Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-Sensitized Solar Cells: A Review
Abstract
:1. Introduction
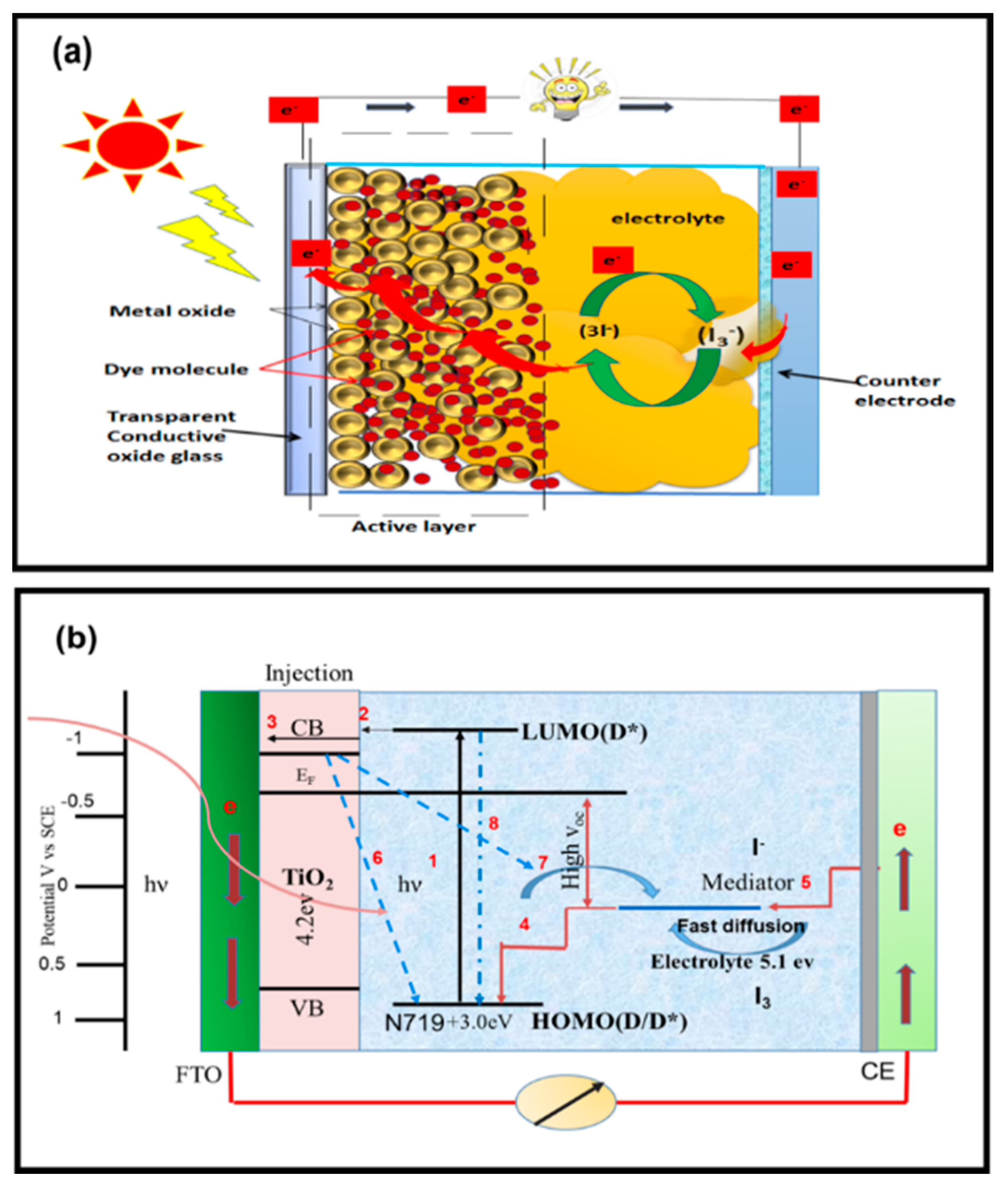
Operating Principle of DSSCs
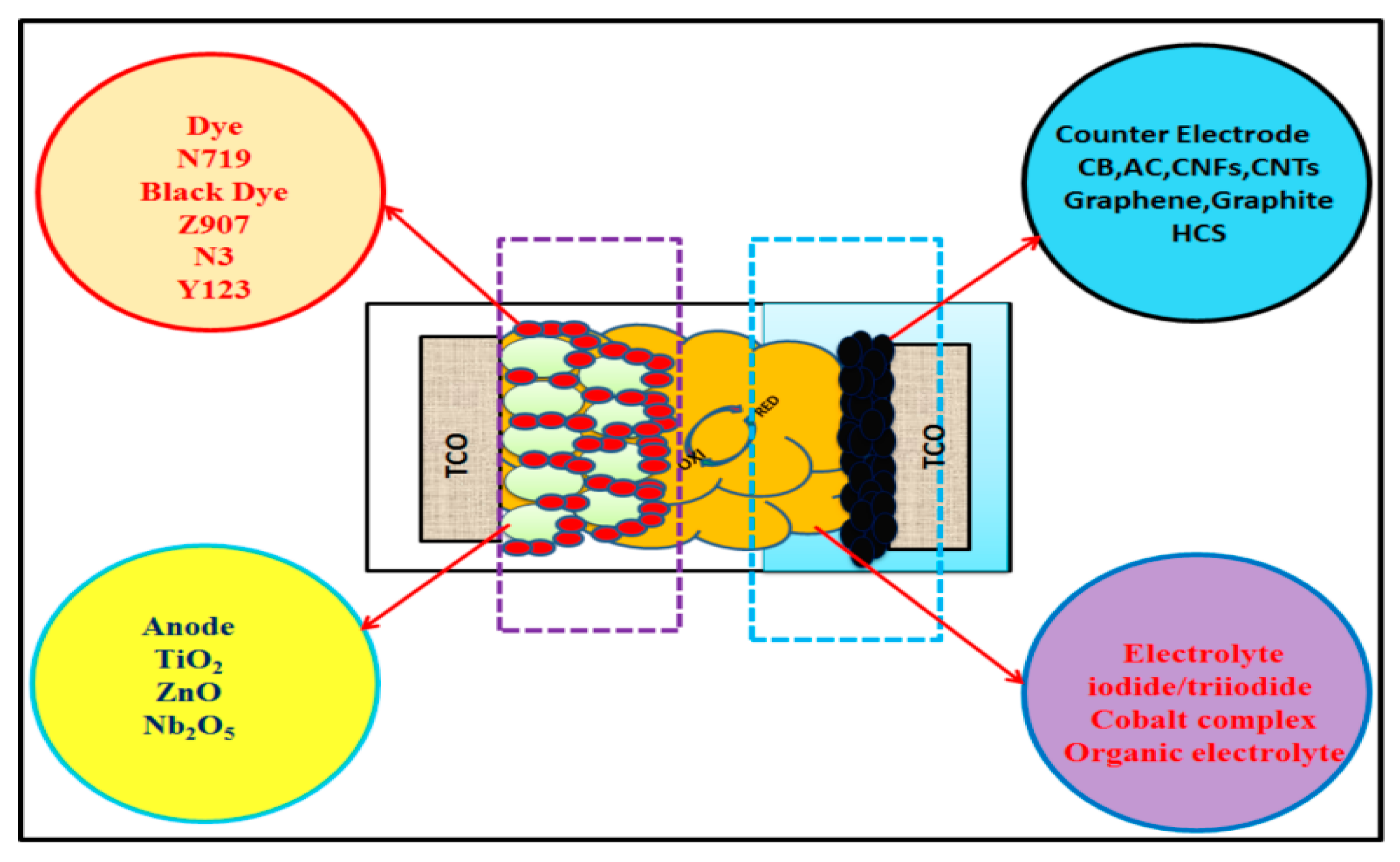
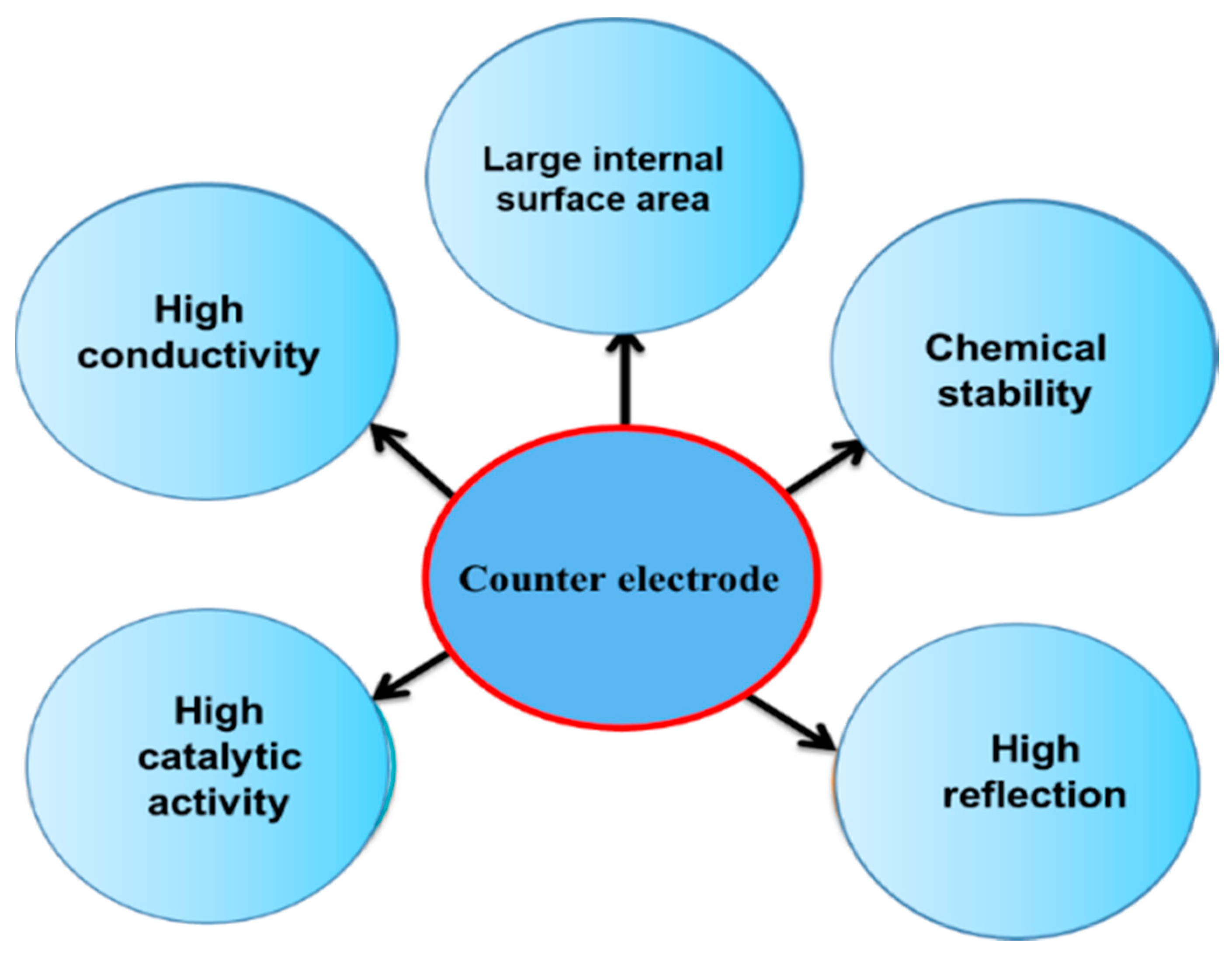
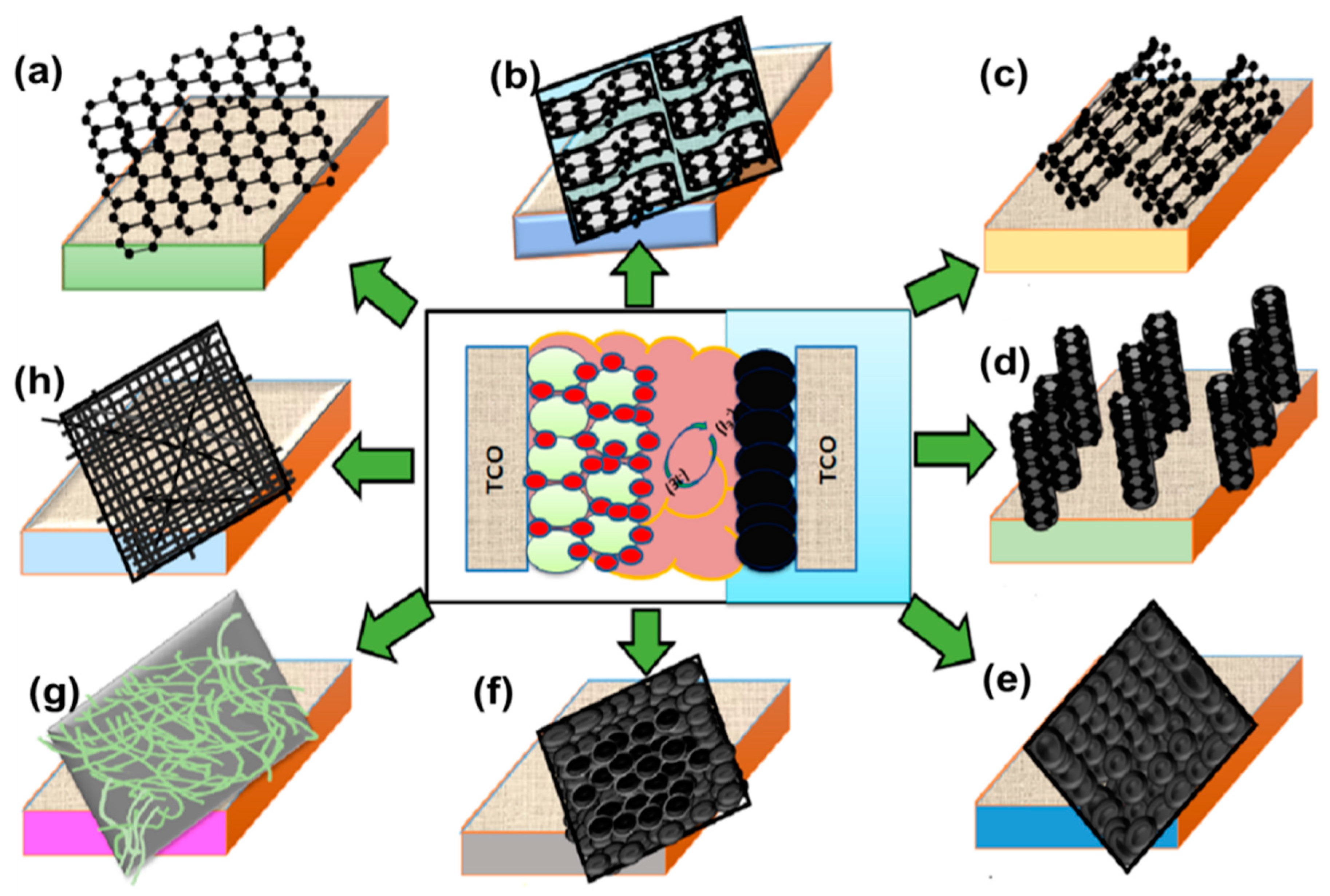
2. Counter Electrodes
3. Carbon-Based Counter Electrodes
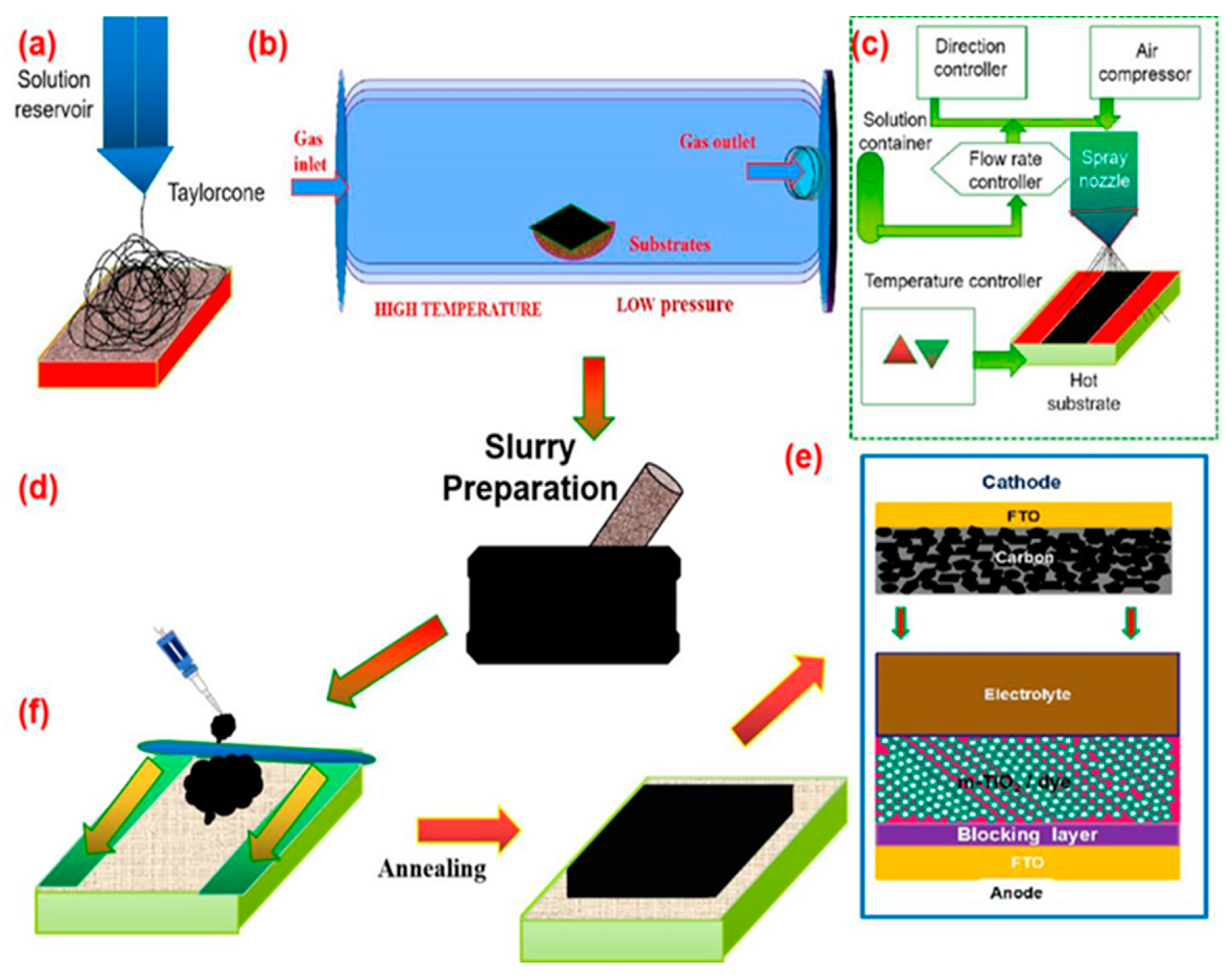
Methods of Carbon-Based Counter Electrodes
4. Carbon Black Nanoparticles and Hybridization
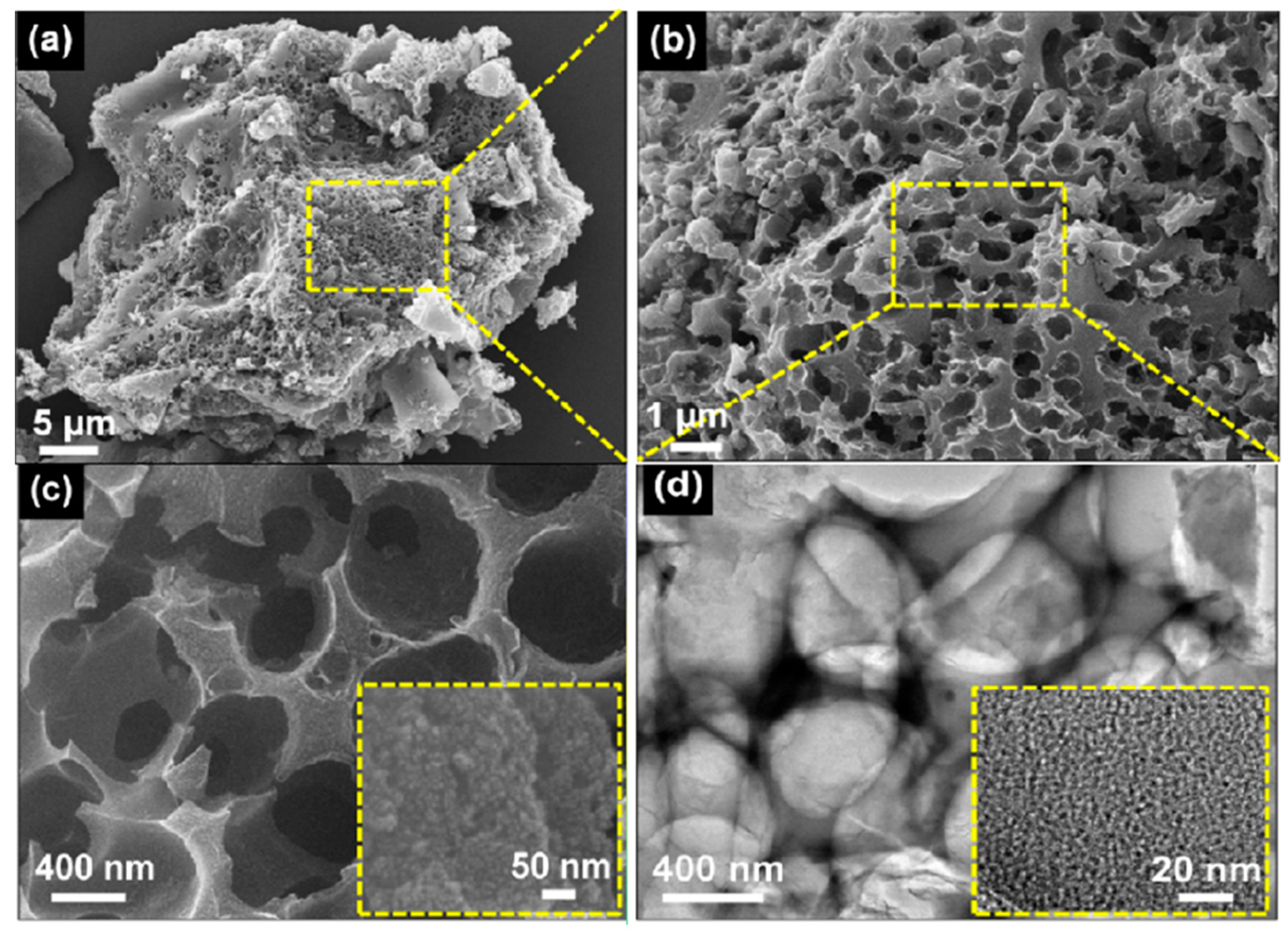
5. Activated Carbon and Hybridized Activated Carbon
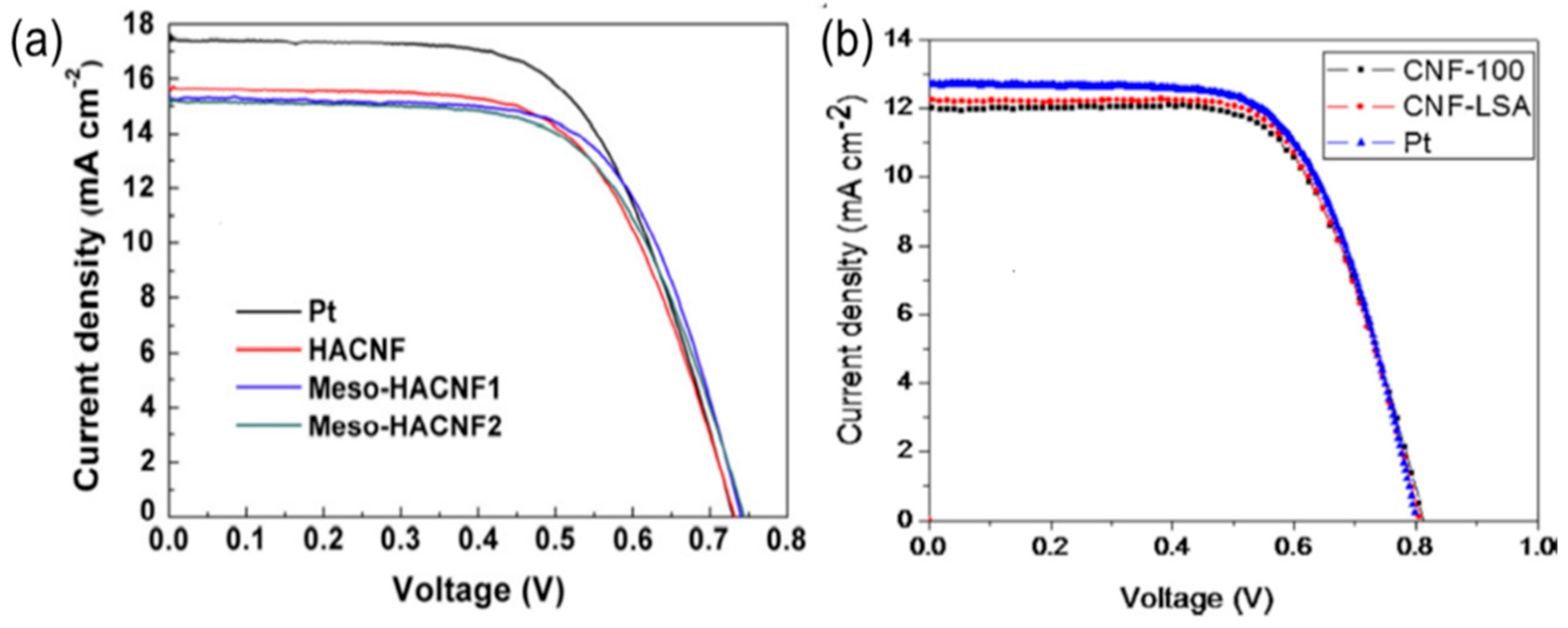
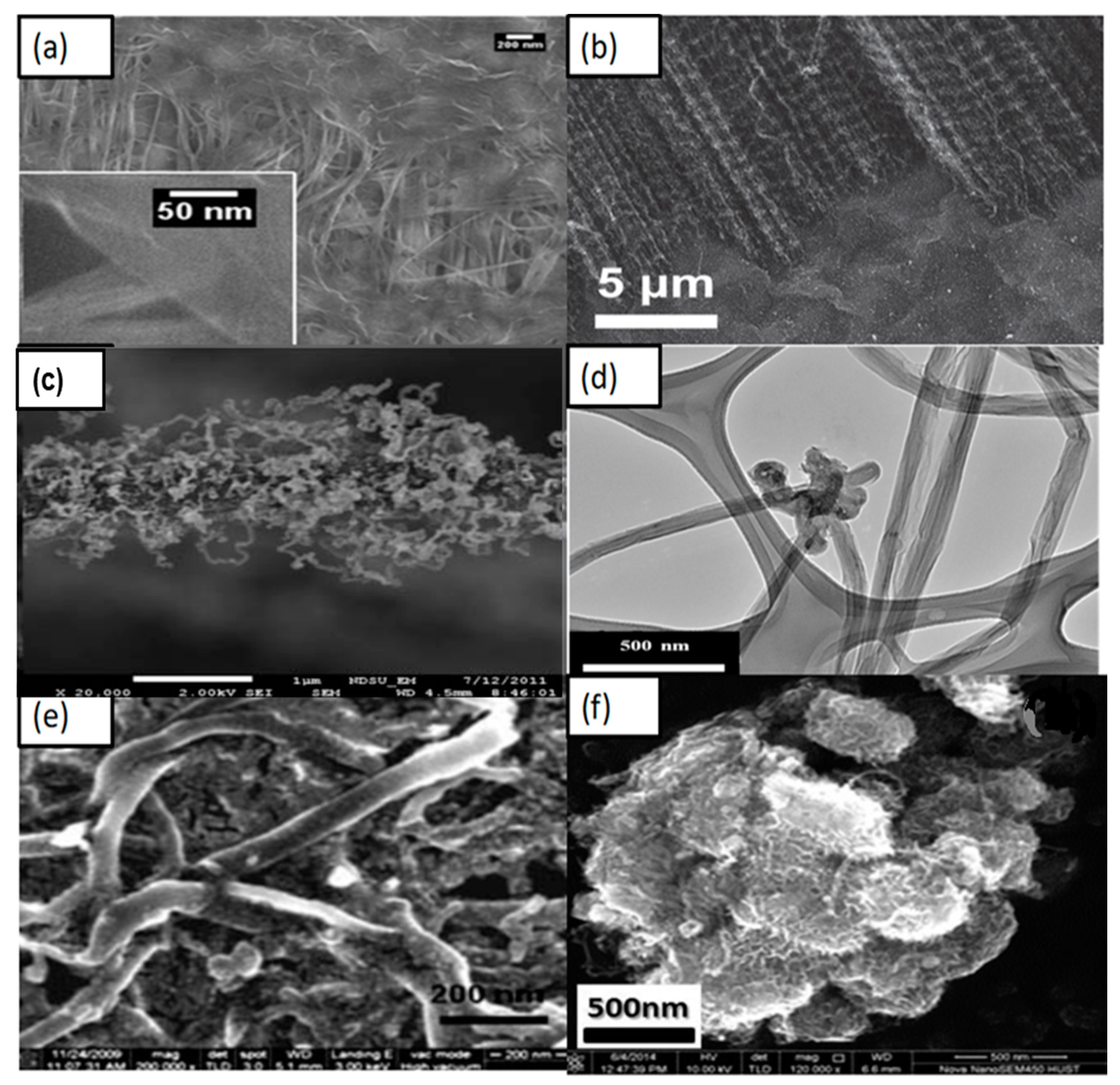
6. Carbon Nanofibers and Hybridization as CEs for DSSC
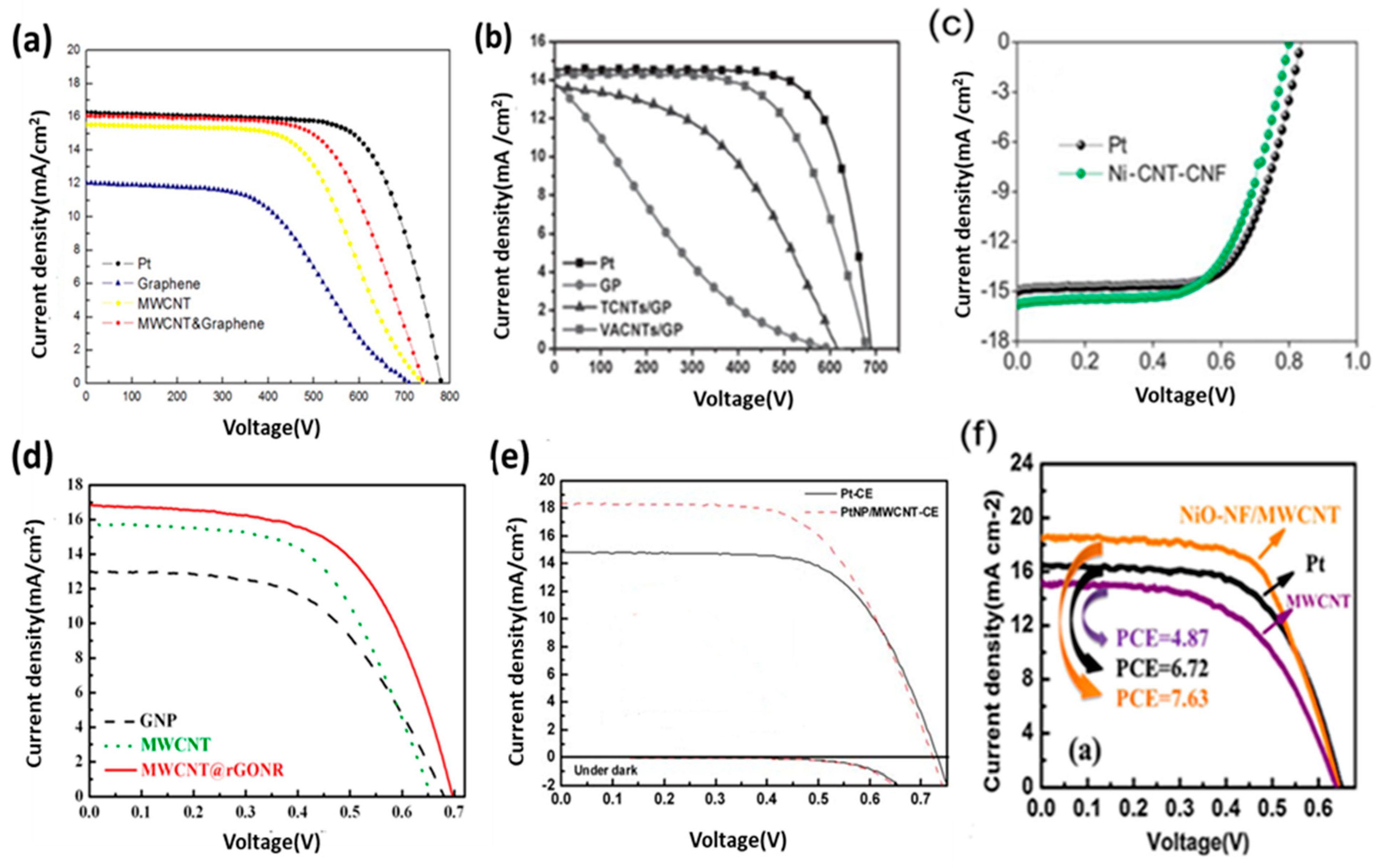
7. Carbon Nanotubes and Their Hybridizations as CEs for DSSC
8. Two-Dimensional Graphite/Graphene and Hybrid-Graphene Electrodes as CEs for DSSC
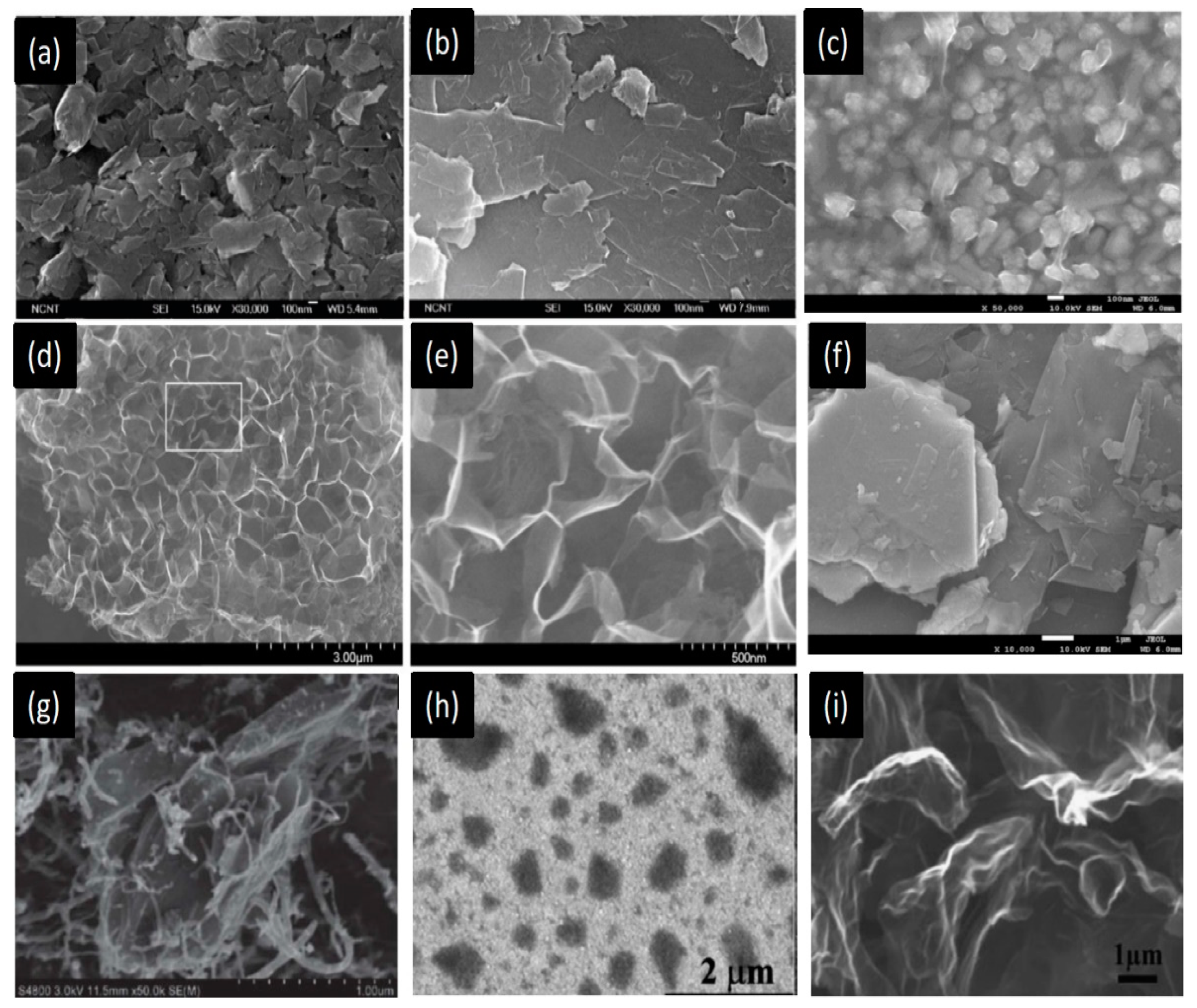
9. Summary
10. Challenges and Future Direction in the Hybrid Carbon Nanostructured CEs for DSSCs
- (1)
- The carbon CE and hybrid-based CE are comprehensively reviewed for DSSC application according to the recent study.
- (2)
- The desired properties of a CE are briefly explained with emphasis on the importance of charge transfer resistance.
- (3)
- Photovoltaic performance of various low-cost carbon-based counter electrodes and their composite as CEs for DSSC are tabulated.
- (4)
- The various synthesis and fabrication techniques for a high-performance CE are also discussed.
Author Contributions
Funding
Conflicts of Interest
References
- Faninger, G. The Potential of Solar Heat in the Future Energy System. Available online: https://www.iea-shc.org/Data/Sites/1/publications/Potential_of_Solar_Thermal_Technologies_2010.pdf (accessed on 15 June 2020).
- Green, M.A.; Dunlop, E.D.; Levi, D.H.; Hohl-Ebinger, J.; Yoshita, M.; Ho-Baillie, A.W. Solar cell efficiency tables (Version 55). Prog. Photovolt. Res. Appl. 2019, 28, 3–15. [Google Scholar] [CrossRef]
- Bullock, J.; Hettick, M.; Geissbühler, J.; Ong, A.J.; Allen, T.; Sutter-Fella, C.M.; Chen, T.; Ota, H.; Schaler, E.W.; De Wolf, S.; et al. Efficient silicon solar cells with dopant-free “asymmetric heterocontacts. Nat. Energy 2016, 1, 15031. [Google Scholar] [CrossRef]
- Green, M.A. Thin-film solar cells: Review of materials, technologies and commercial status. J. Mater. Sci. 2007, 18, 15–19. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygili, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lighting. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Gao, P.; Scopelliti, R.; Mosconi, E.; Graetzel, M.; Nazeeruddin, M.K. Material for e cient perovskite solar cells. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Ramli, A.M.; Razali, M.Z.; Ludin, N.A. Performance Enhancement of Dye Sensitized Solar Cell Using Graphene Oxide Doped Titanium Dioxide Photoelectrode. Malays. J. Anal. Sci. 2017, 21, 928–940. [Google Scholar] [CrossRef]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Ye, M.; Wen, X.; Wang, M.; Iocozzia, J.; Zhang, N.; Lin, C.; Lin, Z. Recent advances in dye-sensitized solar cells: From photoanodes, sensitizers and electrolytes to counter electrodes. Mater. Today 2015, 18, 155–162. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Ludin, N.A.; Ghaffari, B.V.; Sopian, K.; Karim, N.A.; Miyake, M. An Efficient Metal-Free Hydrophilic Carbon as a Counter Electrode for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2016, 2016, 5186762. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Huang, Y.; Fan, L.; Luo, G. Electrolytes in Dye-Sensitized Solar Cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Mahalingam, S.; Abdullah, H.; Shaari, S.; Muchtar, A.; Asshari, I. Structural, Morphological, and Electron Transport Studies of Annealing Dependent In2O3 Dye-Sensitized Solar Cell. Sci. World J. 2015, 2015, 403848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauch, A.; Georg, A. Diffusion in the electrolyte and charge-transfer reaction at the platinum electrode in dye-sensitized solar cells. Electrochim. Acta 2001, 46, 3457–3466. [Google Scholar] [CrossRef]
