Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of g-C3N4
- heating rate (°C min−1);
- target temperature (°C);
- time of annealing (h);
- type of precursor: dicyandiamide (D), thiourea (T), melamine (M).
2.2. Characterization of g-C3N4
2.3. Photocatalytic Activity Measurements
3. Results and Discussion
3.1. Efficiency of Synthesis and Surface Area of g-C3N4
3.2. Morphological Characterizations (XRD and SEM)
3.3. Elemental Analysis
3.4. XPS Analysis
3.5. Structure Characterization (FTIR)
3.6. Thermogravimetric Analysis
3.7. Structure Characterization (UV–Vis Analysis)
3.8. Photocatalytic Activity
4. Conclusions
- -
- the yield of synthesis of carbon nitride decreases in the order: M ≈ D > T > U;
- -
- higher temperature decreases the synthesis efficiency regardless of the precursor used;
- -
- the size of SSA is inversely proportional to the efficiency of the synthesis and decreases in the order: M < D < T < U;
- -
- higher synthesis temperature gives carbon nitrides with higher SSA;
- -
- higher synthesis temperatures generally reduce BG.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.C.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.C.; Antonietti, M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew. Chem. Int. Ed. 2012, 51, 68–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Bai, X. A Review on quantum dots modified g-C3N4-based photocatalysts with improved photocatalytic activity. Catalysts 2020, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.P.; Zhang, Y.H.; Pan, Q.W.; Qiu, J.R. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Wen, J.Q.; Xie, J.; Chen, X.B.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Jorge, A.B.; Martin, D.J.; Dhanoa, M.T.S.; Rahman, A.S.; Makwana, N.; Tang, J.W.; Sella, A.; Cora, F.; Firth, S.; Darr, J.A.; et al. H2 and O2 evolution from water half-splitting reactions by graphitic carbon nitride materials. J. Phys. Chem. C 2013, 117, 7178–7185. [Google Scholar] [CrossRef]
