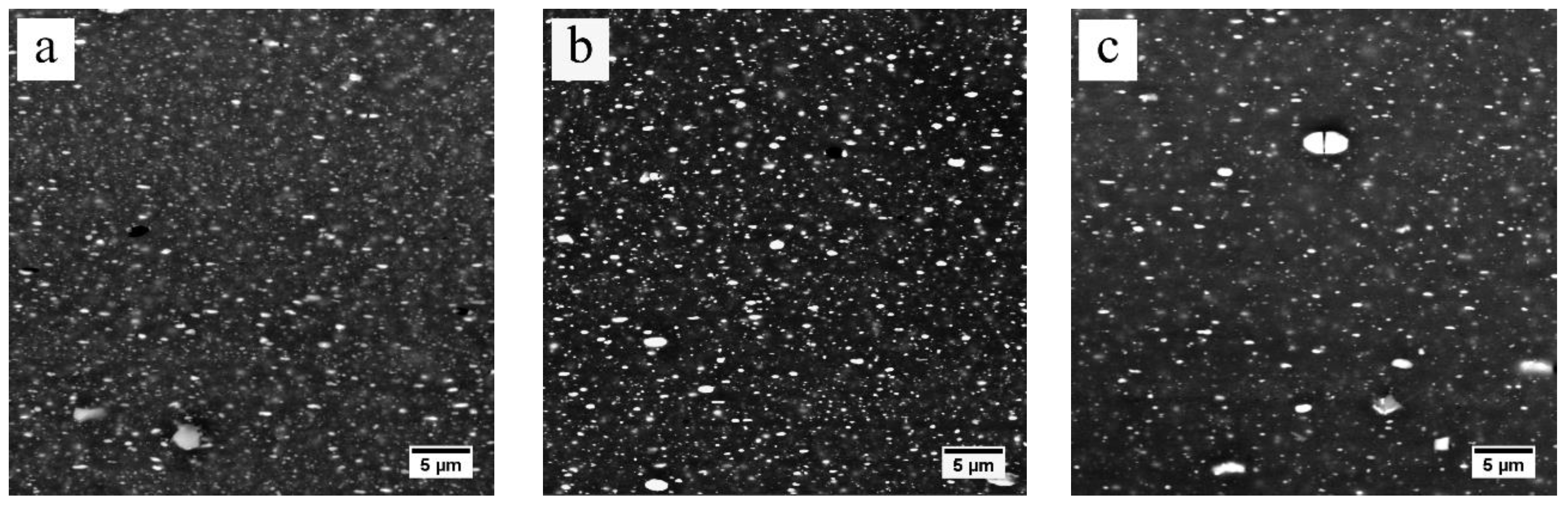
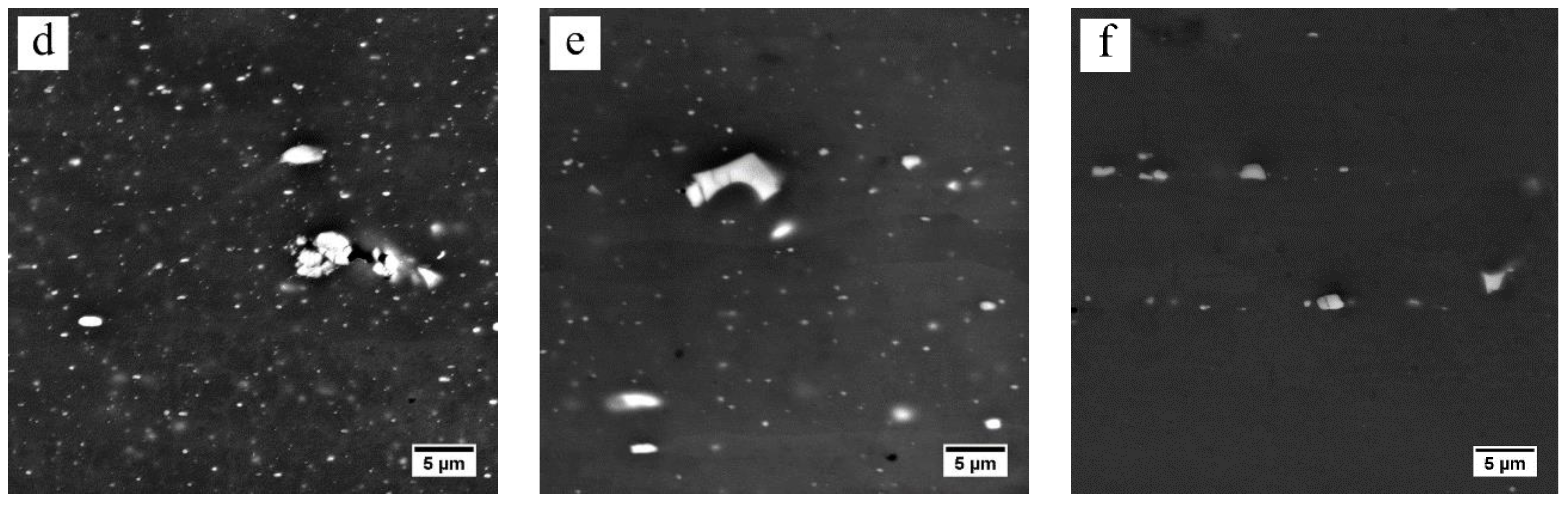
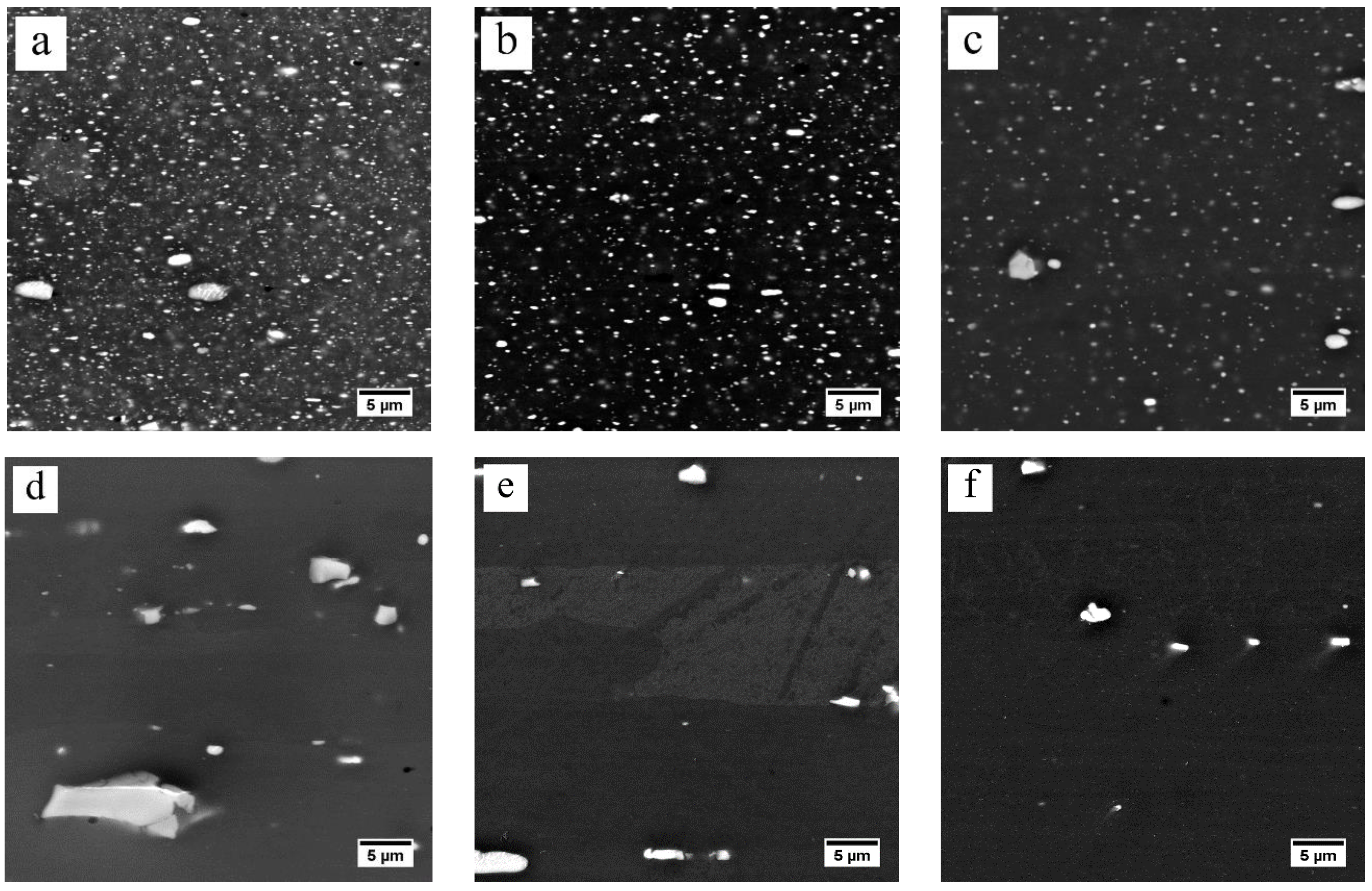
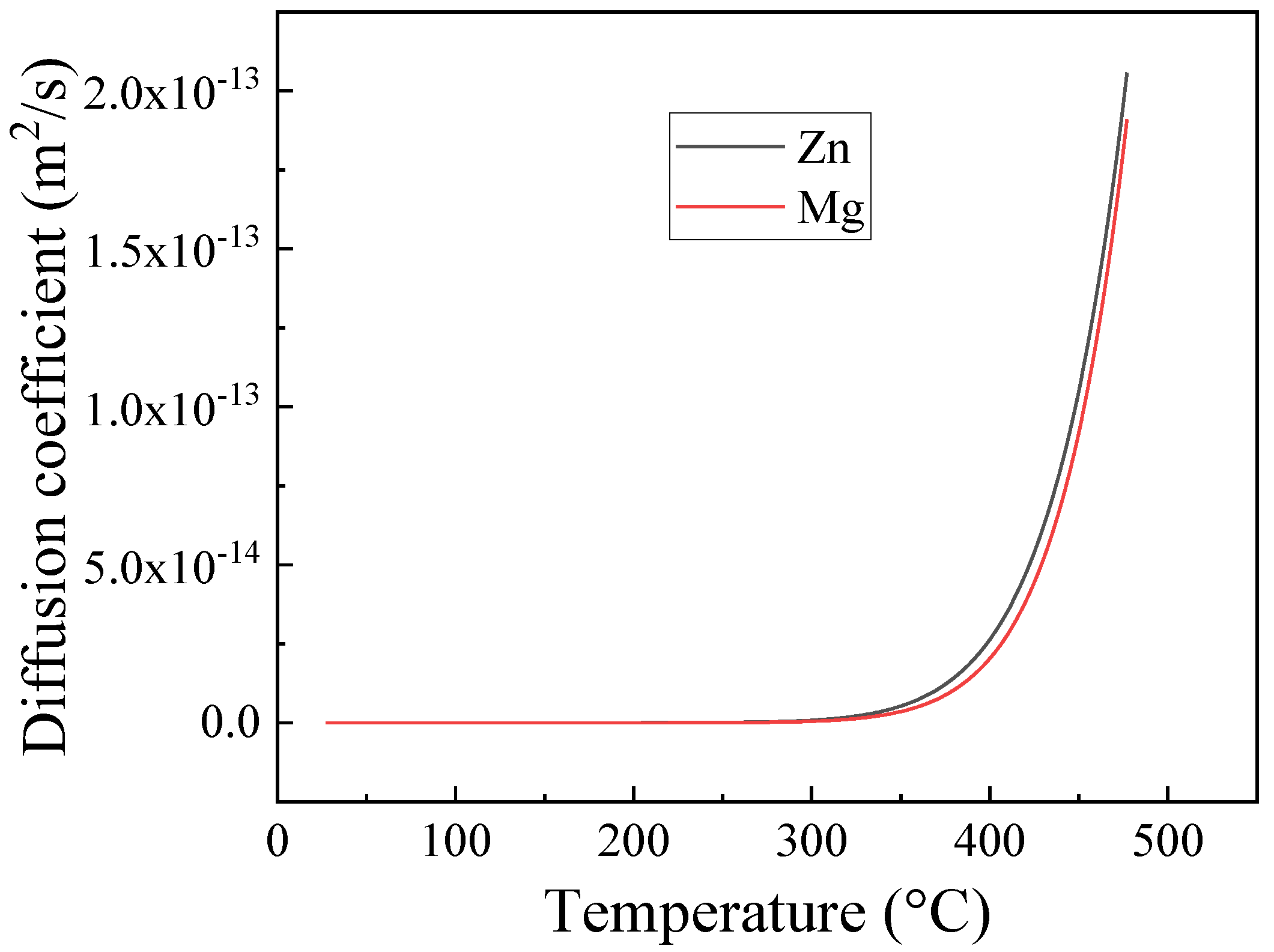
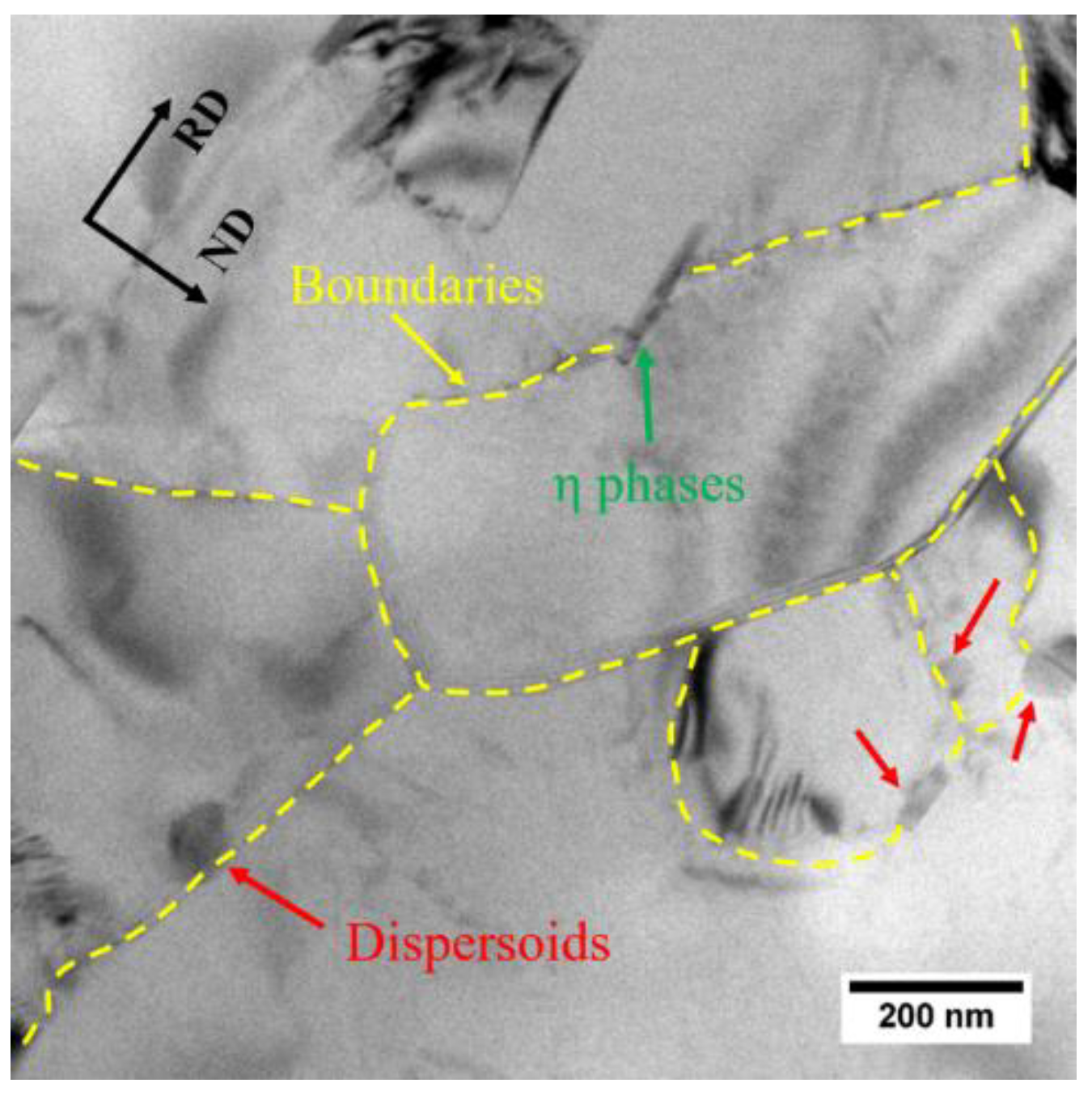
3.2. Dissolution of Equilibrium Precipitates (η)
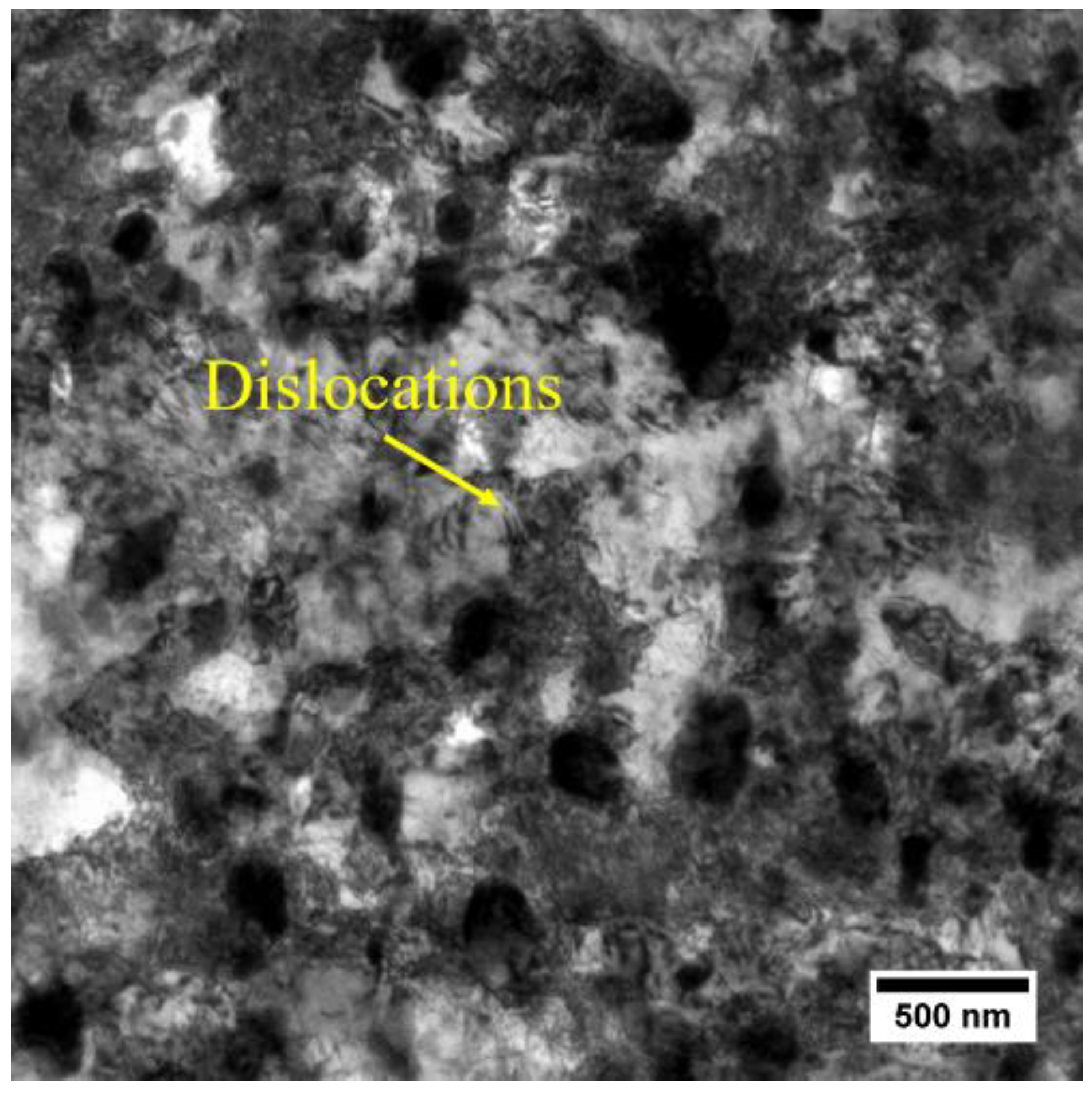
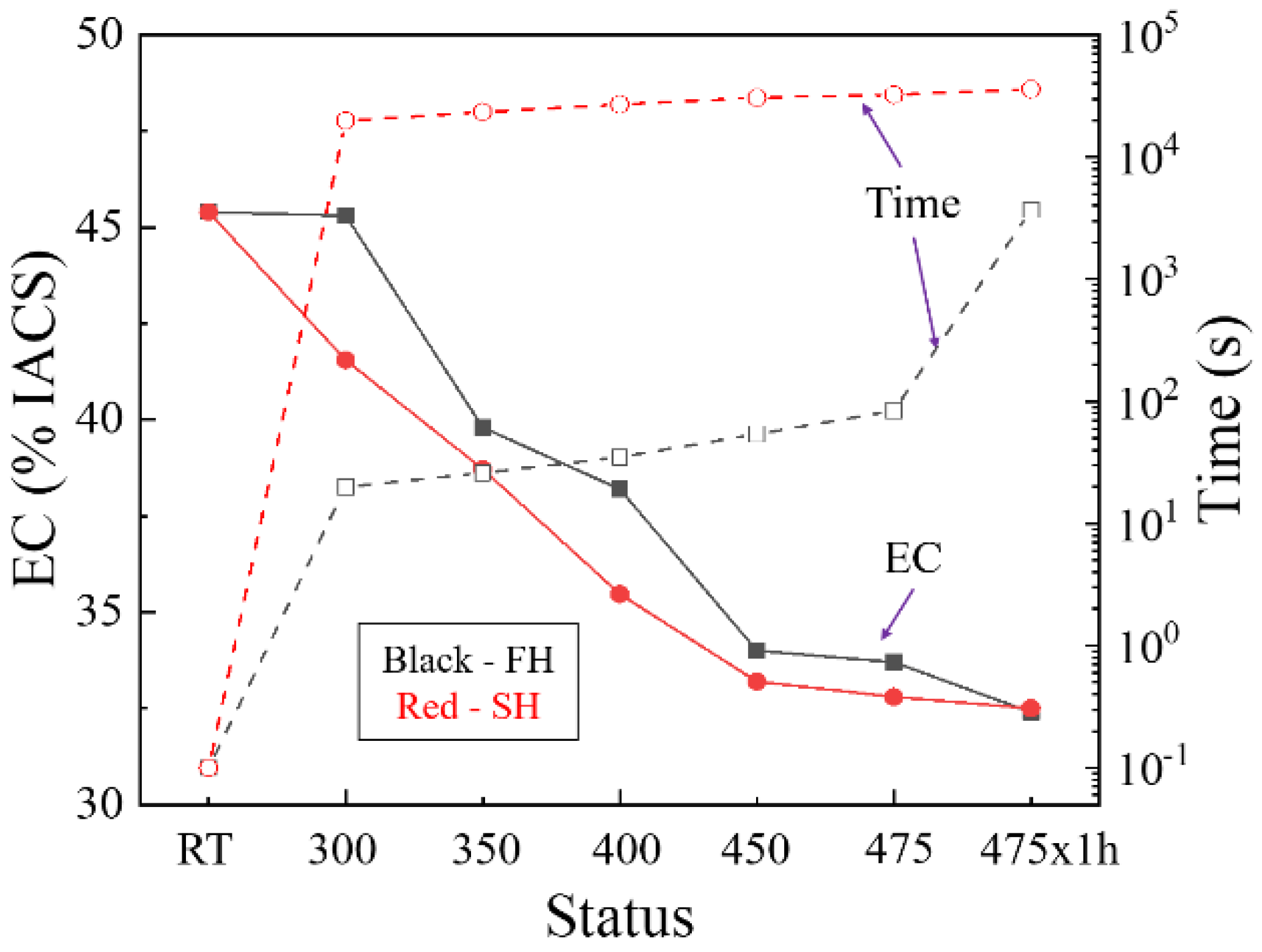
