The Influence of Co Additive on the Sintering, Mechanical Properties, Cytocompatibility, and Digital Light Processing Based Stereolithography of 3Y-TZP-5Al2O3 Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powders Synthesis and Ceramic Sintering
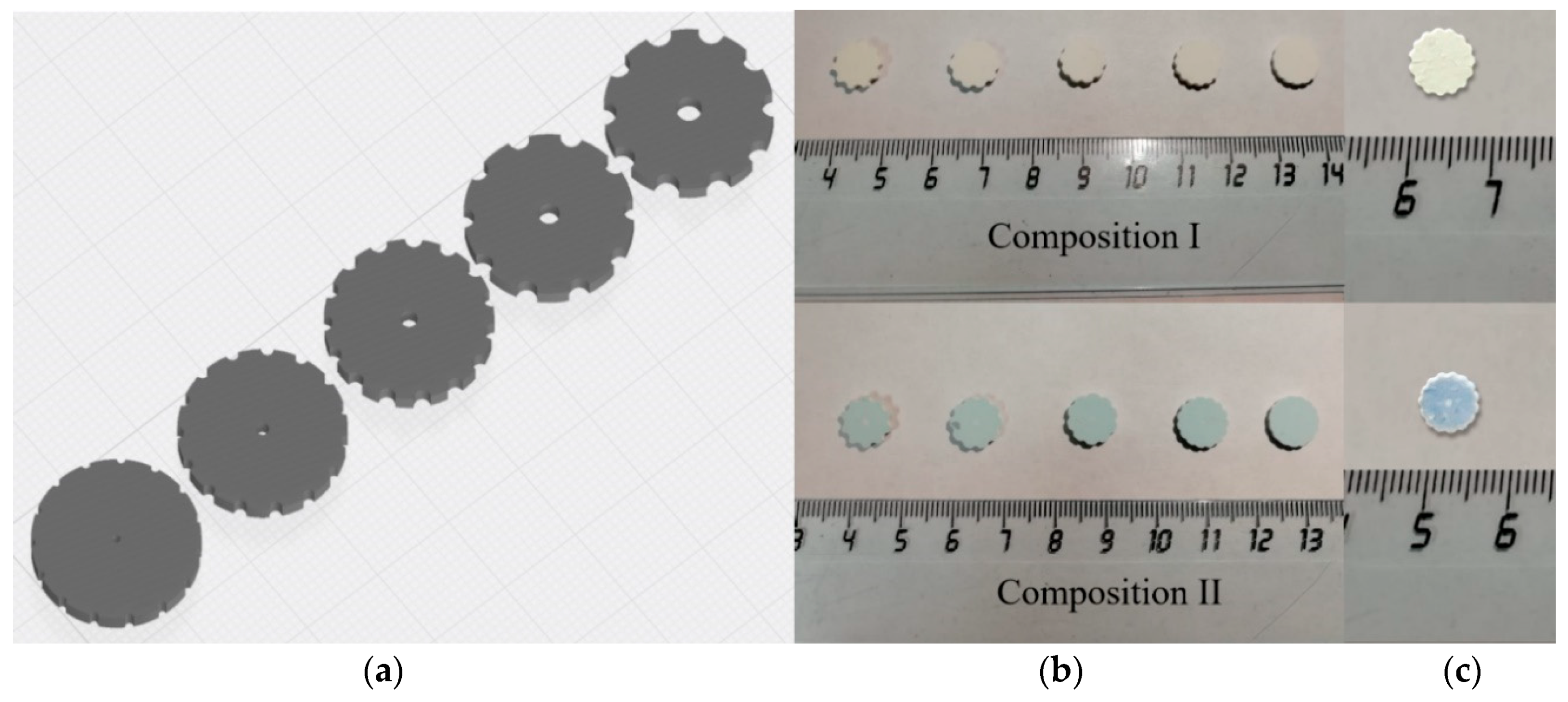
2.2. Ceramic Samples DLP Printing and Sintering
2.3. Characterization Techniques
2.4. Cytotoxicity Studies
3. Results
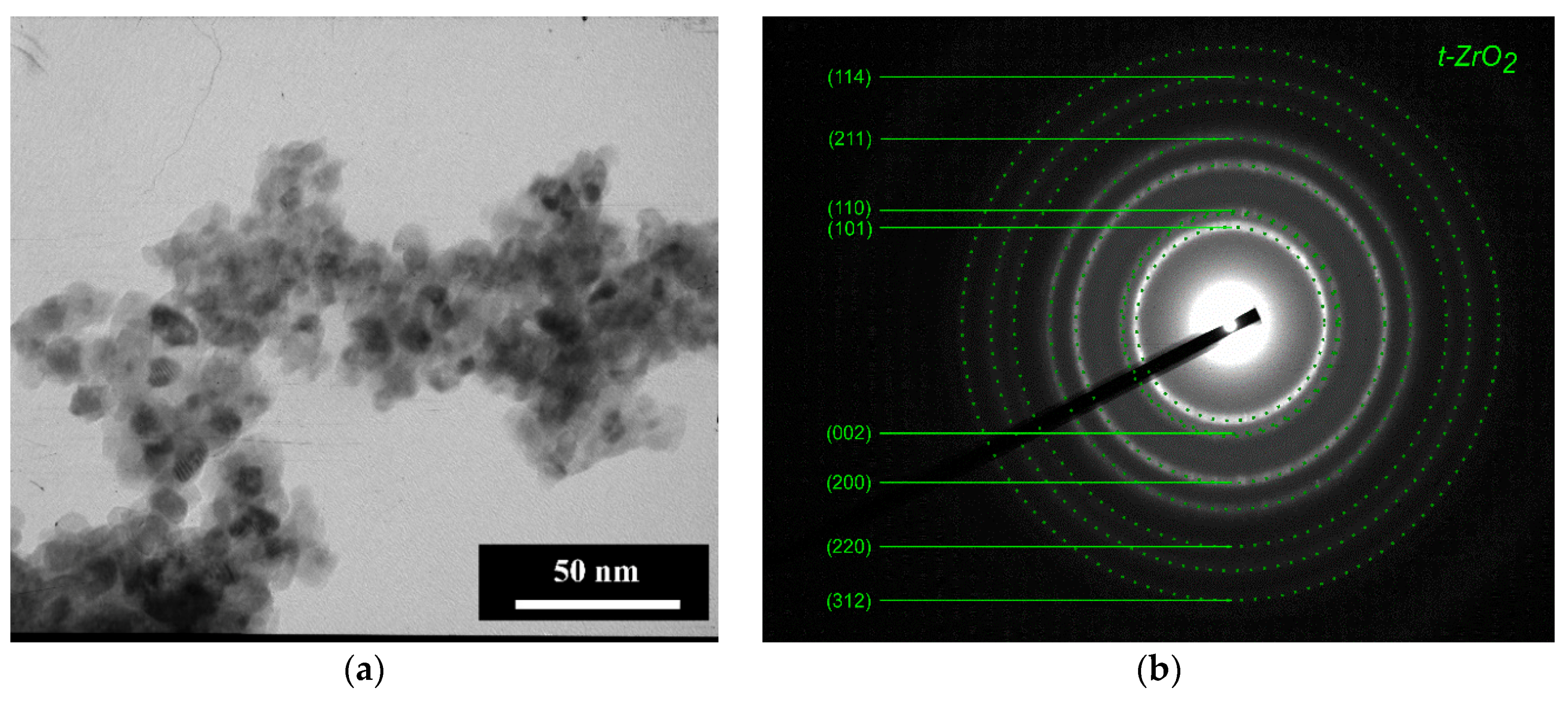
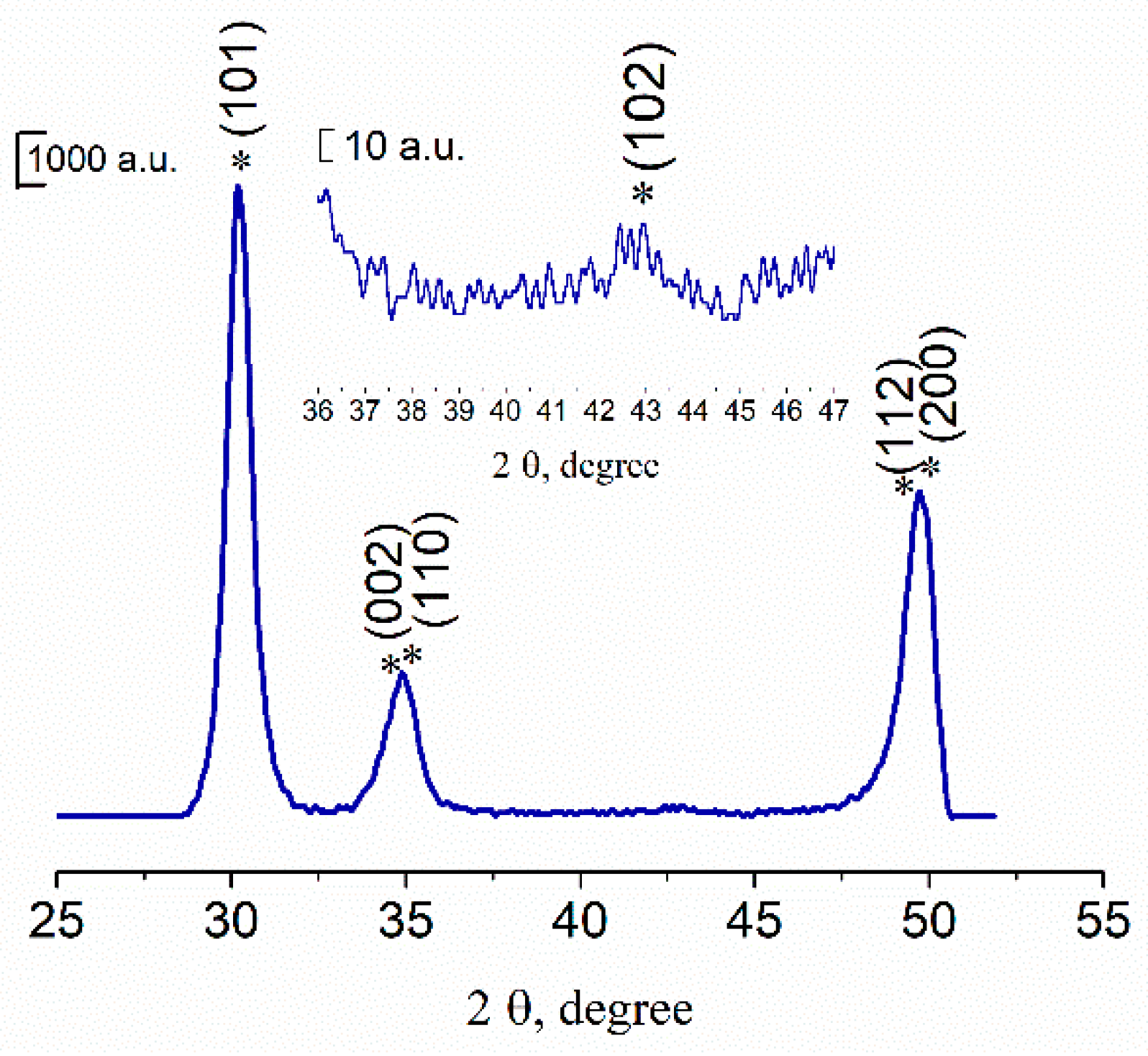