- Chen, Z.P.; Vorobyeva, E.; Mitchell, S.; Fako, E.; Lopez, N.; Collins, S.M.; Leary, R.K.; Midgley, P.A.; Hauert, R.; Perez-Ramirez, J. Single-atom heterogeneous catalysts based on distinct carbon nitride scaffolds. Natl. Sci. Rev. 2018, 5, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Sun, J.; Wang, J.Q.; Yang, Z.F.; Cheng, W.G.; Zhang, S.J. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal. Sci. Technol. 2014, 4, 1556–1562. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, L.; Fu, L.; Liu, C.; Yu, B.; Yang, F.; Hu, Y. Sodium alginate-templated synthesis of g-C3N4/carbon spheres/Cu ternary nanohybrids for fire safety application. J. Colloid Interface Sci. 2019, 539, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Fu, L.B.; Chen, X.L.; Guo, J.; Yang, F.Q.; Wang, J.G.; Zheng, Y.Y.; Hu, Y. Hypophosphite/graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Zhang, G.G.; Zhang, J.S.; Zhang, M.W.; Wang, X.C. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Cao, S.W.; Yu, J.G. g-C3N4-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Goettmann, F.; Fischer, A.; Antonietti, M.; Thomas, A. Chemical synthesis of mesoporous carbon nitrides using hard templates and their use as a metal-free catalyst for friedel-crafts reaction of benzene. Angew. Chem. Int. Ed. 2006, 45, 4467–4471. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Mane, G.P.; Mano, A.; Anand, C.; Dhawale, D.S.; Mori, T.; Vinu, A. Synthesis of nitrogen-rich mesoporous carbon nitride with tunable pores, band gaps and nitrogen content from a single aminoguanidine precursor. ChemSusChem 2012, 5, 700–708. [Google Scholar] [CrossRef]
- Thomas, A.; Goettmann, F.; Antonietti, M. Hard templates for soft materials: Creating nanostructured organic materials. Chem. Mater. 2008, 20, 738–755. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, C.; Shen, Y.F.; Zhou, Z.X.; Yu, J.C.; Li, Y.; Wei, W.; Liu, S.Q.; Zhang, Y.J. Environment-friendly preparation of porous graphite-phase polymeric carbon nitride using calcium carbonate as templates, and enhanced photoelectrochemical activity. J. Mater. Chem. A 2015, 3, 5126–5131. [Google Scholar] [CrossRef]
- Chen, X.F.; Jun, Y.S.; Takanabe, K.; Maeda, K.; Domen, K.; Fu, X.Z.; Antonietti, M.; Wang, X.C. Ordered mesoporous SBA-15 type graphitic carbon nitride: A semiconductor host structure for photocatalytic hydrogen evolution with visible light. Chem. Mater. 2009, 21, 4093–4095. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Y.W.; Sun, G.; Luo, N.; Zhang, B.; Zhang, Z.Y. Synthesis of a flower-like g-C3N4/ZnO hierarchical structure with improved CH4 sensing properties. Nanomaterials 2019, 9, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.B.; Yuan, X.Z.; Pan, Y.; Liang, J.; Zeng, G.M.; Wu, Z.B.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, H.Y.; Sun, H.Q.; Liu, S.M.; Tade, M.O.; Wang, S.B.; Jin, W.Q. Recent advances in non-metal modification of graphitic carbon nitride for photocatalysis: A historic review. Catal. Sci. Technol. 2016, 6, 7002–7023. [Google Scholar] [CrossRef]
- Xu, M.Q.; Chai, B.; Yan, J.T.; Wang, H.B.; Ren, Z.D.; Paik, K.W. Facile synthesis of fluorine doped graphitic carbon nitride with enhanced visible light photocatalytic activity. Nano 2016, 11, 11. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Mori, T.; Ye, J.H.; Antonietti, M. Phosphorus-doped carbon nitride solid: Enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Q.; Liu, B.S.; Cheng, B.; Ho, W.K.; Yu, J.G. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.R.; Yao, J.; Wang, X.C.; Antonietti, M. Synthesis of boron doped polymeric carbon nitride solids and their use as metal-free catalysts for aliphatic C-H bond oxidation. Chem. Sci. 2011, 2, 446–450. [Google Scholar] [CrossRef]
- Liu, G.; Niu, P.; Sun, C.H.; Smith, S.C.; Chen, Z.G.; Lu, G.Q.; Cheng, H.M. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4. J. Am. Chem. Soc. 2010, 132, 11642–11648. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.W.; Shi, R.; Zhu, Y.F. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Maslana, K.; Kalenczuk, R.J.; Zielinska, B.; Mijowska, E. Synthesis and characterization of nitrogen-doped carbon nanotubes derived from g-C3N4. Materials 2020, 13, 1349. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhang, Z.S.; Li, C.H. A comparison of graphitic carbon nitrides synthesized from different precursors through pyrolysis. J. Photochem. Photobiol. A: Chem. 2017, 332, 32–44. [Google Scholar] [CrossRef]
- Zhang, W.D.; Zhang, Q.; Dong, F.; Zhao, Z.W. The multiple effects of precursors on the properties of polymeric carbon nitride. Int. J. Photoenergy 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.H.; Ma, Y.; Fan, J.M.; Xue, Y.Q.; Chang, H.H.; Masubuchi, Y.; Yin, S. Synthesis of graphitic carbon nitride from different precursors by fractional thermal polymerization method and their visible light induced photocatalytic activities. J. Alloys Compd. 2018, 735, 1297–1305. [Google Scholar] [CrossRef]