- Elata, H.; Rutenium, N.; Sel, P. Co-sensitization of natural sensitizers extracted from rengas (Gluta spp.) and mengkulang (Heritiera elata) wood with ruthenium dye (N719) to enhance the performance of dye-sensitized solar cells. Malays. J. Anal. Sci. 2018, 22, 95–106. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Che Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Grätzel, C.; Zakeeruddin, S.M.; Grätzel, M. Recent developments in redox electrolytes for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 9394–9405. [Google Scholar] [CrossRef]
- Gra, M. Solar Energy Conversion by Dye-Sensitized Photovoltaic Cells. Inor. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Molecular Photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Ardo, S.; Meyer, G.J. Photodriven heterogeneous charge transfer with transition-metal compounds anchored to TiO2 semiconductor surfaces. Chem. Soc. Rev. 2009, 38, 115–164. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, Y.; Tang, Q.; Yue, G.; Lin, J.; Huang, M.; Meng, L. Bifacial dye-sensitized solar cells: A strategy to enhance overall efficiency based on transparent polyaniline electrode. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papageorgiou, N.; Maier, W.F.; Grätzel, M. An Iodine/Triiodide Reduction Electrocatalyst for Aqueous and Organic Media. J. Electrochem. Soc. 1997, 144, 876. [Google Scholar] [CrossRef]
- Kouhnavard, M.; Ludin, N.A.; Ghaffari, B.V.; Sopian, K.; Ikeda, S. Carbonaceous Materials and Their Advances as a Counter Electrode in Dye-Sensitized Solar Cells: Challenges and Prospects. ChemSusChem 2015, 8, 1510–1533. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Is it time to shoot for the sun? Science 2005, 309, 548–551. [Google Scholar] [CrossRef]
- Dsscs, Z.; Giannouli, M.; Govatsi, K.; Syrrokostas, G.; Leftheriotis, G. Factors Affecting the Power Conversion Efficiency in ZnO DSSCs: Nanowire vs. nanoparticles. Materials 2018, 11, 411. [Google Scholar] [CrossRef] [Green Version]
- Pysch, D.; Mette, A.; Glunz, S.W. A review and comparison of different methods to determine the series resistance of solar cells. Solar Energy Mater. Solar Cells 2007, 91, 1698–1706. [Google Scholar] [CrossRef]
- Yin, X.; Xue, Z.; Liu, B. Electrophoretic deposition of Pt nanoparticles on plastic substrates as counter electrode for flexible dye-sensitized solar cells. J. Power Sour. 2011, 196, 2422–2426. [Google Scholar] [CrossRef]
- Trancik, J.E.; Barton, S.C.; Hone, J. Transparent and Catalytic CNT films.pdf. Nano Lett. 2008, 1, 19–24. [Google Scholar] [CrossRef]
- Reid, L.F.; Dooley, K.J. Expandable crack inhibitors and methods of using the same. U.S. Patent 20120304577, 6 December 2012. [Google Scholar]
- Longo, C.; De Paoli, M.A. Dye-Sensitized Solar Cells: A Successful Combination of Materials. J. Braz. Chem. Soc. 2003, 14, 889–901. [Google Scholar] [CrossRef]
- Efendi, R.; Syifa, J.N.A. Status Kesehatan Pasar Ditinjau Dari Aspek Lokasi Dan Bangunan Pada Pasar Ciputat Dan Pasar Modern BSD Kota Tangerang Selatan. Jurnal Kesehatan Indonesia. 2019, 12, 1–29. [Google Scholar] [CrossRef]
- Wu, M.; Ma, T. Low-cost pt-free counter electrode catalysts in dye-sensitized solar cells. Green Energy Technol 2014, 77–87. [Google Scholar] [CrossRef]
- Tang, Q.; Duan, J.; Duan, Y.; He, B.; Yu, L. Recent advances in alloy counter electrodes for dye-sensitized solar cells. A critical review. Electrochim. Acta 2015, 178, 886–899. [Google Scholar] [CrossRef]
- Olsen, E.; Hagen, G.; Lindquist, S.E. Dissolution of platinum in methoxy propionitrile containing LiI/I2. Sol. Energy Mater. Sol. Cells 2000, 63, 267–273. [Google Scholar] [CrossRef]
- Murakami, T.N.; Grätzel, M. Counter electrodes for DSC: Application of functional materials as catalysts. Inorg. Chim. Acta 2008, 361, 572–580. [Google Scholar] [CrossRef]
- Wang, M.; Chamberland, N.; Breau, L.; Moser, J.E.; Humphry-Baker, R.; Marsan, B.; Zakeeruddin, S.M.; Grätzel, M. An organic redox electrolyte to rival triiodide/iodide in dye-sensitized solar cells. Nat. Chem. 2010, 2, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yella, A.; Mai, C.L.; Zakeeruddin, S.M.; Chang, S.N.; Hsieh, C.H.; Yeh, C.Y.; Grätzel, M. Molecular engineering of push-pull porphyrin dyes for highly efficient dye-sensitized solar cells: The role of benzene spacers. Angew. Chem. Int. Ed. 2014, 53, 2973–2977. [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef]