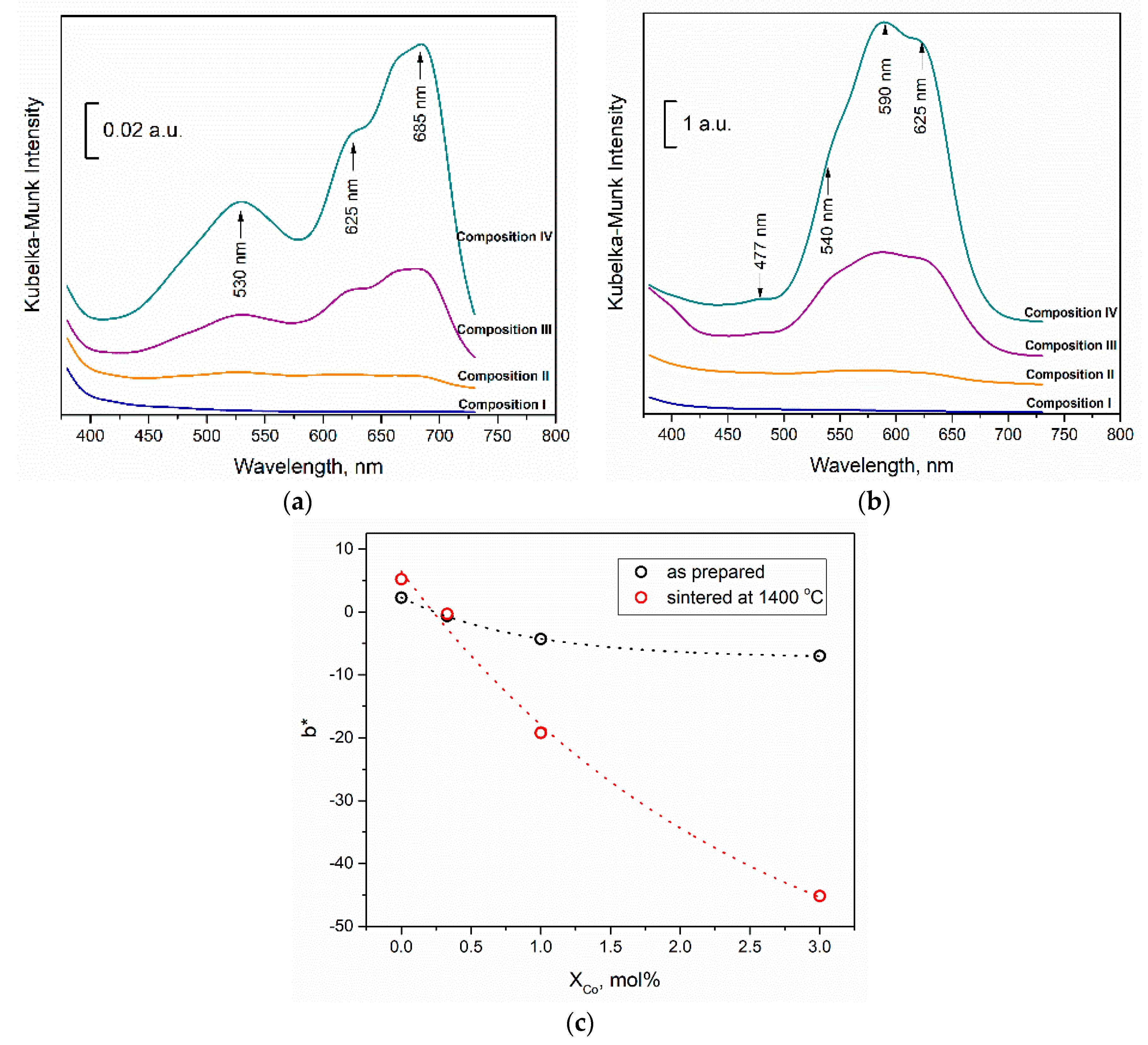
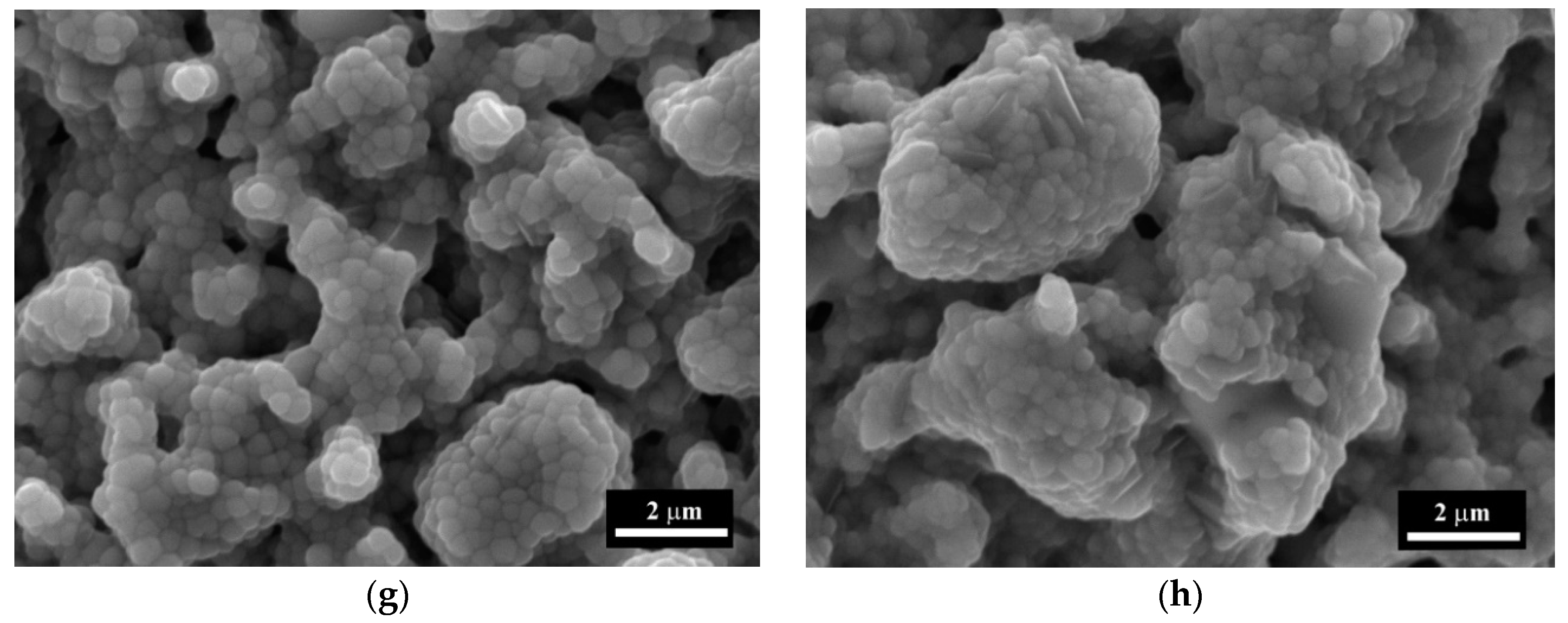
3.1. Powders Morphology, Surface Area, and Phase Composition before Co Introduction
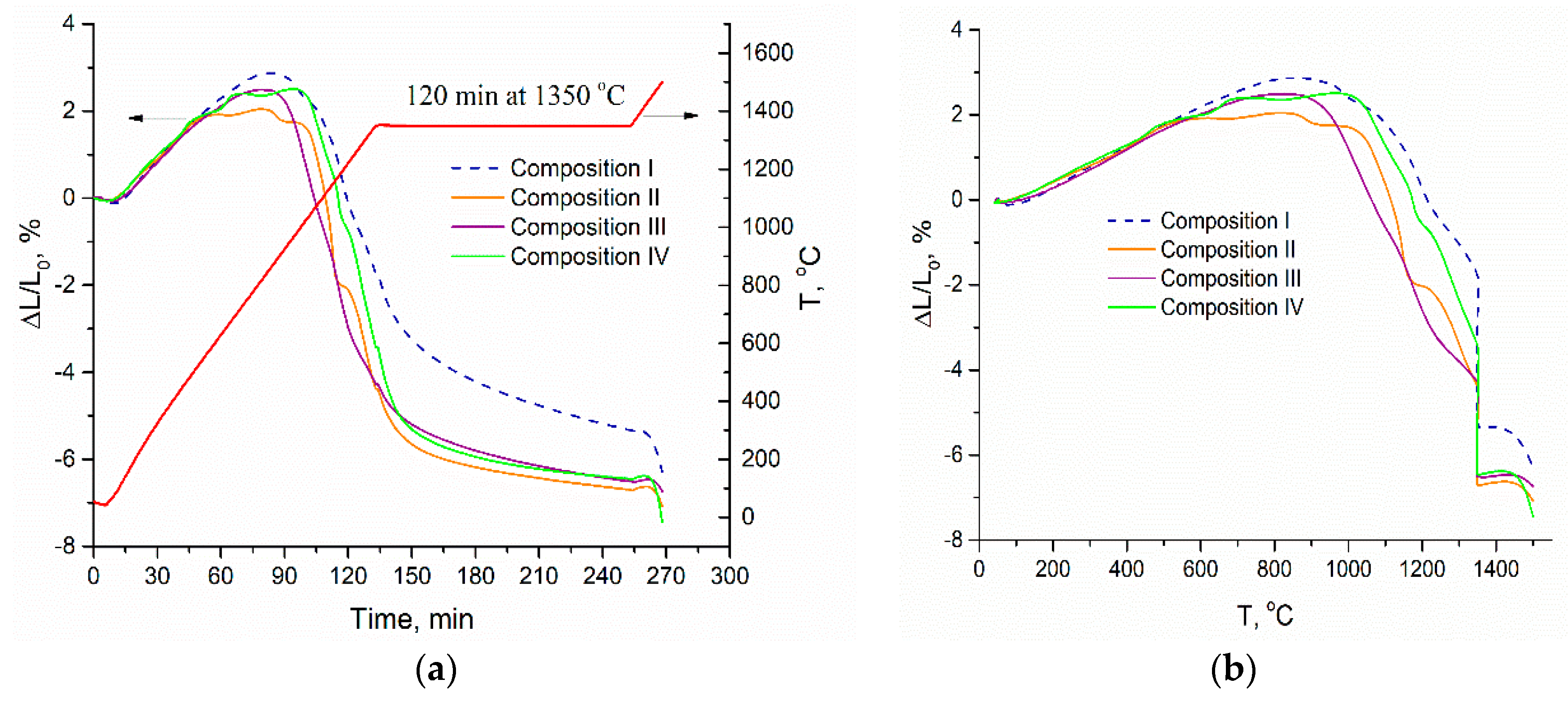
3.2. Dilatometric Study of the Ceramic Samples
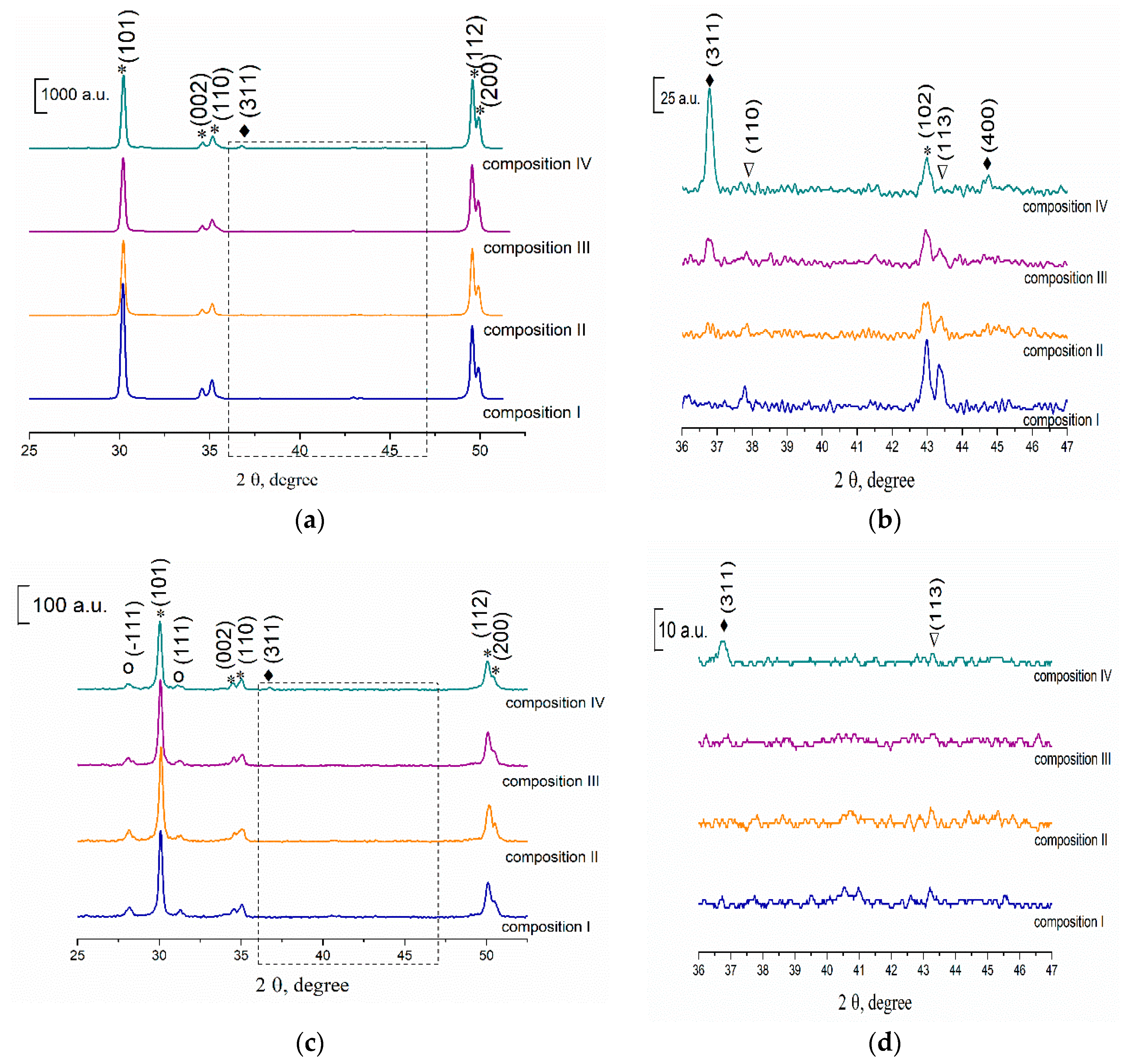
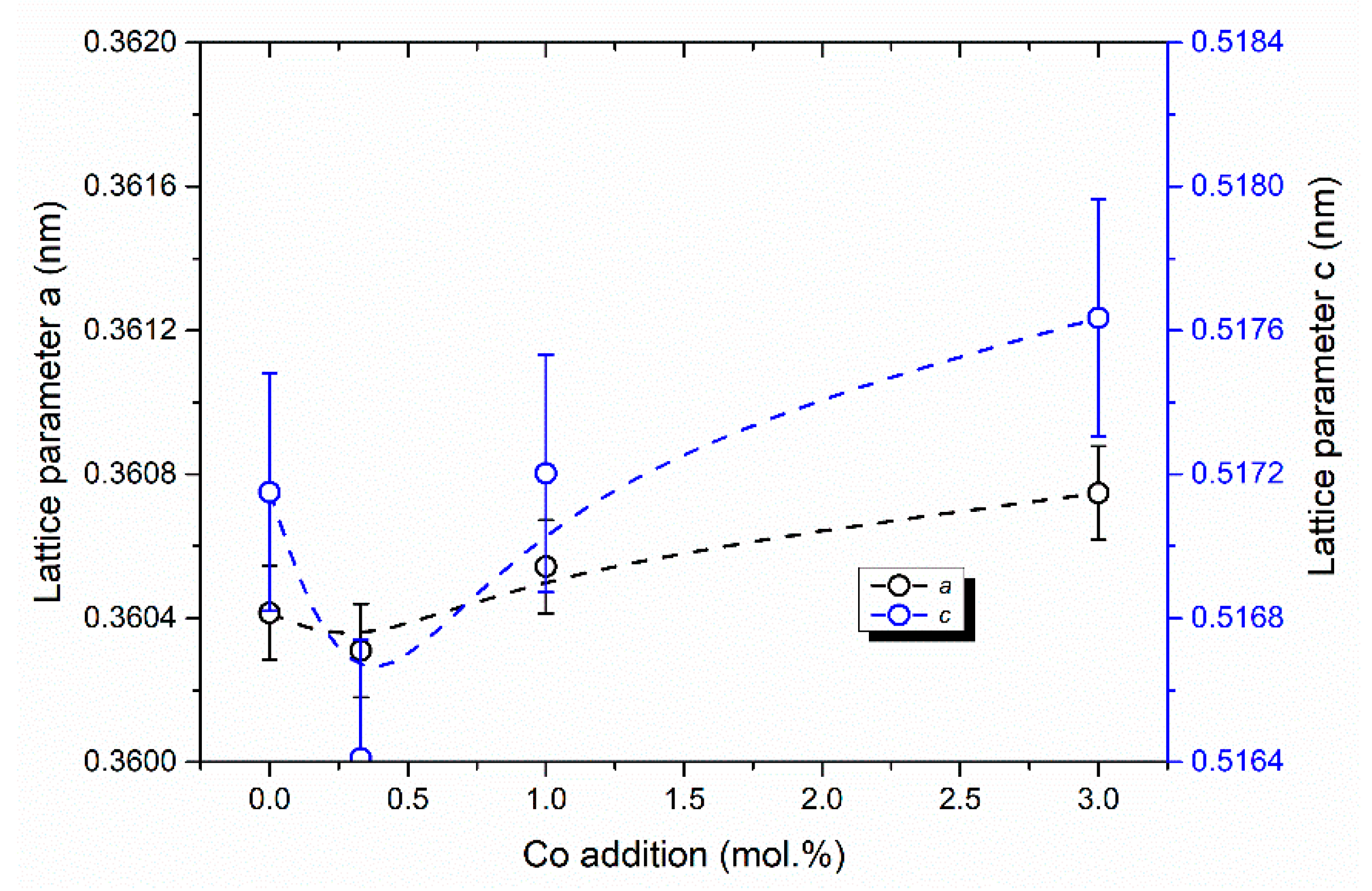
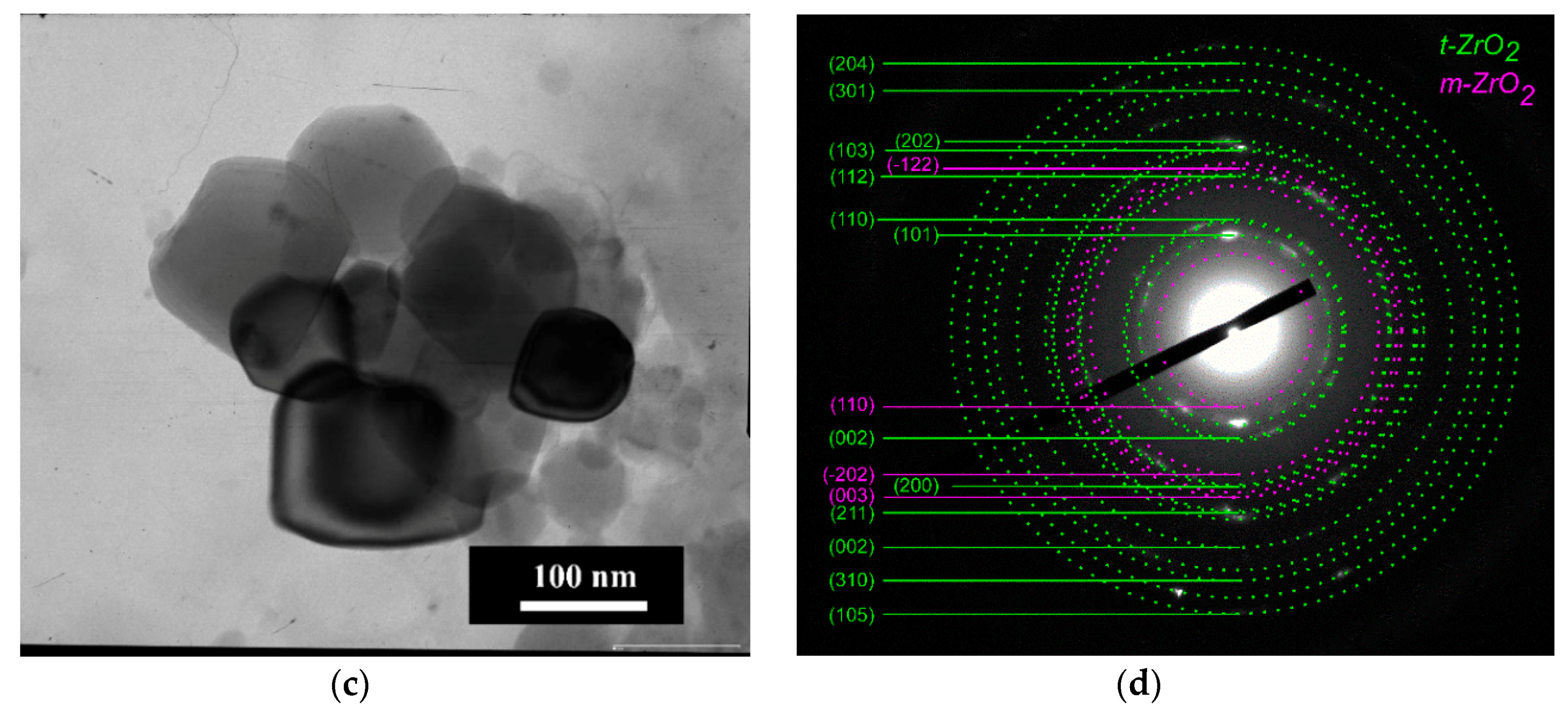
3.3. Phase Composition of the Ceramics Samples
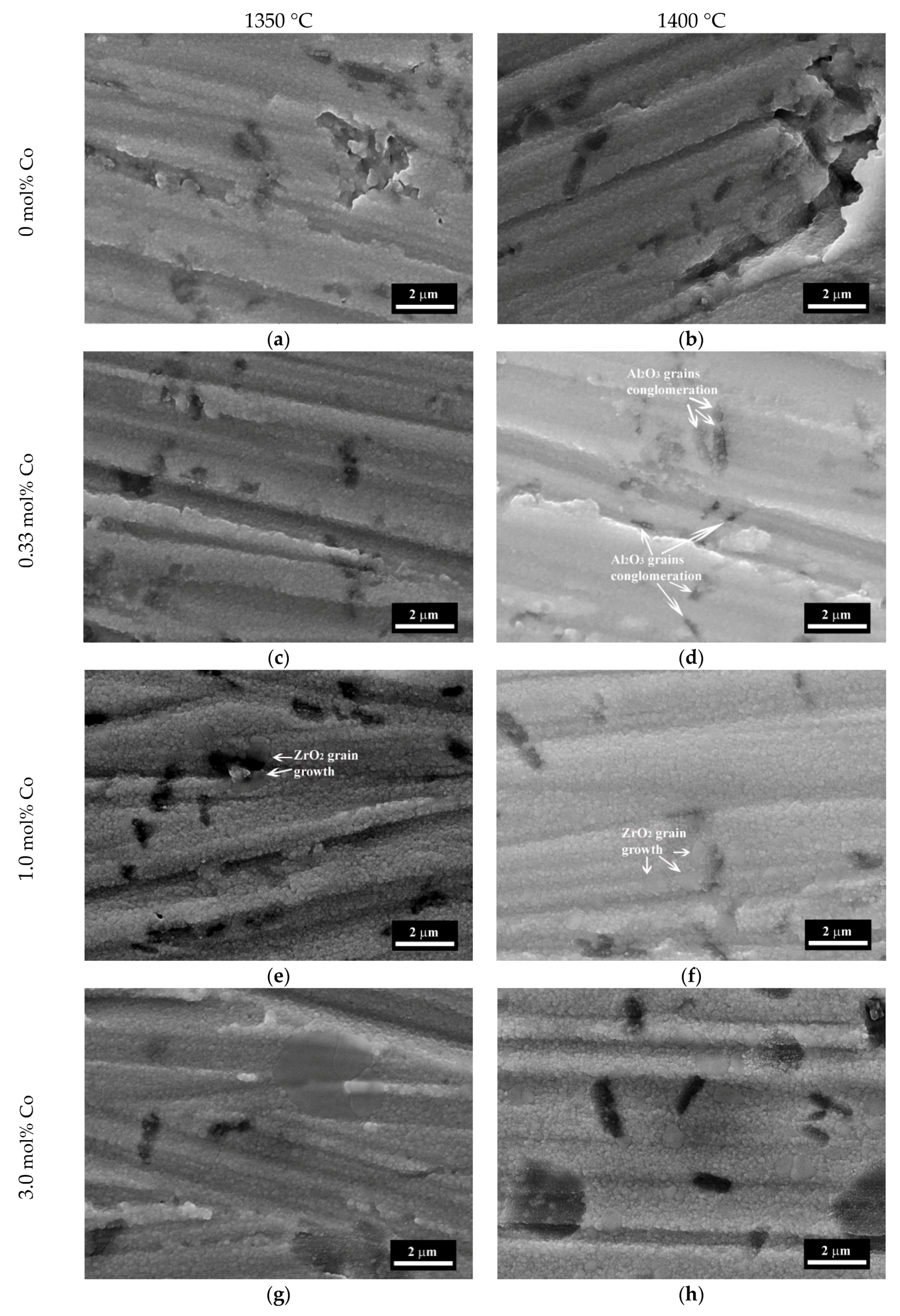
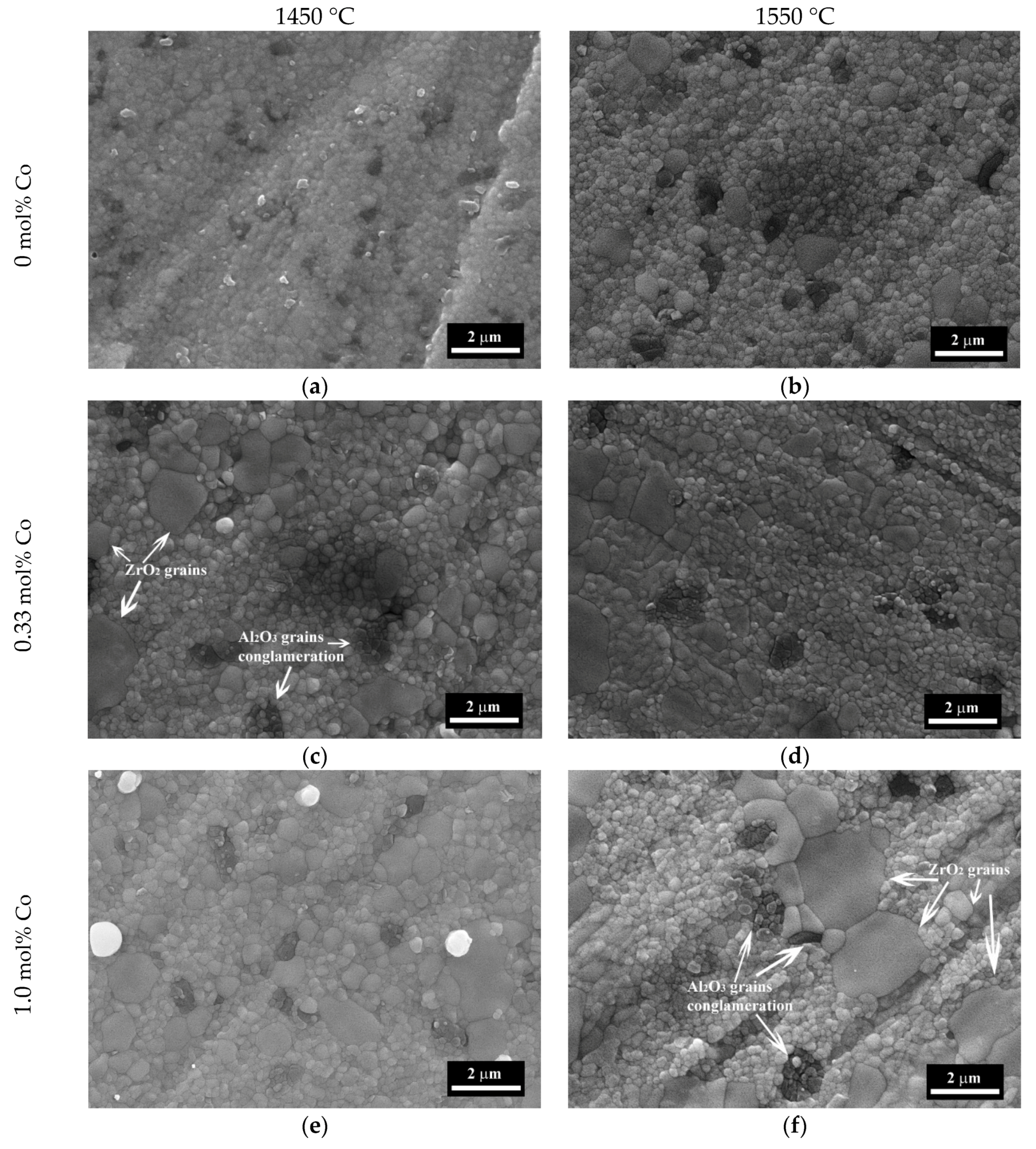