- Devthade, V.; Kulhari, D.; Umare, S.S. Role of precursors on photocatalytic behavior of graphitic carbon nitride. Mater. Today: Proc. 2018, 5, 9203–9210. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Chiarello, G.L.; Pedroni, M.; Livraghi, S.; Giamello, E.; Selli, E. High photocatalytic hydrogen production on Cu(II) pre-grafted Pt/TiO2. Appl. Catal. B Environ. 2017, 209, 417–428. [Google Scholar] [CrossRef]
- Koci, K.; Troppova, I.; Edelmannova, M.; Starostka, J.; Matejova, L.; Lang, J.; Reli, M.; Drobna, H.; Rokicinska, A.; Kustrowski, P.; et al. Photocatalytic decomposition of methanol over La/TiO2 materials. Environ. Sci. Pollut. Res. 2018, 25, 34818–34825. [Google Scholar] [CrossRef]
- Edelmannova, M.; Dubnova, L.; Reli, M.; Meinhardova, V.; Huo, P.W.; Stangar, U.L.; Capek, L.; Koci, K. The role of fluorine in F-La/TiO2 photocatalysts on photocatalytic decomposition of methanol-water solution. Materials 2019, 12, 2867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs. thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B Environ. 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Lau, V.W.H.; Moudrakovski, I.; Botari, T.; Weinberger, S.; Mesch, M.B.; Duppel, V.; Senker, J.; Blum, V.; Lotsch, B.V. Rational design of carbon nitride photocatalysts by identification of cyanamide defects as catalytically relevant sites. Nat. Commun. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, B.V.; Doblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking melon by a complementary approach employing electron diffraction, solid-state NMR spectroscopy, and theoretical calculations-structural characterization of a carbon nitride polymer. Chem. Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, B.V.; Schnick, W. New light on an old story: Formation of melam during thermal condensation of melamine. Chem. Eur. J. 2007, 13, 4956–4968. [Google Scholar] [CrossRef]