- Huang, K.C.; Wang, Y.C.; Dong, R.X.; Tsai, W.C.; Tsai, K.W.; Wang, C.C.; Chen, Y.H.; Vittal, R.; Lin, J.J.; Ho, K.C. A high performance dye-sensitized solar cell with a novel nanocomposite film of PtNP/MWCNT on the counter electrode. J. Mater. Chem. 2010, 20, 4067–4073. [Google Scholar] [CrossRef] [Green Version]
- Poudel, P.; Zhang, L.; Joshi, P.; Venkatesan, S.; Fong, H.; Qiao, Q. Enhanced performance in dye-sensitized solar cells via carbon nanofibers-platinum composite counter electrodes. Nanoscale 2012, 4, 4726–4730. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sudhagar, P.; Verma, V.; Song, D.; Ito, E.; Lee, S.Y.; Kang, Y.S.; Choi, W. Amplifying charge-transfer characteristics of graphene for triiodide reduction in dye-sensitized solar cells. Adv. Funct. Mater. 2011, 21, 3729–3736. [Google Scholar] [CrossRef]
- Thomas, S.; Deepak, T.G.; Anjusree, G.S.; Arun, T.A.; Nair, S.V.; Nair, A.S. A review on counter electrode materials in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 4474–4490. [Google Scholar] [CrossRef]
- Yun, S.; Hagfeldt, A.; Ma, T. Pt-free counter electrode for dye-sensitized solar cells with high efficiency. Adv. Mater. 2014, 26, 6210–6237. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.D.; Lodermeyer, F.; Casillas, R.; Guldi, D.M. Recent advances in multifunctional nanocarbons used in dye-sensitized solar cells. Energy Environ. Sci. 2014, 7, 1281–1296. [Google Scholar] [CrossRef] [Green Version]
- Roy-Mayhew, J.D.; Aksay, I.A. Graphene materials and their use in dye-sensitized solar cells. Chem. Rev. 2014, 114, 6323–6348. [Google Scholar] [CrossRef]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended graphene. Nat. Nanotechnol. 2008, 3, 491–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unarunotai, S.; Murata, Y.; Chialvo, C.E.; Mason, N.; Petrov, I.; Nuzzo, R.G.; Moore, J.S.; Rogers, J.A. Conjugated carbon monolayer membranes: Methods for synthesis and integration. Adv. Mater. 2010, 22, 1072–1077. [Google Scholar] [CrossRef]
- Jeon, I.; Seo, S.; Sato, Y.; Delacou, C.; Anisimov, A.; Suenaga, K.; Kauppinen, E.I.; Maruyama, S.; Matsuo, Y. Perovskite solar cells using carbon nanotubes both as cathode and as anode. J. Phy. Chem. C 2017, 121, 25743–25749. [Google Scholar] [CrossRef]
- Kim, J.M.; Rhee, S.W. Electrochemical properties of porous carbon black layer as an electron injector into iodide redox couple. Electrochim. Acta 2012, 83, 264–270. [Google Scholar] [CrossRef]
- Cha, S.M.; Nagaraju, G.; Sekhar, S.C.; Bharat, L.K.; Yu, J.S. Fallen leaves derived honeycomb-like porous carbon as a metal-free and low-cost counter electrode for dye-sensitized solar cells with excellent tri-iodide reduction. J. Coll. Interface Sci. 2018, 513, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, G.; Bojan, K.; Rhee, S.W. Sub-micrometer-sized graphite as a conducting and catalytic counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2011, 3, 857–862. [Google Scholar] [CrossRef]
- Mei, X.; Cho, S.J.; Fan, B.; Ouyang, J. High-performance dye-sensitized solar cells with gel-coated binder-free carbon nanotube films as counter electrode. Nanotechnology 2010, 21, 395202. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D honeycomb-like structured graphene and its high efficiency as a counter-electrode catalyst for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Pan, L.; Lu, T.; Xu, T.; Sun, Z. Electrophoretic deposition of reduced graphene-carbon nanotubes composite films as counter electrodes of dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 14869–14875. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, B.K.; Lee, W.J. Electrospun activated carbon nanofibers with hollow core/highly mesoporous shell structure as counter electrodes for dye-sensitized solar cells. J. Power Sour. 2013, 239, 122–127. [Google Scholar] [CrossRef]