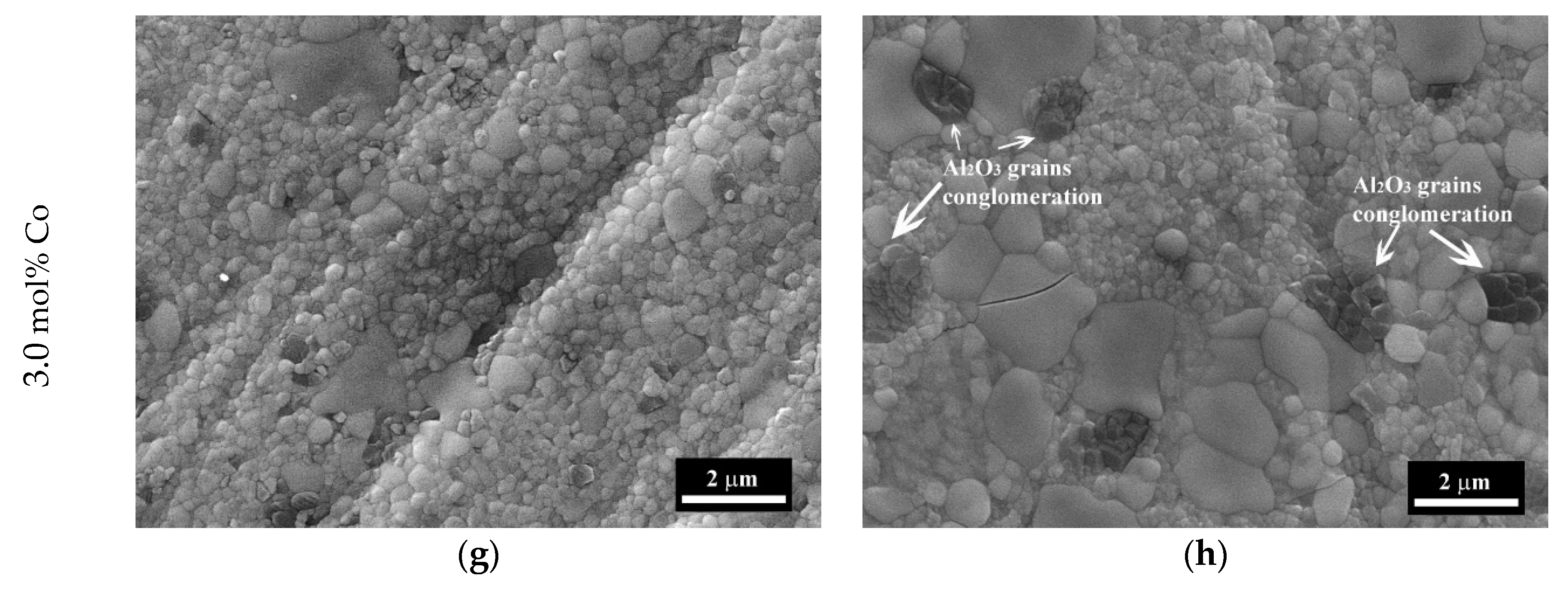
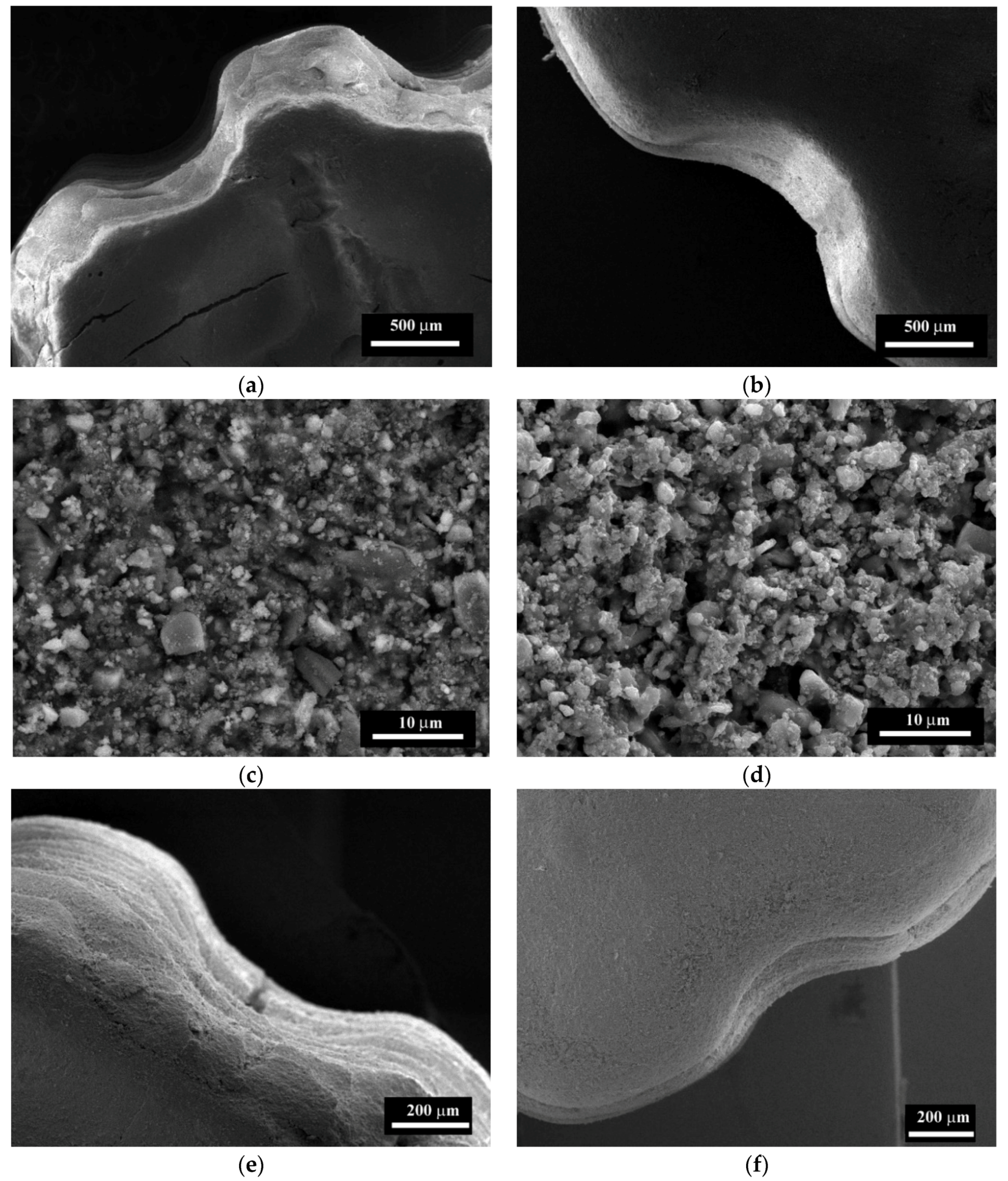
3.4. The Microstructure Investigations
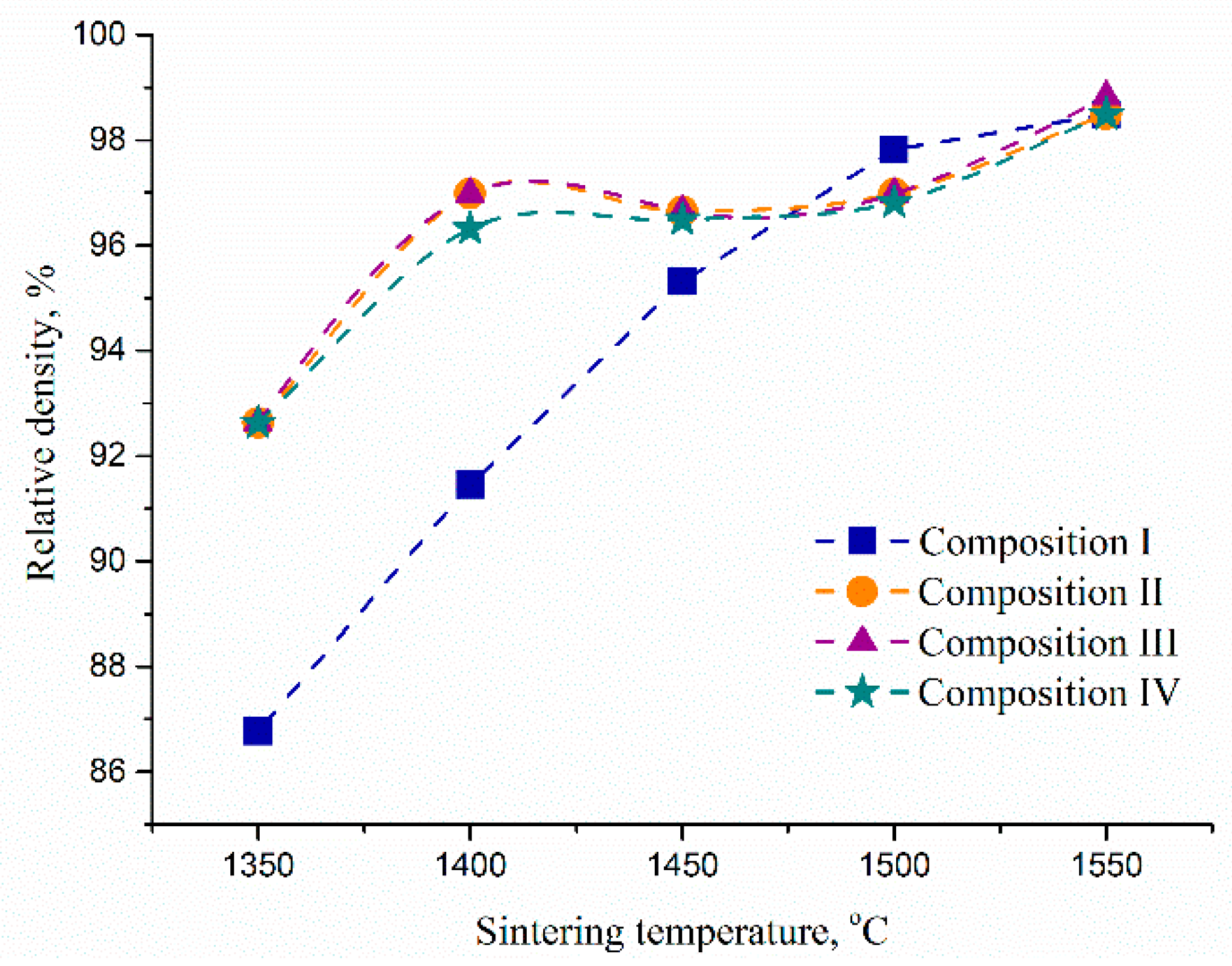
3.5. The Porosity, Density and Mechanical Properties of Ceramic Materials
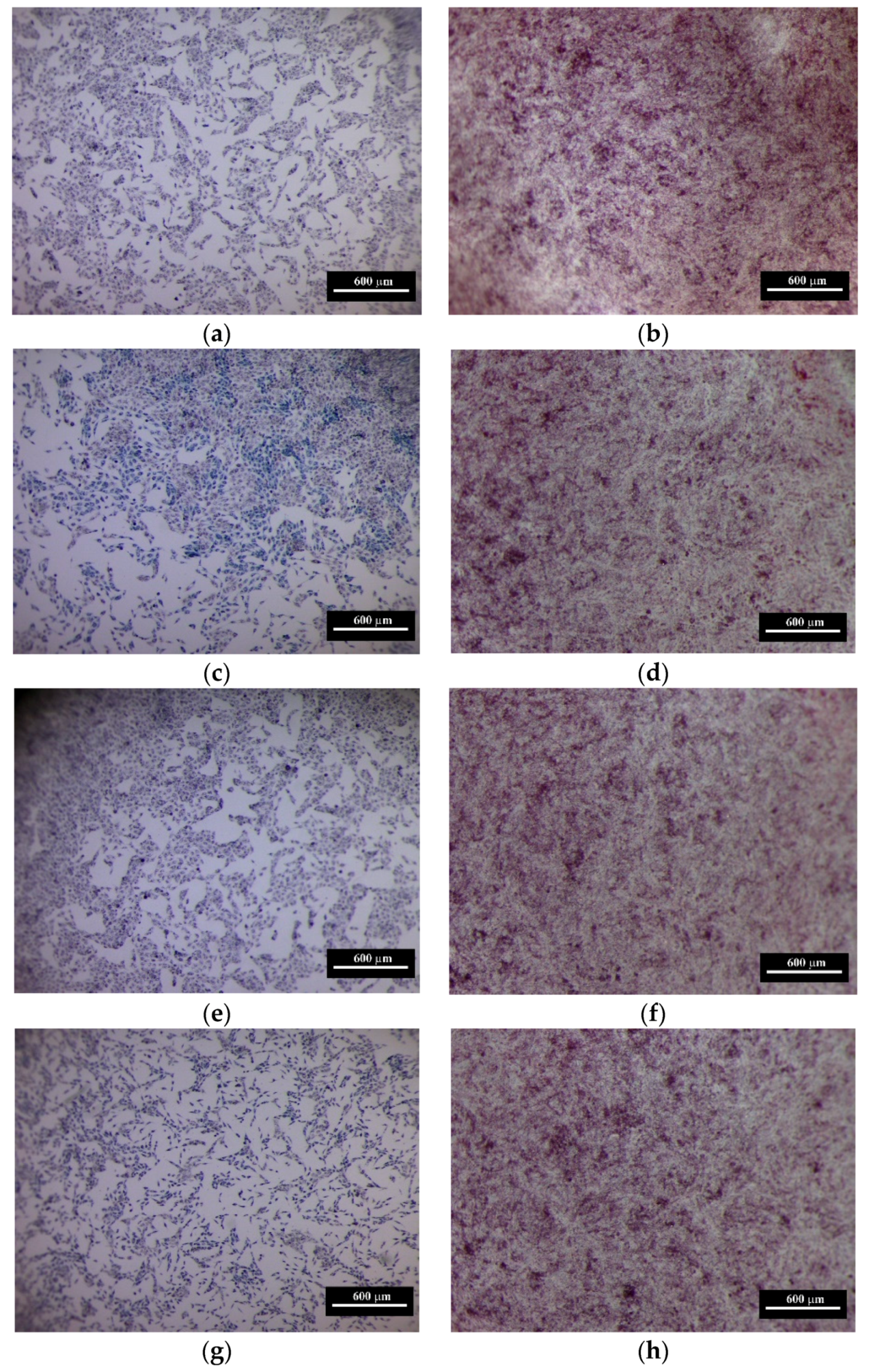
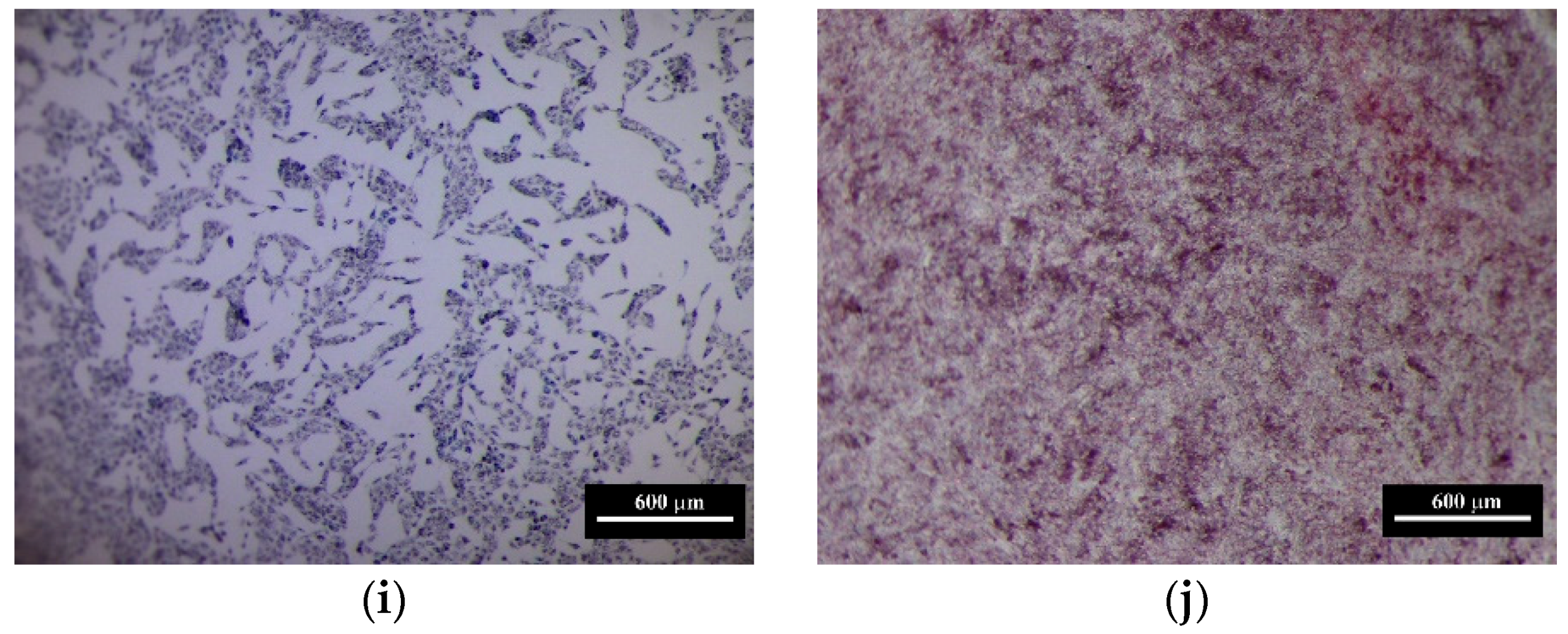
3.6. The Results of In Vitro Investigations
3.7. The DLP Printed and Sintered Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manicone, P.; Rossi