- Akaike, K.; Aoyama, K.; Dekubo, S.; Onishi, A.; Kanai, K. Characterizing electronic structure near the energy gap of graphitic carbon nitride based on rational interpretation of chemical analysis. Chem. Mater. 2018, 30, 2341–2352. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 nanoparticles in mesoporous silica host matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Miller, T.S.; Jorge, A.B.; Suter, T.M.; Sella, A.; Cora, F.; McMillan, P.F. Carbon nitrides: Synthesis and characterization of a new class of functional materials. Phys. Chem. Chem. Phys. 2017, 19, 15613–15638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via XRD and neutron diffraction. Chem. Mater. 2015, 27, 2612–2618. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Wu, Z.; Wang, Y.T.; Lin, C.S. Enhanced visible-light photocatalytic activity from graphene-like boron nitride anchored on graphitic carbon nitride sheets. J. Mater. Sci. 2017, 52, 9477–9490. [Google Scholar] [CrossRef]
- Wang, Y.O.; Bayazit, M.K.; Moniz, S.J.A.; Ruan, Q.S.; Lau, C.C.; Martsinovich, N.; Tang, J.W. Linker-controlled polymeric photocatalyst for highly efficient hydrogen evolution from water. Energy Environ. Sci. 2017, 10, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Han, Y.; Chen, S.Y.; Crumlin, E.J.; Yang, B.; Li, Y.M.; Liu, Z. CO2 activation on Ni(111) and Ni(100) surfaces in the presence of H2O: An ambient-pressure X-ray photoelectron spectroscopy study. J. Phys. Chem. C 2019, 123, 12176–12182. [Google Scholar] [CrossRef]
- Tao, F.; Wang, Z.H.; Qiao, M.H.; Liu, Q.; Sim, W.S.; Xu, G.Q. Covalent attachment of acetonitrile on Si(100) through Si-C and Si-N linkages. J. Chem. Phys. 2001, 115, 8563–8569. [Google Scholar] [CrossRef] [Green Version]
- Brant, P.; Enemark, J.H.; Balch, A.L. X-ray photoelectron-spectra of palladium and platinum complexes of carbenoid and related ligands. J. Organomet. Chem. 1976, 114, 99–106. [Google Scholar] [CrossRef]
- Lalitha, S.; Manoharan, P.T. X-ray photoelectron spectroscopic studies on some dithiolate complexes. J. Electron Spectrosc. Relat. Phenom. 1989, 49, 61–75. [Google Scholar] [CrossRef]
- Wu, C.R.; Salaneck, W.R.; Ritsko, J.J.; Bredas, J.L. X-ray photoelectron-spectroscopy of polyacrylonitrile. Synth. Met. 1986, 16, 147–159. [Google Scholar] [CrossRef]