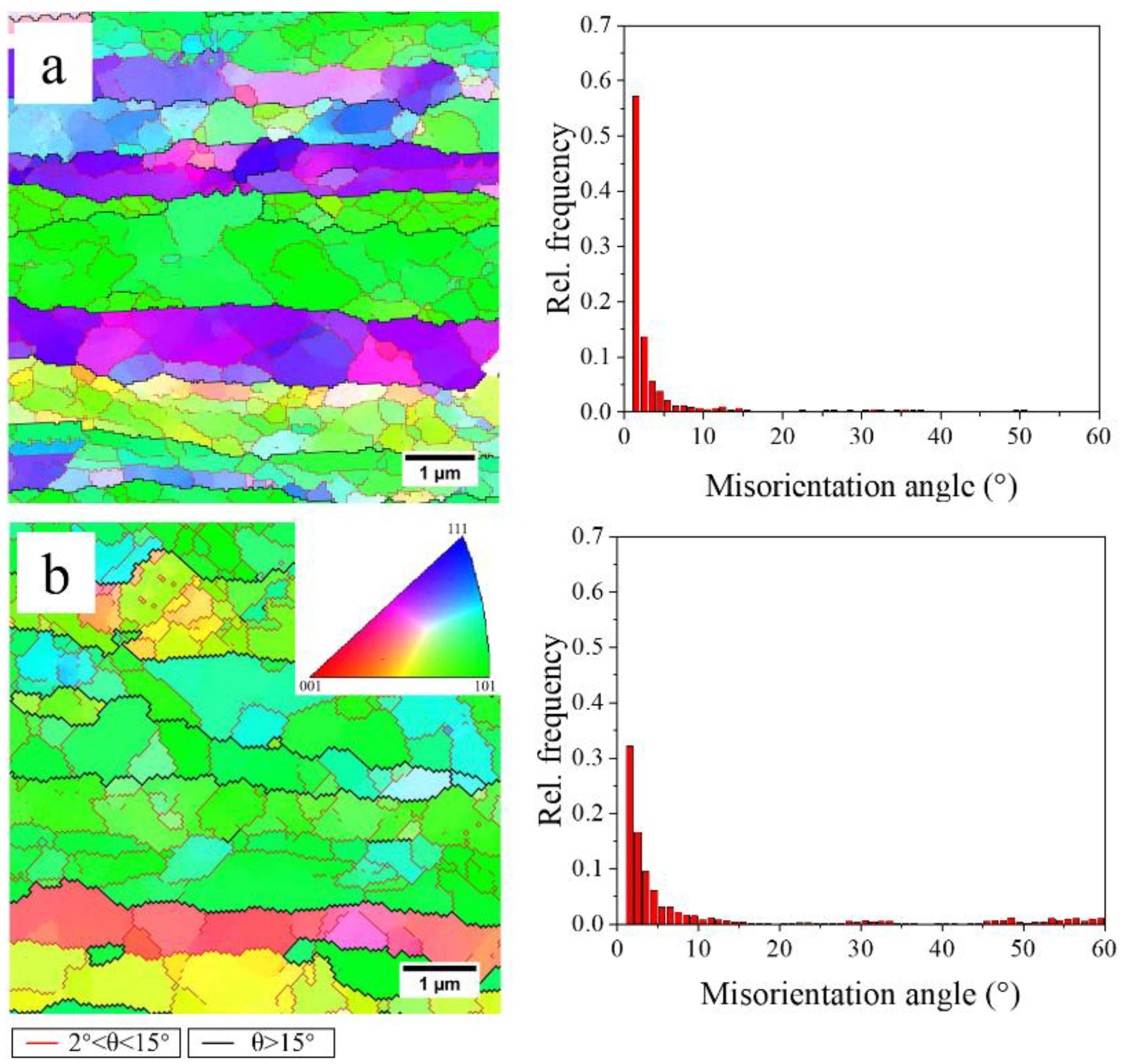
3.3. Recovery Prior to Recrystallization
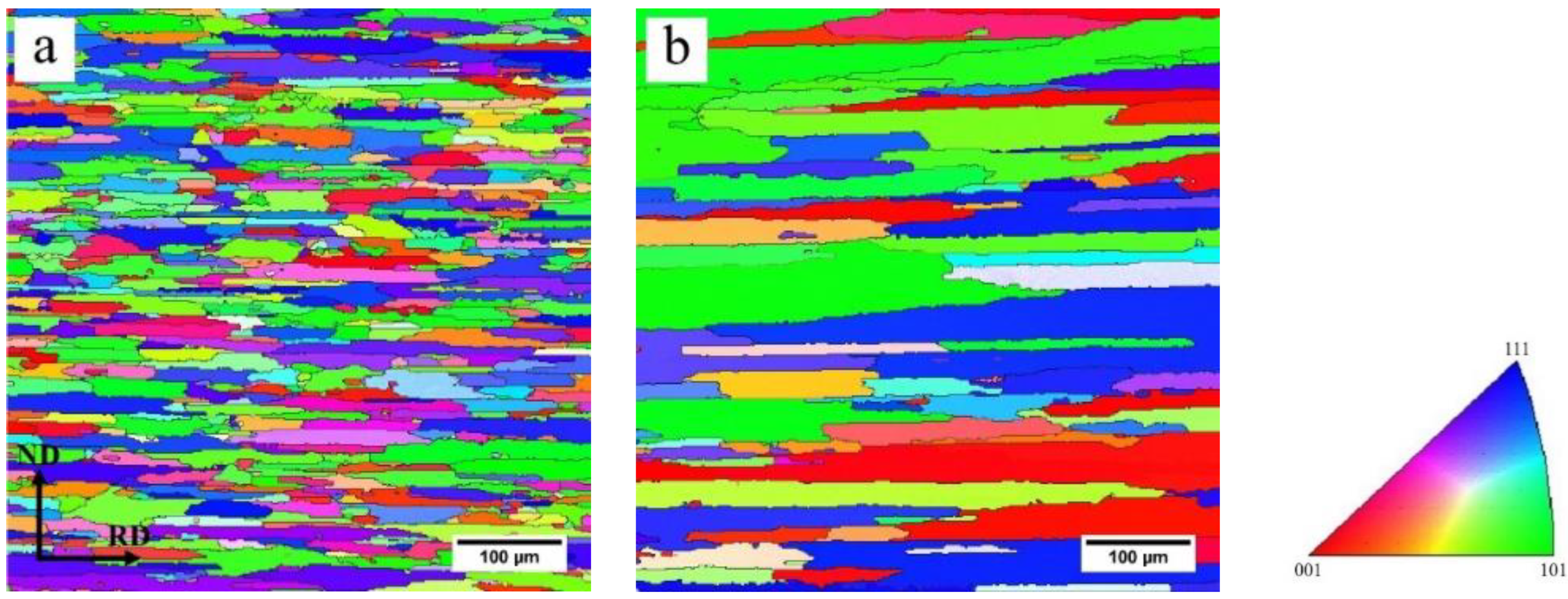
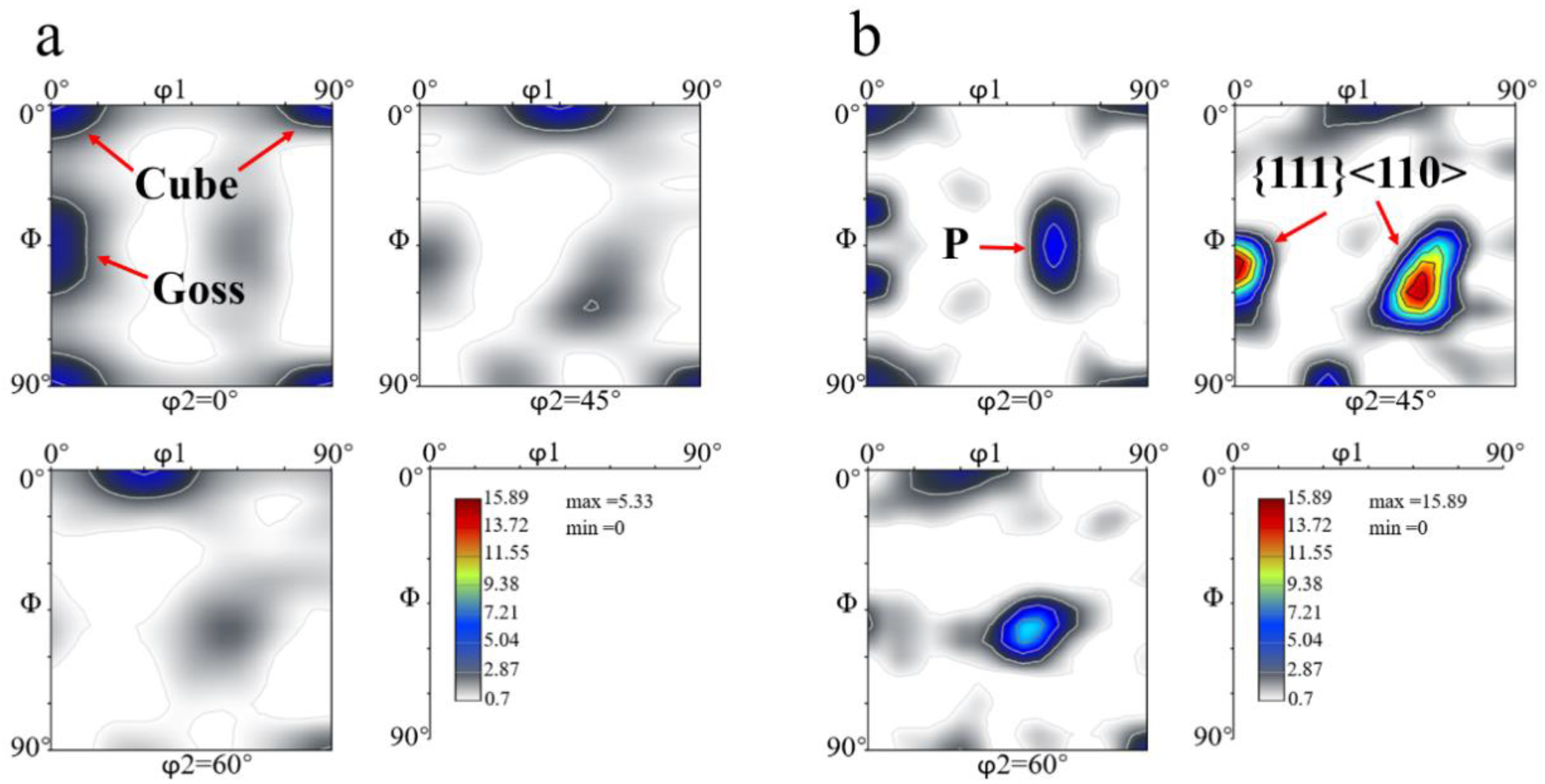
3.4. Grain Structures and Textures after Solution Treatment
3.5. Tensile Properties
4. Discussion
5. Conclusions
- The heating rate influenced the dissolution of equilibrium η particles during the heating stage of the solution treatment and the final microstructure of the sheet at the time of recrystallization. The dissolution of η particles mainly happened during the heating process and these particles still remained when recrystallization began. Since the η particles gradually dissolved into the matrix, the recrystallization during heating process may be mainly affected by prior recovery and the sub-micron and indissoluble dispersoids.
- Recovery during the heating process consumes some of the stored energy for recrystallization. A high degree of recovery caused by the long time at low temperature results in the low nucleation rate for recrystallization and coarser grains in the slow-heated sample after solution treatment. The finer grains and higher recrystallized fractions in the fast-heated sample are attributed to more nucleation sites for recrystallization, due to the higher stored energy after recovery.
- The elongated nature of the recrystallized grains is caused by the variation in the pinning pressure of the migration of the boundaries toward the RD and ND, due to the shape and distribution of dispersoids and possibly η phases. The variation is enhanced by the long time for (sub)grain growth during the slow heating process, resulting in the strongly elongated grains.