Iommetti, P.; Raffaelli, L. An overview of zirconia ceramics: Basic properties and clinical applications. J. Dent. 2007, 35, 819–826. [Google Scholar] [CrossRef]
- Afzal, A. Implantable zirconia bioceramics for bone repair and replacement: A chronological review. Mater. Express 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Kagawa, M.; Kikuchi, M.; Syono, Y.; Nagae, T. Stability of Ultrafine Tetragonal ZrO2 Coprecipitated with Al2O3 by the Spray-ICP Technique. J. Am. Ceram. Soc. 1983, 66, 751–754. [Google Scholar] [CrossRef]
- Wu, Z.K.; Li, N.; Jian, C.; Zhao, W.Q.; Yan, J.Z. Low temperature degradation of Al2O3-doped 3Y-TZP sintered at various temperatures. Ceram. Int. 2013, 39, 7199–7204. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Daguano, J.; Santos, C.; Souza, R.; Balestra, R.; Strecker, K.; Elias, C. Properties of ZrO2–Al2O3 composite as a function of isothermal holding time. Int. J. Refract. Met. Hard Mater. 2007, 25, 374–379. [Google Scholar] [CrossRef]
- Abden, M.; Afroze, J.; Qadir, M.; Gafur, M.; Chowdhury, F. Correlation among composition, microstructure and mechanical properties of ZrO2 (Y2O3)/Al2O3 composite ceramics. Int. J. Mater. Eng. Innov. 2015, 6, 170. [Google Scholar] [CrossRef]
- Hwang, K.; Zhao, J.; Kim, J.; Lee, J. Dispersion of Nano Size ZrO2 in Al2O3/ZrO2 Ceramics by Hydrolysis. Procedia Manuf. 2015, 2, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, M.; Wu, H.; He, F.; Chen, Y.; Wu, S. Cure behavior of colorful ZrO2 