- Wu, G.P.; Lu, C.X.; Wu, X.P.; Zhang, S.C.; Fu, H.; Ling, L.C. X-ray photoelectron spectroscopy investigation into thermal degradation and stabilization of polyacrylonitrile fibers. J. Appl. Polym. Sci. 2004, 94, 1705–1709. [Google Scholar] [CrossRef]
- Dante, R.C.; Martin-Ramos, P.; Correa-Guimaraes, A.; Martin-Gil, J. Synthesis of graphitic carbon nitride by reaction of melamine and uric acid. Mater. Chem. Phys. 2011, 130, 1094–1102. [Google Scholar] [CrossRef]
- Lau, V.W.H.; Mesch, M.B.; Duppel, V.; Blum, V.; Senker, J.; Lotsch, B.V. Low-molecular-weight carbon nitrides for solar hydrogen evolution. J. Am. Chem. Soc. 2015, 137, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Lotsch, B.V.; Schnick, W. From triazines to heptazines: Novel nonmetal tricyanomelaminates as precursors for graphitic carbon nitride materials. Chem. Mater. 2006, 18, 1891–1900. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.R.; Wang, J.J.; Gillan, E.G. Rapid, facile synthesis of nitrogen-rich carbon nitride powders. J. Mater. Chem. 2002, 12, 2463–2469. [Google Scholar] [CrossRef]
- Wei, X.Q.; Qiu, Y.; Duan, W.Y.; Liu, Z.X. Cathodic and anodic photocurrents generation from melem and its derivatives. RSC Adv. 2015, 5, 26675–26679. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.S.; Qian, M.; Zhou, S.Y.; Xue, A.L.; Zhang, L.L.; Zhao, Y.J.; Xing, W.H. Simple synthesis of high specific surface carbon nitride for adsorption-enhanced photocatalytic performance. Nanoscale Res. Lett. 2018, 13, 7. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Shi, Y.Q.; Huang, Z.Q.; Duan, L.J.; Tai, Q.L.; Hu, Y. Novel graphite-like carbon nitride/organic aluminum diethylhypophosphites nanohybrid: Preparation and enhancement on thermal stability and flame retardancy of polystyrene. Compos. Part A Appl. Sci. Manuf. 2017, 99, 149–156. [Google Scholar] [CrossRef]
- Sano, T.; Sato, H.; Hori, T.; Hirakawa, T.; Teramoto, Y.; Koike, K. Effects of polymeric- and electronic-structure of graphitic carbon nitride (g-C3N4) on oxidative photocatalysis. Mol. Catal. 2019, 474, 8. [Google Scholar] [CrossRef]
- Zuluaga, S.; Liu, L.H.; Shafiq, N.; Rupich, S.M.; Veyan, J.F.; Chabal, Y.J.; Thonhauser, T. Structural band-gap tuning in g-C3N4. Phys. Chem. Chem. Phys. 2015, 17, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Tyborski, T.; Merschjann, C.; Orthmann, S.; Yang, F.; Lux-Steiner, M.C.; Schedel-Niedrig, T. Tunable optical transition in polymeric carbon nitrides synthesized via bulk thermal condensation. J. Phys. Condens. Matter 2012, 24, 4. [Google Scholar] [CrossRef]