- Typical recrystallization textures, such as Cube, Goss and P, as well as a shear {111}<110> texture, were found in both fast-heated and slow-heated samples after solution treatment. The sharp textures in the slow-heated sample led to the strong anisotropic tensile properties (high longitudinal yield strength, high Δr) after artificial aging to T6 temper.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminum alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Hirsch, J. Aluminum in innovative light-weight car design. Mater. Trans. 2011, 52, 818–824. [Google Scholar] [CrossRef]
- Couturier, L.; Deschamps, A.; De Geuser, F.; Fazeli, F.; Poole, W.J. An investigation of the strain dependence of dynamic precipitation in an Al-Zn-Mg-Cu alloy. Scr. Mater. 2017, 136, 120–123. [Google Scholar] [CrossRef]
- Shin, J.; Kim, T.; Kim, D.; Kim, D.; Kim, K. Castability and mechanical properties of new 7xxx aluminum alloys for automotive chassis/body applications. J. Alloy. Compd. 2017, 698, 577–590. [Google Scholar] [CrossRef]
- Kumar, M.; Sotirov, N.; Chimani, C.M. Investigations on warm forming of AW-7020-T6 alloy sheet. J. Mater. Process. Technol. 2014, 214, 1769–1776. [Google Scholar] [CrossRef]
- Kumar, M.; Ross, N.G. Influence of temper on the performance of a high-strength Al-Zn-Mg alloy sheet in the warm forming processing chain. J. Mater. Process. Technol. 2016, 231, 189–198. [Google Scholar] [CrossRef]
- Huo, W.T.; Hou, L.G.; Zhang, Y.S.; Zhang, J.S. Warm formability and post-forming microstructure/property of high-strength AA 7075-T6 Al alloy. Mater. Sci. Eng. A 2016, 675, 44–54. [Google Scholar] [CrossRef]
- Engler, O.; Hirsch, J. Texture control by thermomechanical processing of AA6xxx Al-Mg-Si sheet alloys for automotive applications-a review. Mater. Sci. Eng. A 2002, 336, 249–262. [Google Scholar] [CrossRef]
- Lee, Y.S.; Koh, D.H.; Kim, H.W.; Ahn, Y.S. Improved bake-hardening response of Al-Zn-Mg-Cu alloy through pre-aging treatment. Scr. Mater. 2018, 147, 45–49. [Google Scholar] [CrossRef]
- He, Y.; Jia, Z.H.; Sanders, R.E.; Liu, Y.Y.; Ding, L.P.; Xing, Y.; Liu, Q. Quantitative study of dissolution of Mg2Si during solution treatment in AA6014 alloy. J. Alloy. Compd. 2017, 703, 272–279. [Google Scholar] [CrossRef]
- Shen, F.H.; Wang, B.; Yi, D.Q.; Liu, H.Q.; Tang, C.; Shou, W.B. Effects of heating rate during solid-solution treatment on microstructure and fatigue properties of AA2524 T3 Al-Cu-Mg sheet. Mater. Des. 2016, 104, 116–125. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.; Cao, L.; Luo, J.; Zhang, J.; Zhuang, L. Effect of heating rate on mechanical property, microstructure and texture evolution of Al-Mg-Si-Cu alloy during solution treatment. Mater. Sci. Eng. A 2015, 621, 8–17. [Google Scholar] [CrossRef]
- Robson, J.D.; Prangnell, P.B. Predicting recrystallized volume fraction in aluminum alloy 7050 hot rolled plate. Mater. Sci. Technol. 2002, 18, 607–614. [Google Scholar] [CrossRef]
- Bennett, T.A.; Petrov, R.H.; Kestens, L.A.I.; Zhuang, L.Z.; de Smet, P. The effect of particle-stimulated nucleation on texture banding in an aluminum alloy. Scr. Mater. 2010, 63, 461–464. [Google Scholar] [CrossRef]
- Hatch, J.D. Aluminum: Properties and Physical Metallurgy, 1st ed.; AMS International: Cleveland, OH, USA, 1984; pp. 123–124. [Google Scholar]
- Kim, K.C.; Nam, S.W. Effects of Mn-dispersoids on the fatigue mechanism in an Al-Zn-Mg alloy. Mater. Sci. Eng. A 1998, 244, 257–262. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Rohrer, G.S.; Rollett, A.D. Recrystallization and Related Annealing Phenomena, 3rd ed.; Elsevier: Oxford, UK, 2017; pp. 169–213. [Google Scholar]
- Park, D.S.; Nam, S.W. On the characteristics of Mn dispersoid in Al-Zn-Mg alloys. J. Mater. Sci. Lett. 1994, 13, 716–718. [Google Scholar] [CrossRef]
- Wu, L.M.; Wang, W.H.; Hsu, Y.F.; Trong, S. Effects of homogenization treatment on recrystallization behavior and dispersoid distribution in an Al-Zn-Mg-Sc-Zr alloy. J. Alloy. Compd. 2008, 456, 163–169. [Google Scholar] [CrossRef]