- Sun, K.C.; Memon, A.A.; Arbab, A.A.; Sahito, I.A.; Kim, M.S.; Yeo, S.Y.; Choi, Y.O.; Kim, Y.S.; Jeong, S.H. Electrocatalytic porous nanocomposite of graphite nanoplatelets anchored with exfoliated activated carbon filler as counter electrode for dye sensitized solar cells. Sol. Energy 2018, 167, 95–101. [Google Scholar] [CrossRef]
- Arbab, A.A.; Sun, K.C.; Sahito, I.A.; Qadir, M.B.; Choi, Y.S.; Jeong, S.H. A Novel Activated-Charcoal-Doped Multiwalled Carbon Nanotube Hybrid for Quasi-Solid-State Dye-Sensitized Solar Cell Outperforming Pt Electrode. ACS Appl. Mater. Interfaces 2016, 8, 7471–7482. [Google Scholar] [CrossRef]
- Ito, S.; Baranwal, A.K. Carbon Counter Electrodes for Dye-Sensitized and Perovskite Solar Cells; Wiley Online Library: Hoboken, NJ, USA, 2018; Volume 2, pp. 457–485. [Google Scholar] [CrossRef]
- Joshi, P.; Xie, Y.; Ropp, M.; Galipeau, D.; Bailey, S.; Qiao, Q. Dye-sensitized solar cells based on low cost nanoscale carbon/TiO 2 composite counter electrode. Energy Environ. Sci. 2009, 2, 426–429. [Google Scholar] [CrossRef]
- Murakami, T.N.; Ito, S.; Wang, Q.; Nazeeruddin, M.K.; Bessho, T.; Cesar, I.; Liska, P.; Humphry-Baker, R.; Comte, P.; Péchy, P.; et al. Highly Efficient Dye-Sensitized Solar Cells Based on Carbon Black Counter Electrodes. J. Electrochem. Soc. 2006, 153, A2255. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Lin, J.; Huang, M.; Huang, Y.; Li, Q. High-performance and low platinum loading Pt/Carbon black counter electrode for dye-sensitized solar cells. Sol. Energy 2009, 83, 845–849. [Google Scholar] [CrossRef]
- Li, G.R.; Wang, F.; Song, J.; Xiong, F.Y.; Gao, X.P. TiN-conductive carbon black composite as counter electrode for dye-sensitized solar cells. Electrochim. Acta 2012, 65, 216–220. [Google Scholar] [CrossRef]
- Miao, X.; Pan, K.; Pan, Q.; Zhou, W.; Wang, L.; Liao, Y.; Tian, G.; Wang, G. Highly crystalline graphene/carbon black composite counter electrodes with controllable content: Synthesis, characterization and application in dye-sensitized solar cells. Electrochim. Acta 2013, 96, 155–163. [Google Scholar] [CrossRef]
- Wu, C.S.; Chang, T.W.; Teng, H.; Lee, Y.L. High performance carbon black counter electrodes for dye-sensitized solar cells. Energy 2016, 115, 513–518. [Google Scholar] [CrossRef]
- Li, C.T.; Lee, C.T.; Li, S.R.; Lee, C.P.; Chiu, I.T.; Vittal, R.; Wu, N.L.; Sun, S.S.; Ho, K.C. Composite films of carbon black nanoparticles and sulfonated-polythiophene as flexible counter electrodes for dye-sensitized solar cells. J. Power Sour. 2016, 302, 155–163. [Google Scholar] [CrossRef]
- Kitamura, K.; Shiratori, S. Layer-by-layer self-assembled mesoporous PEDOT-PSS and carbon black hybrid films for platinum free dye-sensitized-solar-cell counter electrodes. Nanotechnology 2011, 22, 195703. [Google Scholar] [CrossRef]
- Al-bahrani, M.R.; Liu, L.; Ahmad, W.; Tao, J.; Tu, F.; Cheng, Z.; Gao, Y. NiO-NF/MWCNT nanocomposite catalyst as a counter electrode for high performance dye-sensitized solar cells. Appl. Surf. Sci. 2015, 331, 333–338. [Google Scholar] [CrossRef]
- Lee, K.M.; Chiu, W.H.; Wei, H.Y.; Hu, C.W.; Suryanarayanan, V.; Hsieh, W.F.; Ho, K.C. Effects of mesoscopic poly(3,4-ethylenedioxythiophene) films as counter electrodes for dye-sensitized solar cells. Thin Solid Films 2010, 518, 1716–1721. [Google Scholar] [CrossRef]
- Zussman, E.; Chen, X.; Ding, W.; Calabri, L.; Dikin, D.A.; Quintana, J.P.; Ruoff, R.S. Mechanical and structural characterization of electrospun PAN-derived carbon nanofibers. Carbon 2005, 43, 2175–2185. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Game, O.; Bhange, S.N.; Kumari, T.; Ogale, S.B.; Kurungot, S. Enhanced catalytic activity of polyethylenedioxythiophene towards tri-iodide reduction in DSSCs via 1-dimensional alignment using hollow carbon nanofibers. Nanoscale 2014, 6, 10332–10339. [Google Scholar] [CrossRef]
- Yen, M.Y.; Teng, C.C.; Hsiao, M.C.; Liu, P.I.; Chuang, W.P.; Ma, C.C.M.; Hsieh, C.K.; Tsai, M.C.; Tsai, C.H. Platinum nanoparticles/graphene composite catalyst as a novel composite counter electrode for high performance dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 12880–12888. [Google Scholar] [CrossRef]
- Yue, G.; Wu, J.; Xiao, Y.; Lin, J.; Huang, M.; Lan, Z.; Fan, L. Functionalized graphene/poly(3,4-ethylenedioxythiophene): Polystyrenesulfonate as counter electrode catalyst for dye-sensitized solar cells. Energy 2013, 54, 315–321. [Google Scholar] [CrossRef]