- Koci, K.; Reli, M.; Edelmannova, M.; Troppova, I.; Drobna, H.; Rokicinska, A.; Kustrowski, P.; Dvoranova, D.; Capek, L. Photocatalytic hydrogen production from methanol over Nd/TiO2. J. Photochem. Photobiol. A Chem. 2018, 366, 55–64. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yin, S.; Dong, Q.; Guo, C.S.; Kimura, T.; Matsushita, J.; Sato, T. Photocatalytic properties of Nd and C codoped TiO2 with the whole range of visible light absorption. J. Phys. Chem. C 2013, 117, 8345–8352. [Google Scholar] [CrossRef]
- Azam, M.U.; Tahir, M.; Umer, M.; Jaffar, M.M.; Nawawi, M.G.M. Engineering approach to enhance photocatalytic water splitting for dynamic H2 production using La2O3/TiO2 nanocatalyst in a monolith photoreactor. Appl. Surf. Sci. 2019, 484, 1089–1101. [Google Scholar] [CrossRef]
- Cao, J.W.; Zhang, J.Y.; Dong, X.A.; Fu, H.L.; Zhang, X.M.; Lv, X.S.; Li, Y.H.; Jiang, G.M. Defective borate-decorated polymer carbon nitride: Enhanced photocatalytic NO removal, synergy effect and reaction pathway. Appl. Catal. B Environ. 2019, 249, 266–274. [Google Scholar] [CrossRef]
- Yu, Q.B.; Xu, Q.X.; Li, H.Q.; Yang, K.; Li, X.H. Effects of heat treatment on the structure and photocatalytic activity of polymer carbon nitride. J. Mater. Sci. 2019, 54, 14599–14608. [Google Scholar] [CrossRef]
- Martin, D.J.; Qiu, K.P.; Shevlin, S.A.; Handoko, A.D.; Chen, X.W.; Guo, Z.X.; Tang, J.W. Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride. Angew. Chem. Int. Ed. 2014, 53, 9240–9245. [Google Scholar] [CrossRef] [Green Version]
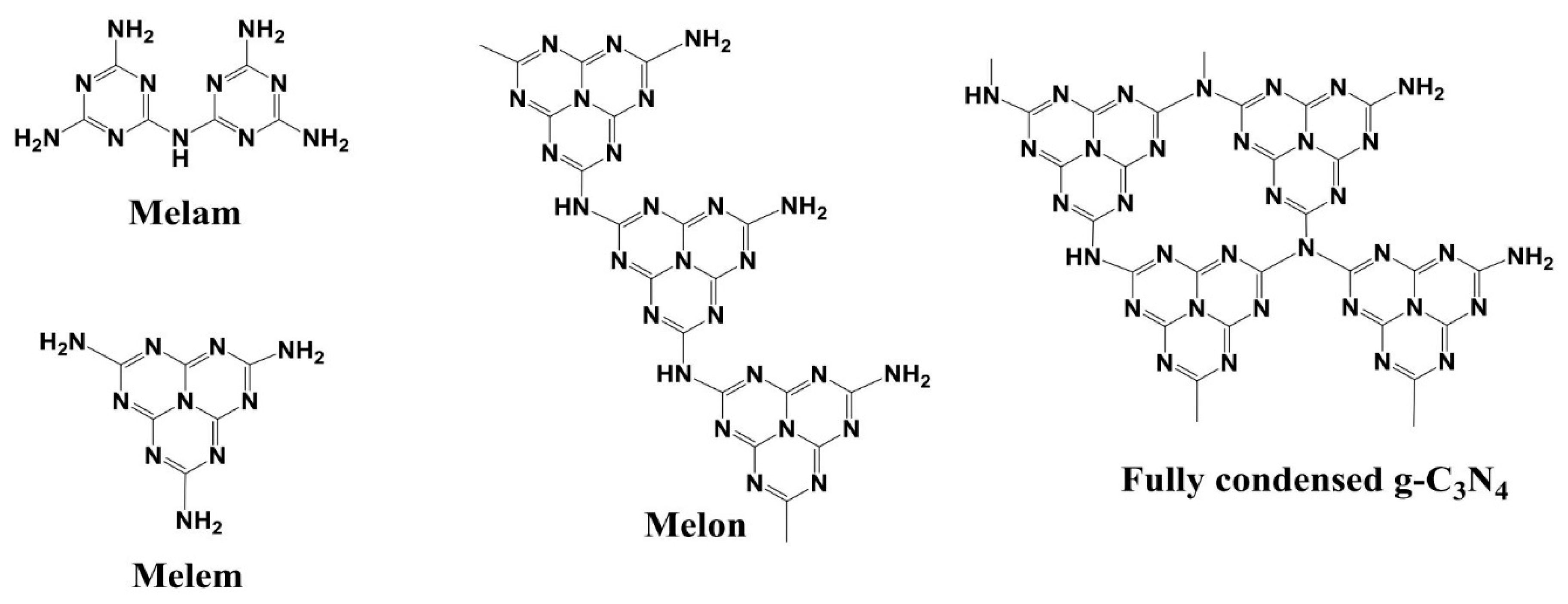


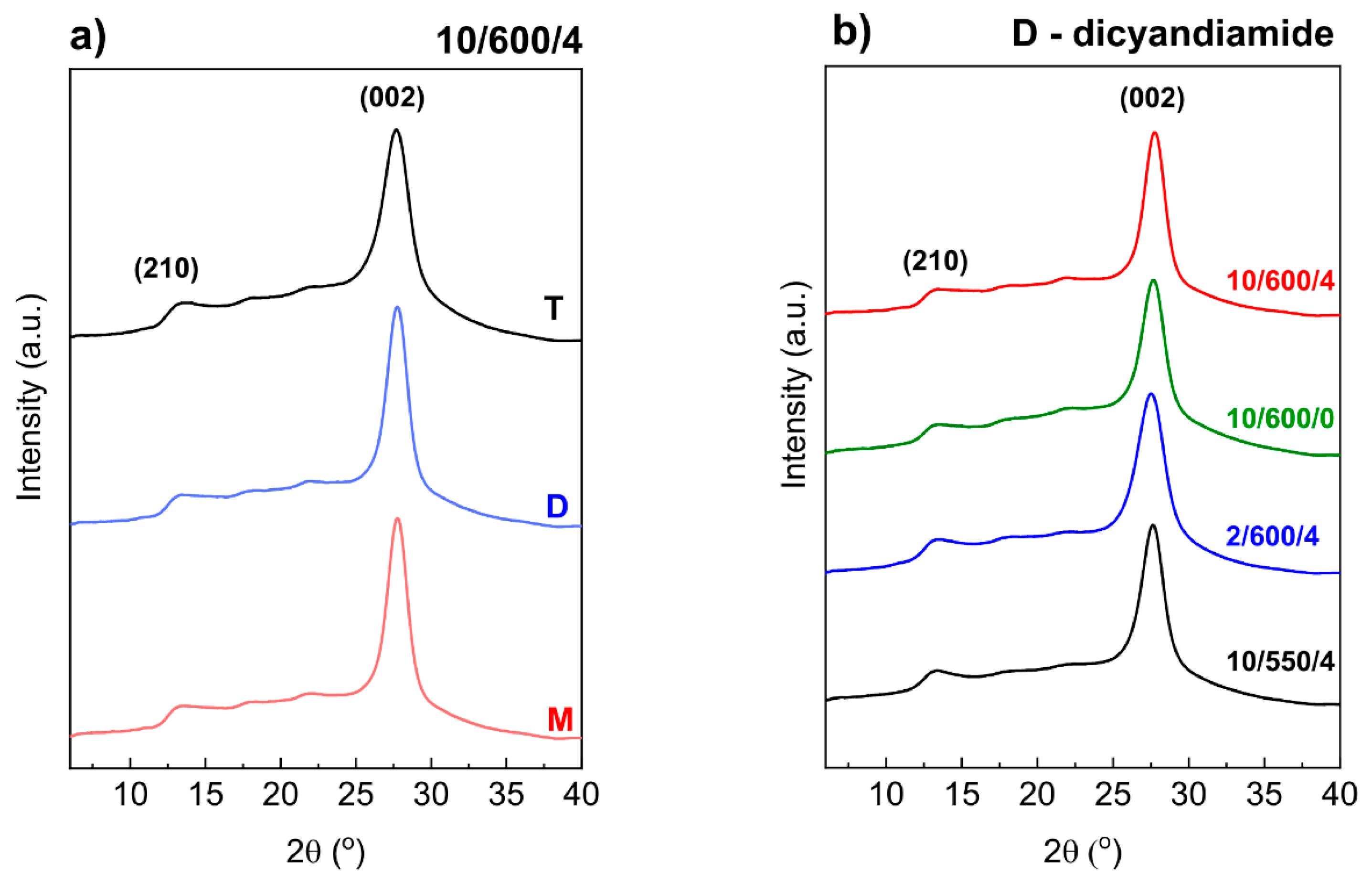
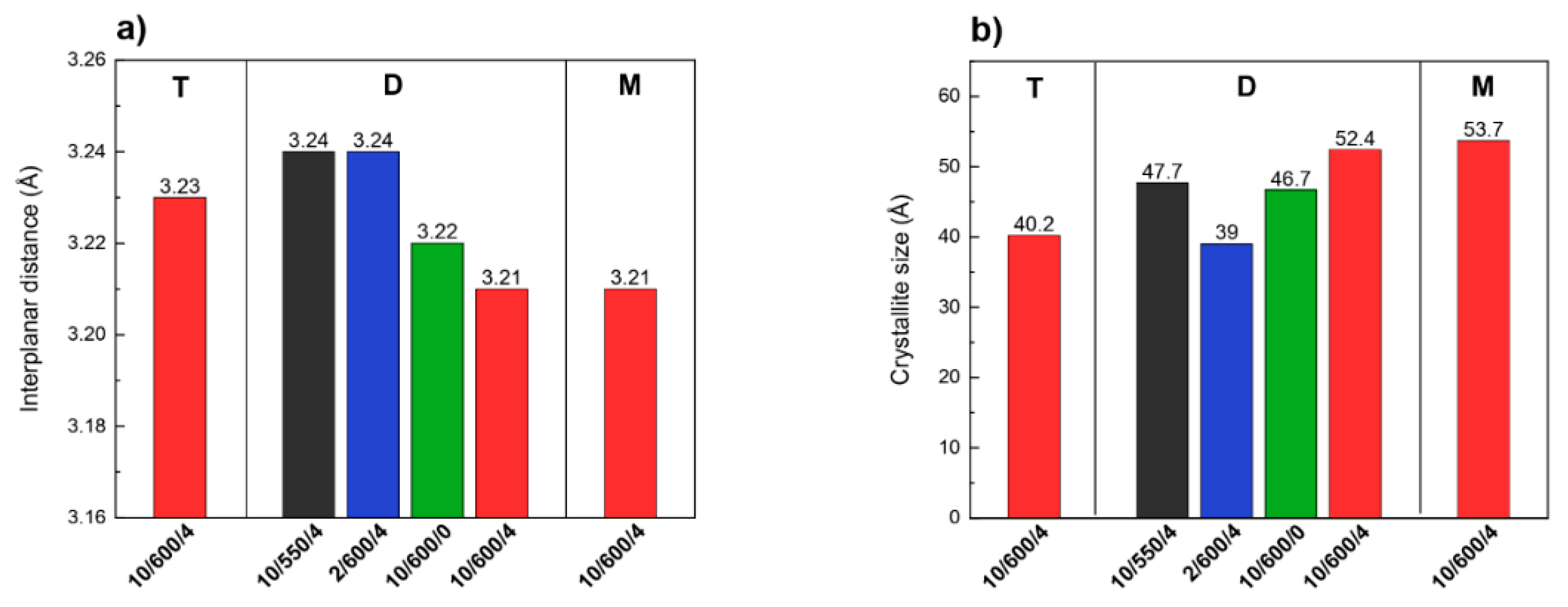

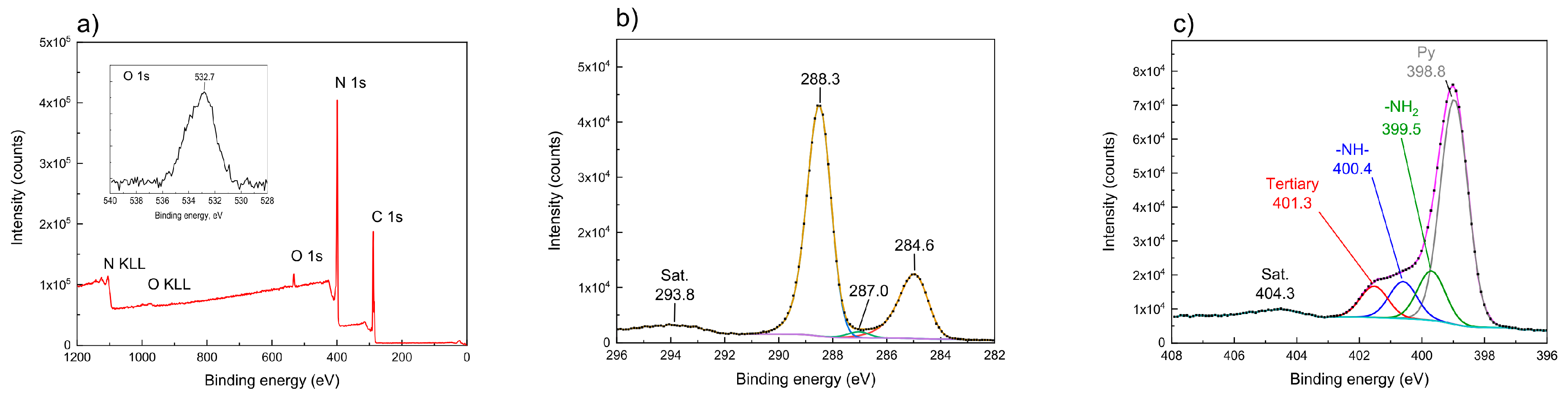

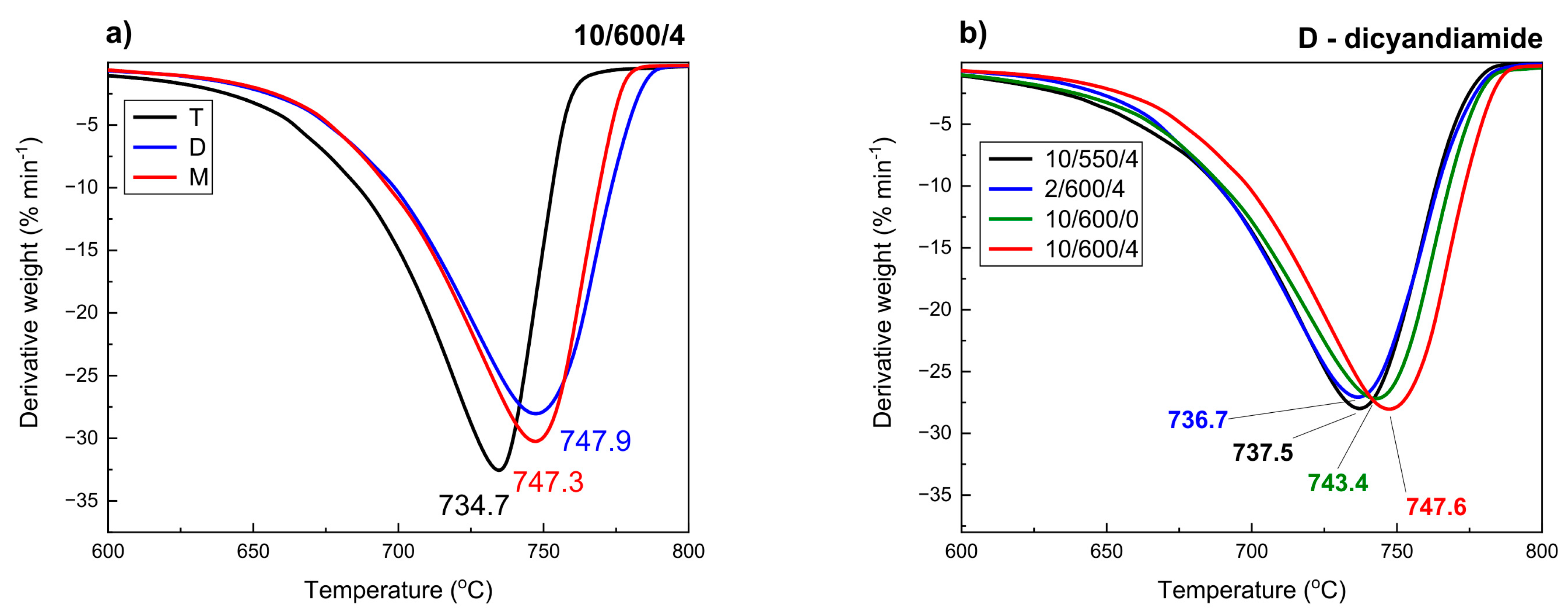

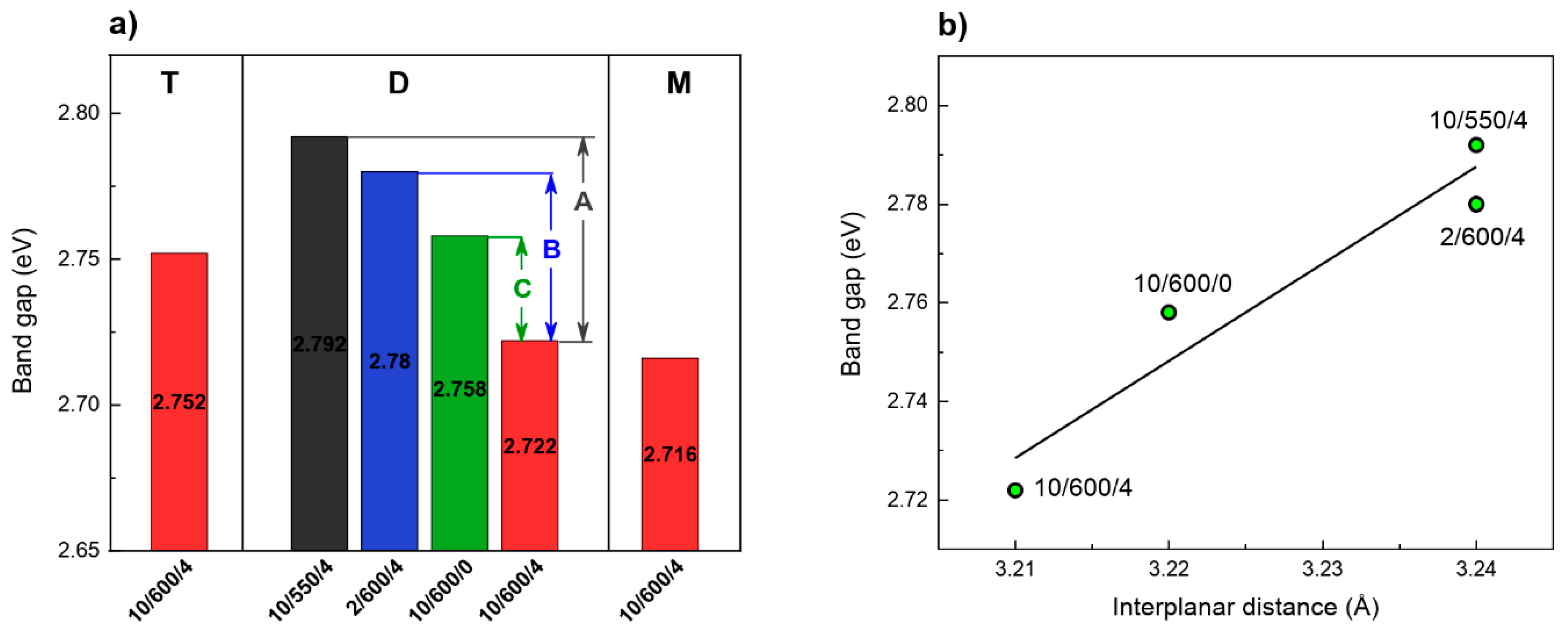
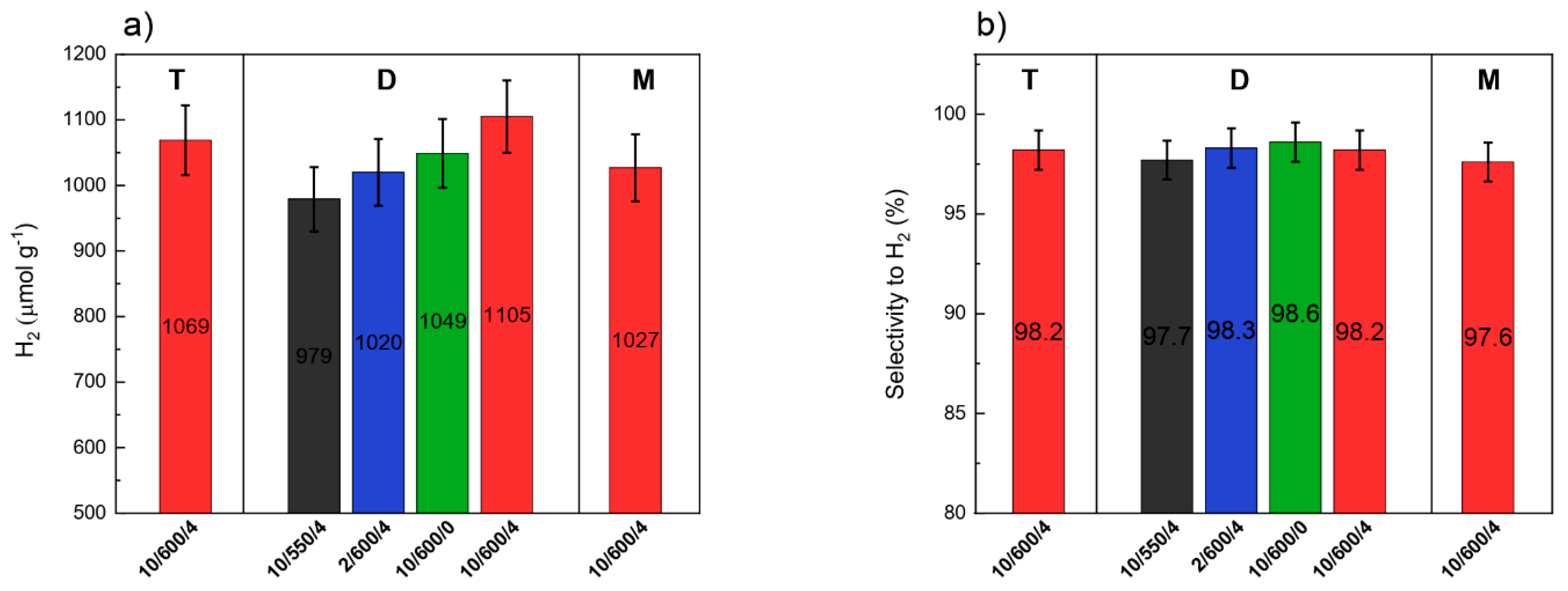
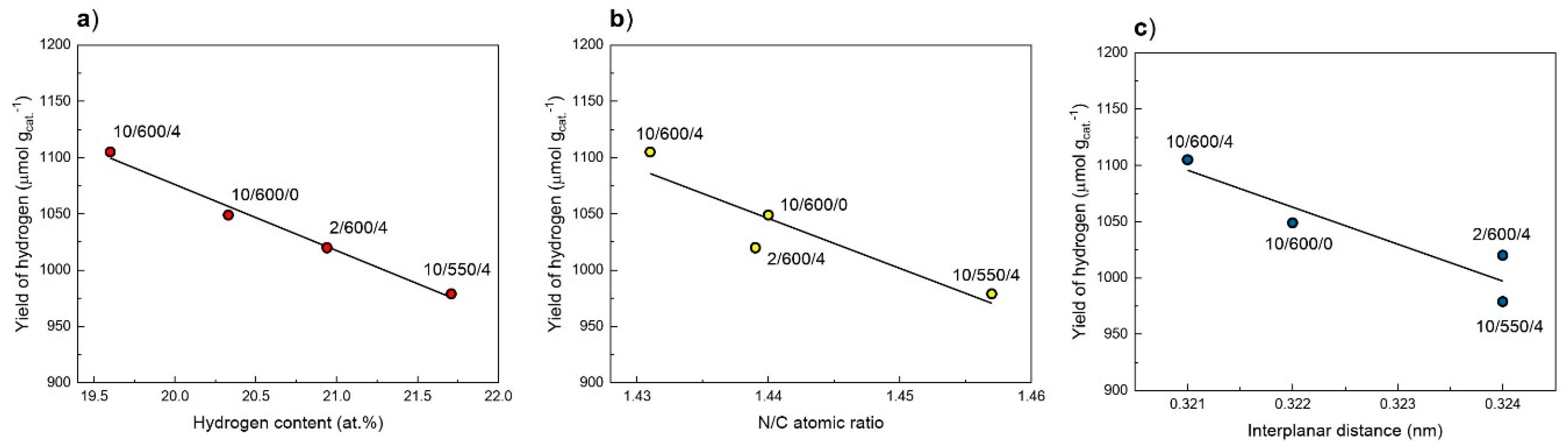
| Preparation Conditions 1 | Surface Area (m2 g−1) | Band Gap (eV) | Reference | ||
|---|---|---|---|---|---|
| Melamine | Urea | Melamine | Urea | ||