- Morere, B.; Maurice, C.; Shahani, R.; Driver, J. The influence of Al3Zr dispersoids on the recrystallization of hot-deformed AA 7010 alloys. Metall. Mater. Trans. A 2001, 32, 625–632. [Google Scholar] [CrossRef]
- Beausir, B.; Fundenberger, J.J. Analysis Tools for Electron and X-ray Diffraction; Université de Lorraine-Metz: Metz, France, 2017. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Ribarik, G.; Joni, B.; Ungar, T. Global optimum of microstructure parameters in the CMWP line-profile-analysis method by combining Marquardt-Levenberg and Monte-Carlo procedures. J. Mater. Sci. Technol. 2019, 35, 1508–1514. [Google Scholar] [CrossRef]
- Ungár, T. Dislocation densities, arrangements and character from X-ray diffraction experiments. Mater. Sci. Eng. A 2001, 309–310, 14–22. [Google Scholar] [CrossRef]
- Ribárik, G.; Ungár, T.; Gubicza, J. MWP-fit: A program for multiple whole-profile fitting of diffraction peak profiles by ab initio theoretical functions. J. Appl. Cryst. 2001, 34, 669–676. [Google Scholar] [CrossRef]
- Vicente Alvarez, M.A.; Santisteban, J.R.; Vizcaíno, P.; Ribárik, G.; Ungar, T. Quantification of dislocations densities in zirconium hydride by X-ray line profile analysis. Acta Mater. 2016, 117, 1–12. [Google Scholar] [CrossRef]
- Jazaeri, H.; Humphreys, F.J. The transition from discontinuous to continuous recrystallization in some aluminum alloys. Acta Mater. 2004, 52, 3251–3262. [Google Scholar] [CrossRef]
- Chen, J.Z.; Zhen, L.; Shao, W.Z.; Dai, S.L.; Cui, Y.X. Through-thickness texture gradient in AA 7055 aluminum alloy. Mater. Lett. 2008, 62, 88–90. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cho, K.K.; Han, J.H.; Shin, M.C. Effect of grain shape and texture on the earings in an Al-Li alloy. Scr. Mater. 2000, 43, 759–764. [Google Scholar] [CrossRef]
- Bampton, C.C.; Wert, J.A.; Mahoney, M.W. Heating rate effects on recrystallized grain size in two Al-Zn-Mg-Cu Alloys. Metall. Trans. A 1982, 13, 193–198. [Google Scholar] [CrossRef]
- Huang, K.; Marthinsen, K. The effect of heating rate on the softening behaviour of a deformed Al-Mn alloy with strong and weak concurrent precipitation. Mater. Charact. 2015, 110, 215–221. [Google Scholar] [CrossRef]
- Zhao, Q.L.; Huang, K.; Li, Y.J.; Marthinsen, K. Orientation preference of recrystallization in supersaturated aluminum alloys influenced by concurrent precipitation. Metall. Mater. Trans. A 2016, 47, 1378–1388. [Google Scholar] [CrossRef]

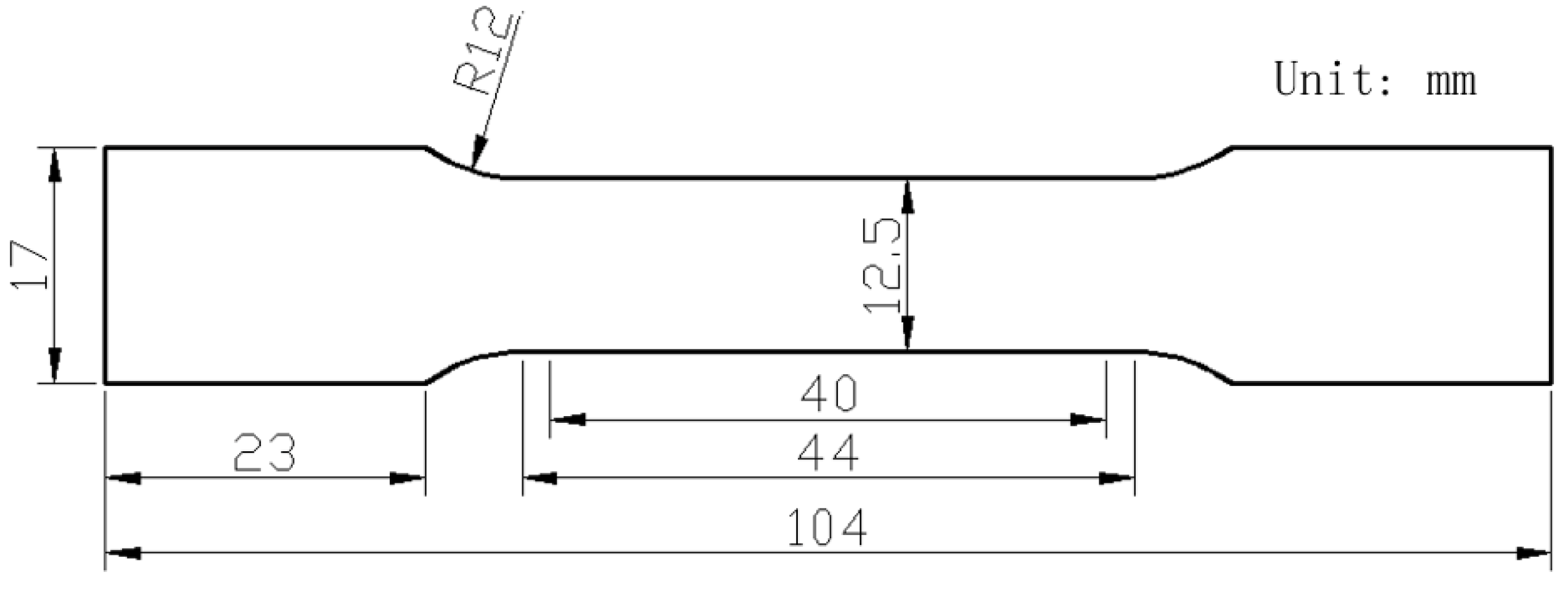


| Alloy | Zn | Mg | Cu | Cr | Mn | Ti | Fe | Si | Al |
|---|---|---|---|---|---|---|---|---|---|
| wt% | 5.57 | 2.29 | 1.46 | 0.20 | 0.08 | 0.04 | 0.30 | 0.08 | Bal. |
| Status | Dislocation Density/1014 m−2 | |
|---|---|---|
| FH | SH | |
| Cold-rolled | 3.28 ± 0.43 | 3.28 ± 0.43 |
| Quenched at 300°C | 1.56 ± 0.31 | 0.63 ± 0.16 |
| Average Grain Size /μm | Grain Aspect Ratio | |
|---|---|---|
| FH | 15.9 ± 3.7 | 4.4 |
| SH | 28.8 ± 5.2 | 6.0 |
| Tensile Directions | Yield Strength/MPa | UTS/MPa | r | Δr | |
|---|---|---|---|---|---|
| FH | RD | 487.7 ± 4.9 | 567.4 ± 1.1 | 0.52 | 0.035 |
| 45° | 482.5 ± 4.9 | 559.9 ± 1.0 | 0.64 | ||
| TD | 508.9 ± 1.5 | 577.0 ± 6.4 | 0.69 | ||
| SH | RD | 499.5 ± 3.7 | 554.8 ± 6.1 | 0.57 | 0.16 |
| 45° | 490.3 ± 5.2 | 546.5 ± 4.7 | 0.65 | ||
| TD | 511.9 ± 4.3 | 575.5 ± 2.3 | 1.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Yang, X.; Robson, J.D.; Sanders, R.E.; Liu, Q. Microstructural Evolution of Cold-Rolled AA7075 Sheet during Solution Treatment. Materials 2020, 13, 2734. https://doi.org/10.3390/ma13122734
Wang L, Yang X, Robson JD, Sanders RE, Liu Q. Microstructural Evolution of Cold-Rolled AA7075 Sheet during Solution Treatment. Materials. 2020; 13(12):2734. https://doi.org/10.3390/ma13122734
Chicago/Turabian StyleWang, Lu, Xiaofang Yang, Joseph D. Robson, Robert E. Sanders, and Qing Liu. 2020. "Microstructural Evolution of Cold-Rolled AA7075 Sheet during Solution Treatment" Materials 13, no. 12: 2734. https://doi.org/10.3390/ma13122734
APA StyleWang, L., Yang, X., Robson, J. D., Sanders, R. E., & Liu, Q. (2020). Microstructural Evolution of Cold-Rolled AA7075 Sheet during Solution Treatment. Materials, 13(12), 2734. https://doi.org/10.3390/ma13122734