- Freitas, F.S.; Gonçalves, A.S.; de Morais, A.; Benedetti, J.E.; Nogueira, A.F. Graphene-like MoS2 as a low-cost counter electrode material for dye-sensitized solar cells. NanoGe J. Energy Sustain. 2013, 1, 11002. [Google Scholar]
- Nagaraju, G.; Lim, J.H.; Cha, S.M.; Yu, J.S. Three-dimensional activated porous carbon with meso/macropore structures derived from fallen pine cone flowers: A low-cost counter electrode material in dye-sensitized solar cells. J. Alloys Compd. 2017, 693, 1297–1304. [Google Scholar] [CrossRef]
- Talip, L.F.A.; Ramli, M.M.; Isa, S.S.M.; Halin, D.S.C.; Mazlan, N.S.; Anhar, N.A.M.; Danial, N.A.; Muda, M.R. Hybrid TiO2-Gigantochloa Albociliata Charcoal in Dye Sensitized Solar Cell. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012086. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.; Wiezorek, J. 20 Renewable Biowastes Derived Carbon Materials as Green Counter Electrodes for Dye-Sensitized Solar Cells. Mater. Chem. Phys. 2018, 204, 294–304. [Google Scholar] [CrossRef]
- Maiaugree, W.; Lowpa, S.; Towannang, M.; Rutphonsan, P.; Tangtrakarn, A.; Pimanpang, S.; Maiaugree, P.; Ratchapolthavisin, N.; Sang-Aroon, W.; Jarernboon, W.; et al. A dye sensitized solar cell using natural counter electrode and natural dye derived from mangosteen peel waste. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Linares-Solano, A.; Lillo-Ródenas, M.A.; Marco-Lozar, J.P.; Kunowsky, M.; Romero-Anaya, A.J. NaOH and KOH for preparing activated carbons used in energy and environmental applications. Res. Appl. Energy Environ. Econ. 2014, 20, 59–92. [Google Scholar]
- Kumarasinghe, K.D.M.S.P.K.; Kumara, G.R.A.; Rajapakse, R.M.G.; Liyanage, D.N.; Tennakone, K. Activated coconut shell charcoal based counter electrode for dye-sensitized solar cells. Org. Electron. 2019, 71, 93–97. [Google Scholar] [CrossRef]
- Environ, E.; Lee, B.; Buchholz, D.B.; Chang, R.P.H. An all carbon counter electrode for dye sensitized solar cells. Environ. Sci. 2012, 6941–6952. [Google Scholar] [CrossRef]
- Kumar, R.; Nemala, S.S.; Mallick, S.; Bhargava, P. High efficiency dye sensitized solar cell made by carbon derived from sucrose. Opt. Mater. 2017, 64, 401–405. [Google Scholar] [CrossRef]
- Wu, M.; Lin, X.; Wang, T.; Qiu, J.; Ma, T. Low-cost dye-sensitized solar cell based on nine kinds of carbon counter electrodes. Energy Environ. Sci. 2011, 4, 2308–2315. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Karthik, P.S.; Himaja, A.L.; Singh, S.P. Carbon-allotropes: Synthesis methods, applications and future perspectives. Carbon Lett. 2014, 15, 219–237. [Google Scholar] [CrossRef] [Green Version]
- Ngo, Q.; Yamada, T.; Suzuki, M.; Ominami, Y.; Cassell, A.M.; Li, J.; Meyyappan, M.; Yang, C.Y. Structural and electrical characterization of carbon nanofibers for interconnect via applications. IEEE Trans. Nanotechnol. 2007, 6, 688–695. [Google Scholar] [CrossRef]
- Wei, C.; Srivastava, D. Nanomechanics of carbon nanofibers: Structural and elastic properties. Appl. Phys. Lett. 2004, 85, 2208–2210. [Google Scholar] [CrossRef]
- Veerappan, G.; Kwon, W.; Rhee, S.W. Carbon-nanofiber counter electrodes for quasi-solid state dye-sensitized solar cells. J. Power Sour. 2011, 196, 10798–10805. [Google Scholar] [CrossRef]
- Joshi, P.; Zhou, Z.; Poudel, P.; Thapa, A.; Wu, X.F.; Qiao, Q. Nickel incorporated carbon nanotube/nanofiber composites as counter electrodes for dye-sensitized solar cells. Nanoscale 2012, 4, 5659–5664. [Google Scholar] [CrossRef]
- Motlak, M.; Barakat, N.A.; Akhtar, M.S.; Hamza, A.M.; Kim, B.S.; Kim, C.S.; Khalil, K.A.; Almajid, A.A. High performance of NiCo nanoparticles-doped carbon nanofibers as counter electrode for dye-sensitized solar cells. Electrochim. Acta 2015, 160, 1–6. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.; Chen, Q.; Galipeau, D.; Fong, H.; Qiao, Q. Electrospun carbon nanofibers as low-cost counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2010, 2, 3572–3577. [Google Scholar] [CrossRef]