| 2/550/2 | 15.4 | 48.1 | - | - | [31] |
| 10/550/2 | 11.2 | 55.1 | 2.58 | 2.69 | [34] |
| 10/550/3 | 14.0 | 153.0 | 2.56 | 2.88 | [32] |
| Symbol of Synthesis Conditions | Precursor 1 | Heating Rate (°C min−1) | Temperature (°C) | Time of Annealing (h) |
|---|---|---|---|---|
| 10/550/4 | D | 10 | 550 | 4 |
| 2/600/4 | D | 2 | 600 | 4 |
| 10/600/0 | D | 10 | 600 | 0 |
| 10/600/4 | D | 10 | 600 | 4 |
| 10/600/4 | T | 10 | 600 | 4 |
| 10/600/4 | M | 10 | 600 | 4 |
| Synthesis Conditions | Precursor | Elemental Analysis | XPS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Atomic Composition | C/N | N/C | C/H | Theoretic Formula | C/N | Theoretic Formula | ||||
| N (at.%) | C (at.%) | H (at.%) | ||||||||
| 10/550/4 | D | 46.42 | 31.86 | 21.71 | 0.686 | 1.457 | 1.467 | C3N4.4H2.0 | 0.688 | C3N4.4 |
| 2/600/4 | D | 46.65 | 32.42 | 20.94 | 0.695 | 1.439 | 1.548 | C3N4.3H1.9 | 0.708 | C3N4.2 |
| 10/600/0 | D | 47.01 | 32.65 | 20.33 | 0.695 | 1.440 | 1.606 | C3N4.3H1.9 | 0.712 | C3N4.2 |
| 10/600/4 | D | 47.32 | 33.08 | 19.60 | 0.699 | 1.431 | 1.688 | C3N4.3H1.8 | 0.702 | C3N4.3 |
| T | 46.54 | 32.33 | 21.13 | 0.695 | 1.440 | 1.530 | C3N4.3H2.0 | 0.674 | C3N4.4 | |
| M | 47.46 | 33.05 | 19.48 | 0.696 | 1.436 | 1.697 | C3N4.3H1.8 | 0.687 | C3N4.4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwin, E.; Kočí, K.; Wojcieszak, R.; Zieliński, M.; Edelmannová, M.; Pietrowski, M. Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability. Materials 2020, 13, 2756. https://doi.org/10.3390/ma13122756
Alwin E, Kočí K, Wojcieszak R, Zieliński M, Edelmannová M, Pietrowski M. Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability. Materials. 2020; 13(12):2756. https://doi.org/10.3390/ma13122756
Chicago/Turabian StyleAlwin, Emilia, Kamila Kočí, Robert Wojcieszak, Michał Zieliński, Miroslava Edelmannová, and Mariusz Pietrowski. 2020. "Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability" Materials 13, no. 12: 2756. https://doi.org/10.3390/ma13122756
APA StyleAlwin, E., Kočí, K., Wojcieszak, R., Zieliński, M., Edelmannová, M., & Pietrowski, M. (2020). Influence of High Temperature Synthesis on the Structure of Graphitic Carbon Nitride and Its Hydrogen Generation Ability. Materials, 13(12), 2756. https://doi.org/10.3390/ma13122756