- Xie, Y.; Joshi, P.; Ropp, M.; Galipeau, D.; Zhang, L.; Fong, H.; You, Y.; Qiao, Q. Structural effects of core-modified porphyrins in dye-sensitized solar cells. J. Porphyr. Phthalocyanines 2009, 13, 903–909. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaguchi, M.; Kumagai, M.; Yanagida, S. Application of carbon nanotubes to counter electrodes of dye-sensitized solar cells. Chem. Lett. 2003, 32, 28–29. [Google Scholar] [CrossRef]
- Heng, L.Y.; Chou, A.; Yu, J.; Chen, Y.; Gooding, J.J. Demonstration of the advantages of using bamboo-like nanotubes for electrochemical biosensor applications compared with single walled carbon nanotubes. Electrochem. Commun. 2005, 7, 1457–1462. [Google Scholar] [CrossRef]
- Lee, W.J.; Ramasamy, E.; Lee, D.Y.; Song, J.S. Efficient dye-sensitized solar cells with catalytic multiwall carbon nanotube counter electrodes. ACS Appl. Mater. Interfaces 2009, 1, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Velten, J.; Mozer, A.J.; Li, D.; Officer, D.; Wallace, G.; Baughman, R.; Zakhidov, A. Carbon nanotube/graphene nanocomposite as efficient counter electrodes in dye-sensitized solar cells. Nanotechnology 2012, 23, 085201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Luo, Y.; Lv, W.; Yu, W.; Wu, S.; Hou, P.; Yang, Q.; Meng, Q.; Liu, C.; Cheng, H.M. Vertically aligned carbon nanotubes grown on graphene paper as electrodes in lithium-ion batteries and dye-sensitized solar cells. Adv. Energy Mater. 2011, 1, 486–490. [Google Scholar] [CrossRef]
- Yeh, M.H.; Lin, L.Y.; Sun, C.L.; Leu, Y.A.; Tsai, J.T.; Yeh, C.Y.; Vittal, R.; Ho, K.C. Multiwalled carbon nanotube@ reduced graphene oxide nanoribbon as the counter electrode for dye-sensitized solar cells. J. Phy. Chem. C 2014, 118, 16626–16634. [Google Scholar] [CrossRef]
- Lan, Z.; Wu, J.; Lin, J.; Huang, M. Morphology controllable fabrication of Pt counter electrodes for highly efficient dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 3948–3954. [Google Scholar] [CrossRef]
- Nam, J.G.; Park, Y.J.; Kim, B.S.; Lee, J.S. Enhancement of the efficiency of dye-sensitized solar cell by utilizing carbon nanotube counter electrode. Scr. Mater. 2010, 62, 148–150. [Google Scholar] [CrossRef]
- de Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Poudel, P.; Qiao, Q. Carbon nanostructure counter electrodes for low cost and stable dye-sensitized solar cells. Nano Energy 2014, 4, 157–175. [Google Scholar] [CrossRef]
- Liu, I.P.; Hou, Y.C.; Li, C.W.; Lee, Y.L. Highly electrocatalytic counter electrodes based on carbon black for cobalt(iii)/(ii)-mediated dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 240–249. [Google Scholar] [CrossRef]
- Grafin, P.; Prestasi, M.; Suria, S. Incorporation of Graphene Into Counter Electrode To Enhance the Performance of Dye-Sensitized Solar Cells. Malays. J. Anal. Sci. 2017, 21, 1120–1126. [Google Scholar] [CrossRef]
- Zhang, J.; Long, H.; Miralles, S.G.; Bisquert, J.; Fabregat-Santiago, F.; Zhang, M. The combination of a polymer-carbon composite electrode with a high-absorptivity ruthenium dye achieves an efficient dye-sensitized solar cell based on a thiolate-disulfide redox couple. Phys. Chem. Chem. Phys. 2012, 14, 7131–7136. [Google Scholar] [CrossRef]
- Ramasamy, E.; Lee, W.J.; Lee, D.Y.; Song, J.S. Spray coated multi-wall carbon nanotube counter electrode for tri-iodide (I3-) reduction in dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1087–1089. [Google Scholar] [CrossRef]
- Dong, J.; Wu, J.; Jia, J.; Fan, L.; Lin, J. Nickel selenide/reduced graphene oxide nanocomposite as counter electrode for high efficient dye-sensitized solar cells. J. Coll. Interface Sci. 2017, 498, 217–222. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Ranjbartoreh, A.R.; Wang, B.; Shen, X.; Wang, G. Advanced mechanical properties of graphene paper. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Xu, G.; He, C.; Wang, T.; Yang, L.; Yang, Z.; Ma, D. Novel catalytic activity for oxygen reduction reaction on MnN4 embedded graphene: A dispersion-corrected density functional theory study. Carbon 2015, 84, 500–508. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. Thermally exfoliated graphene based counter electrode for low cost dye sensitized solar cells. J. Appl. Phys. 2011, 109, 124308. [Google Scholar] [CrossRef]
- Roy-Mayhew, J.D.; Bozym, D.J.; Punckt, C.; Aksay, I.A. Functionalized graphene as a catalytic counter electrode in dye-sensitized solar cells. ACS Nano 2010, 4, 6203–6211. [Google Scholar] [CrossRef] [Green Version]
- Jeon, I.Y.; Kim, H.M.; Choi, I.T.; Lim, K.; Ko, J.; Kim, J.C.; Choi, H.J.; Ju, M.J.; Lee, J.J.; Kim, H.K.; et al. High-performance dye-sensitized solar cells using edge-halogenated graphene nanoplatelets as counter electrodes. Nano Energy 2015, 13, 336–345. [Google Scholar] [CrossRef]
- Cells, S. Electrochemically Reduced Graphene Oxide Multilayer Films as Efficient Counter Electrode for Dye-Sensitized. Sci. Rep. 2013, 3, 1489. [Google Scholar] [CrossRef] [Green Version]
- Yue, G.; Wu, J.; Xiao, Y.; Huang, M.; Lin, J.; Fan, L.; Lan, Z. Platinum/graphene hybrid film as a counter electrode for dye-sensitized solar cells. Electrochim. Acta 2013, 92, 64–70. [Google Scholar] [CrossRef]
- Yue, G.; Lin, J.Y.; Tai, S.Y.; Xiao, Y.; Wu, J. A catalytic composite film of MoS2/graphene flake as a counter electrode for Pt-free dye-sensitized solar cells. Electrochim. Acta 2012, 85, 162–168. [Google Scholar] [CrossRef]
- Hong, W.; Xu, Y.; Lu, G.; Li, C.; Shi, G. Transparent graphene/PEDOT-PSS composite films as counter electrodes of dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1555–1558. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Chen, H.; Wang, R.; Li, D.; Qu, J.; Dai, L. Nitrogen-doped graphene foams as metal-free counter electrodes in high-performance dye-sensitized solar cells. Angew. Chem. Int. Ed. 2012, 51, 12124–12127. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Singh, E.; Nalwa, H.S. Graphene-based dye-sensitized solar cells: A review. Sci. Adv. Mater. 2015, 7, 1863–1912. [Google Scholar] [CrossRef]
- Miao, X.; Pan, K.; Wang, G.; Liao, Y.; Wang, L.; Zhou, W.; Jiang, B.; Pan, Q.; Tian, G. Well-dispersed cos nanoparticles on a functionalized graphene nanosheet surface: A counter electrode of dye-sensitized solar cells. Chem. A Eur. J. 2014, 20, 474–482. [Google Scholar] [CrossRef] [PubMed]






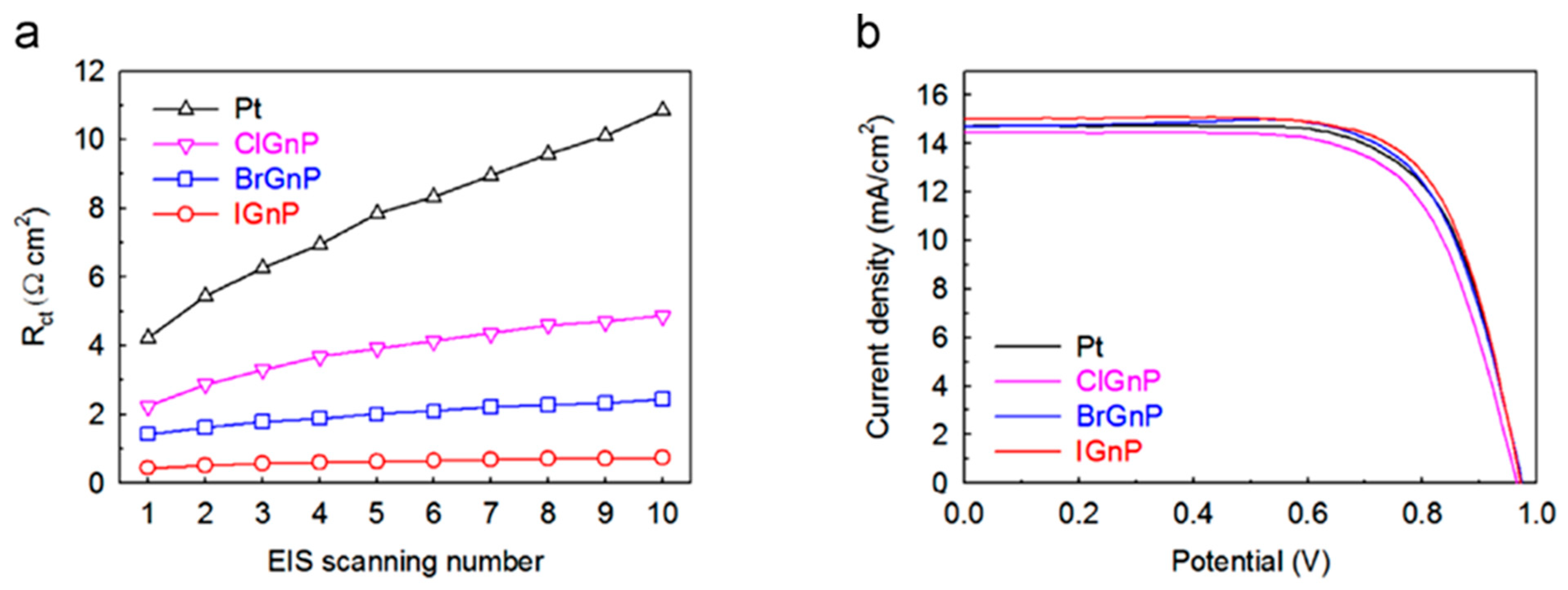
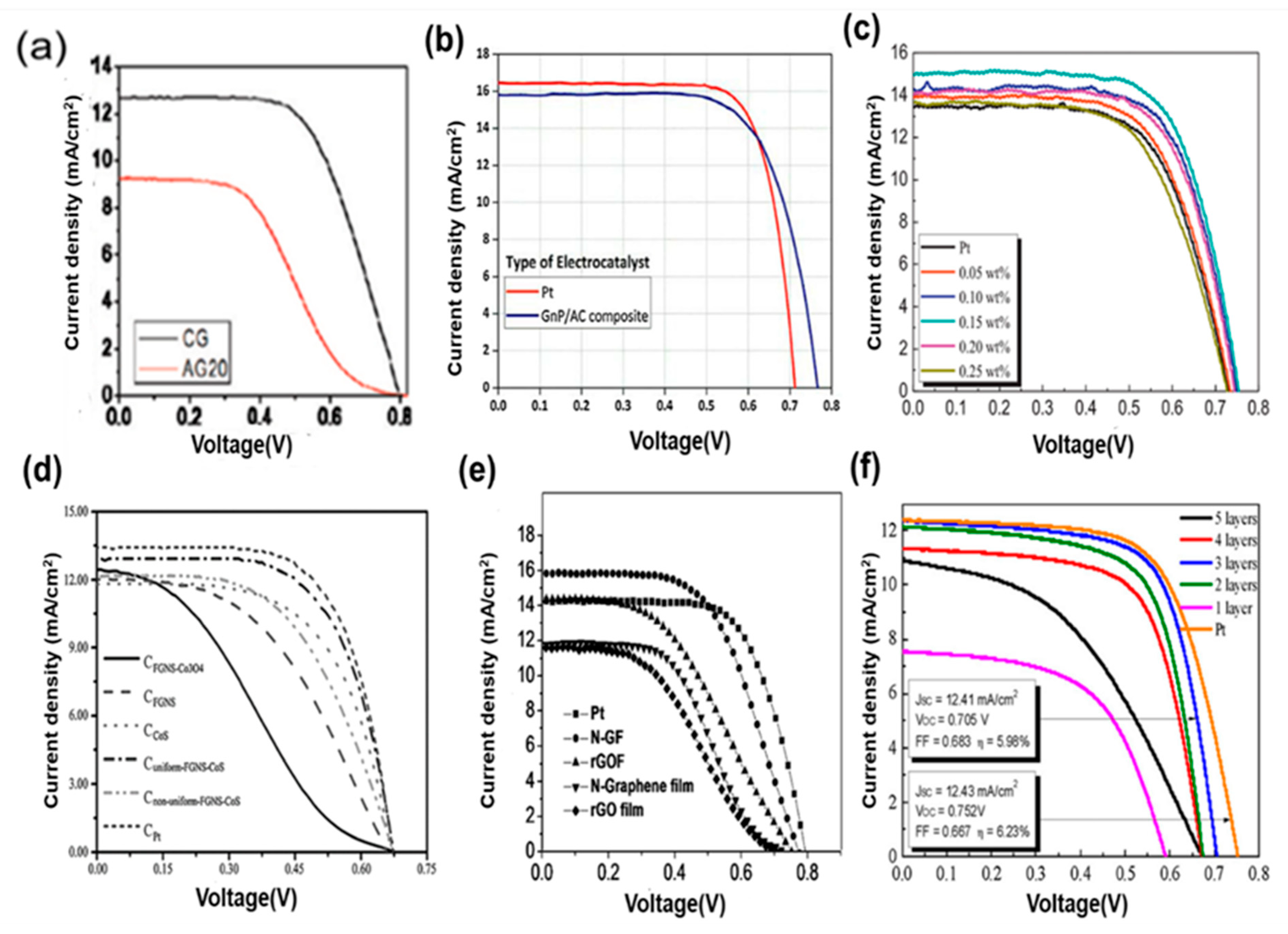
| SL. No. | CE Material | Synthesis | Fabrication | Remark | Ref. |
|---|---|---|---|---|---|
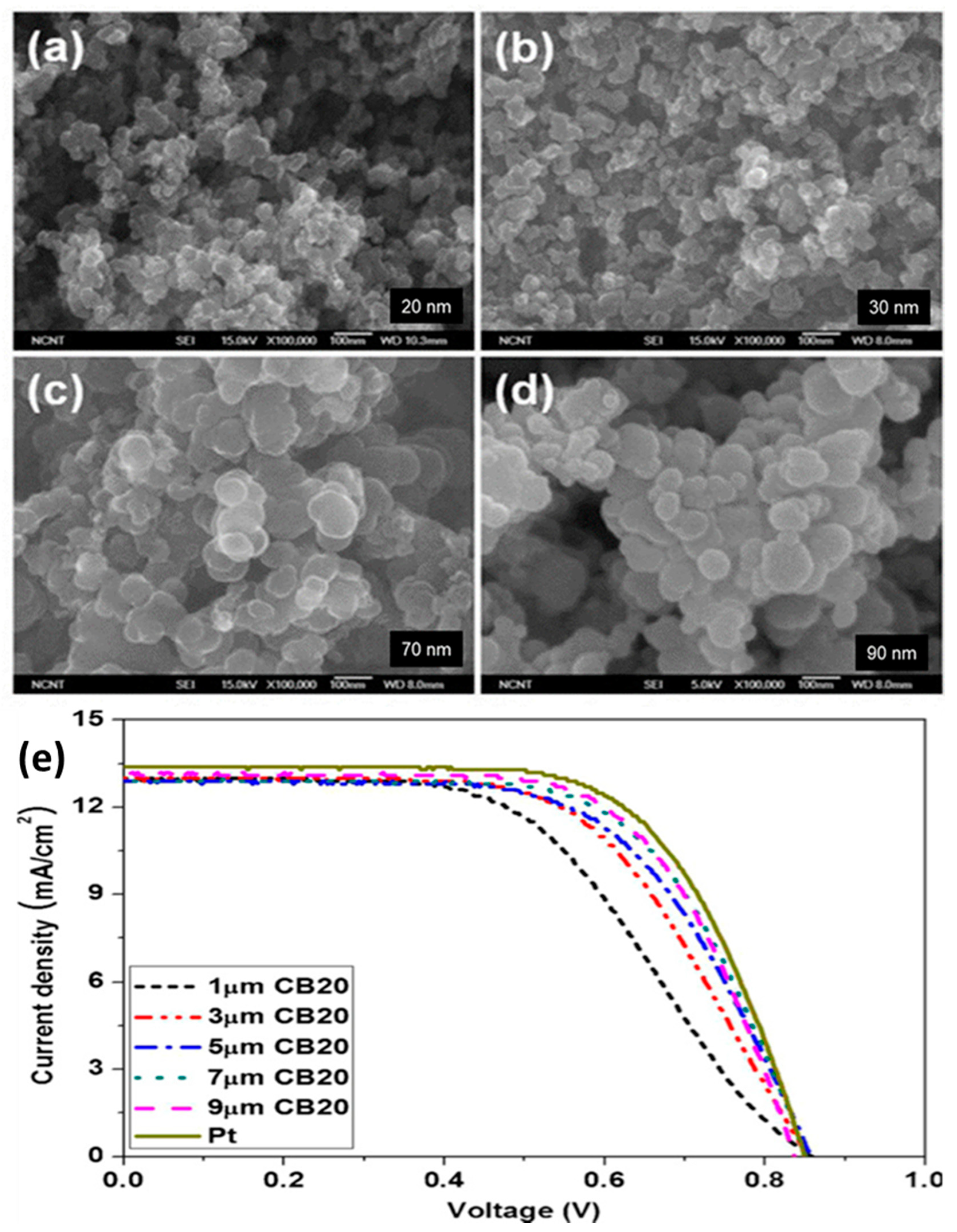
| 1 | carbon black | Combustion of petroleum products. | Doctor blade | Conductivity is low | [51] |
| 2 | AC | Alkali treatment and pyrolysis process | Doctor blade | Conductivity is low | [52] |
| 3 | Graphite | - | Doctor blade | Transparent conductive oxide (TCO) free substrate can be possible | [53] |
| 4 | Carbon nanofiber (CNF) | electrospinning | Doctor blade | Required high thickness. | [42] |
| 5 | CNTs | Chemical vapor deposition (CVD) | Doctor blade | Stability is low | [54] |
| 6 | Graphene | CVD | Doctor blade | Coating on TCO is difficult | [55] |
| 7 | Reduced graphene oxide + CNTs | microwave-assisted reduction | Electrophoretic deposition | Mass production is possible but toxic process | [56] |
| 8 | Hollow activated-carbon nanofiber(HACNF) | concentric electrospinning | Spray-coating | High performance compared with CNF-based CE | [57] |
| 9 | Graphite + AC | - | a bar coating method | - | [58] |
| 10 | AC + multi walled carbon nanotubes (MWCNTs) | enzymatic dispersion | Doctor blade | High fill factor | [59] |
| CE Material | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| Pt + MWCNTs | 0.74 | 18.91 | 62.00 | 8.23 | [41] |
| NiO-Nf/MWCNTs | 0.64 | 18.54 | 63.90 | 7.63 | [69] |
| PEDOT + MWCNTs | 0.72 | 17.00 | 66.01 | 8.08 | [70] |
| Pt + CB | 0.75 | 14.46 | 61.60 | 6.72 | [63] |
| PEDOT/PSS + CB | 0.76 | 10.80 | 57.00 | 4.70 | [68] |
| NiO-Co-doped CNFs | 0.74 | 11.12 | 54.00 | 4.47 | [71] |
| Pt + CNFs | 0.83 | 14.35 | 67.00 | 8.00 | [42] |
| PEDOT + CNFs | 0.72 | 13.96 | 65.19 | 7.16 | [72] |
| Pt + GR | 0.80 | 12.06 | 67.01 | 6.90 | [73] |
| PEDOT/PSS + GR | 0.77 | 15.70 | 65.00 | 7.86 | [74] |
| PEDOT + EXGR | 0.64 | 22.80 | 55.00 | 8.00 | [75] |
| CE Material | Thicknes (µm) | Rct (Ω/cm2) | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Carbon black | 4.8 | 0.47 | 0.77 | 14.74 | 71.30 | 8.35 | [66] |
| Carbon black (20 nm) | 9.0 | 12.80 | 0.84 | 13.10 | 65.60 | 7.20 | [51] |
| Carbon black | 1.4 | 0.39 | 0.88 | 13.44 | 74.01 | 8.81 | [104] |
| AC | 12.0 | 25.90 | 0.70 | 14.99 | 52.59 | 5.52 | [52] |
| AC coconut shell | 52.0 | 1.58 | 0.65 | 19.49 | 62.00 | 7.85 | [81] |
| AC + MWCNTs | 3.0 | 0.60 | 0.76 | 16.07 | 83.00 | 10.05 | [59] |
| Carbon nanofiber | 12.0 | 0.50 | 0.83 | 12.10 | 70.00 | 7.00 | [89] |
| Hollow active carbon nanofiber (HACNF) | 1.6 | 5.40 | 0.73 | 15.40 | 64.00 | 7.21 | [57] |
| SWCNTs | 6.0 | 0.60 | 0.76 | 14.13 | 77.00 | 7.81 | [54] |
| MWCNTs | 6.0 | 0.75 | 0.74 | 14.49 | 71.00 | 7.63 | [54] |
| Reduced graphene oxide | 15.0 | 1.50 | 0.78 | 12.82 | 72.00 | 7.19 | [75] |
| Honeycomb like structure graphene | 20.0 | 20.00 | 0.77 | 27.2 | 37.00 | 7.80 | [55] |
| Graphite carbon from sucrose | 4–5.0 | 1.40 | 0.69 | 19.99 | 72.00 | 9.69 | [83] |
| Graphite + AC | - | 2.19 | 0.77 | 15.80 | 69.99 | 8.48 | [58] |
| Graphite | 9.0 | 5.00 | 0.79 | 12.40 | 61.00 | 6.01 | [105] |
| Large surface polyaromatic hydrocarbon (LPAH) | 3.0 | 2.12 | 0.80 | 11.50 | 80.00 | 8.63 | [45] |
| Carbon + PEDOT | 3.6 | 2.00 | 0.65 | 16.80 | 70.00 | 7.60 | [106] |
| SL No. | CE Material | Advantages | Limitation | Challenges | Remark |
|---|---|---|---|---|---|
| 1 | carbon black | Lower cost than AC | Conductivity is low | Adhesion to the glass substrate | Performance is dependent on the film thickness |
| 2 | AC | Low cost | Conductivity is low | High temperature process | Composite with conductive material has high performance |
| 3 | Graphite | Low-charge transport resistance | Pure graphite showed a poor electrocatalytic ability | Methods for preparing paste | More suitable for large area fabrication technology |
| 4 | Carbon nanofiber | Flexible, lightweight | Larger dimensions compared to carbon nanotubes | Coating on glass substrate | Larger dimension limits the effective surface area, and as a result, a higher thickness is required |
| 5 | CNTs | 1-D is a very high conductivity | Direct coating on substrate is difficult | Coating on substrate is difficult | Vertical coating on substrates showed high efficiency. |
| 6 | Graphene | Highly conductive | Fabrication cost is high | Coating on substrate is difficult | More suitable for flexible DSSCs and transparent DSSCs |
| 7 | Reduced graphene oxide | Low-cost mass production | Hazardous chemical process for the synthesis | A suitable method for preparing a film | Alternative for both large area solid and flexible substrates |
| 8 | Hollow active carbon nanofiber (HACNF) | High catalytic activity | Larger dimensions compared to carbon nanotubes | Preparation method cost is high compared to that of CNF | The larger dimension limits the effective surface area, and as a result, a higher thickness is required |
| 9 | Graphite + AC | High catalytic activity | Defect reaches surface to limit the charge transport process | Stability of composite | Solution preparation is easily compared to another composite |
| 10 | Graphite + CB | High catalytic activity | Less than 10% PCE | Suitable methodology for composite mixing | Low-cost high-efficiency counter electrode could be possible |
| 11 | AC + MWCNTs | High efficiency | Homogeneously mixed matrix | Stability | Composite mixing is a difficult process |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samantaray, M.R.; Mondal, A.K.; Murugadoss, G.; Pitchaimuthu, S.; Das, S.; Bahru, R.; Mohamed, M.A. Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-Sensitized Solar Cells: A Review. Materials 2020, 13, 2779. https://doi.org/10.3390/ma13122779
Samantaray MR, Mondal AK, Murugadoss G, Pitchaimuthu S, Das S, Bahru R, Mohamed MA. Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-Sensitized Solar Cells: A Review. Materials. 2020; 13(12):2779. https://doi.org/10.3390/ma13122779
Chicago/Turabian StyleSamantaray, Manas R., Abhay Kumar Mondal, Govindhasamy Murugadoss, Sudhagar Pitchaimuthu, Santanu Das, Raihana Bahru, and Mohd Ambri Mohamed. 2020. "Synergetic Effects of Hybrid Carbon Nanostructured Counter Electrodes for Dye-Sensitized Solar Cells: A Review" Materials 13, no. 12: 2779. https://doi.org/10.3390/ma13122779