suspensions during Digital light processing (DLP) based stereolithography process. J. Eur. Ceram. Soc. 2019, 39, 4921–4927. [Google Scholar] [CrossRef]
- Hong, J.; Gao, L.; Torre, S.; Miyamoto, H.; Miyamoto, K. Spark plasma sintering and mechanical properties of ZrO2(Y2O3)–Al2O3 composites. Mater. Lett. 2000, 43, 27–31. [Google Scholar] [CrossRef]
- Smirnov, V.; Krylov, A.; Smirnov, S.; Goldberg, M.; Antonova, O.; Shvorneva, L.; Barinov, S. Study of liquid-phase sintering of materials based on zirconium dioxide containing alumina. Inorg. Mater. Appl. Res. 2017, 8, 81–83. [Google Scholar] [CrossRef]
- Oelgardt, C.; Anderson, J.; Heinrich, J.; Messing, G. Sintering, microstructure and mechanical properties of Al2O3–Y2O3–ZrO2 (AYZ) eutectic composition ceramic microcomposites. J. Eur. Ceram. Soc. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Gil-Flores, L.; Salvador, M.; Penaranda-Foix, F.; Fernández, A.; Suarez, M.; Rosa, R.; Veronesi, P.; Leonelli, C.; Borrell, A. Microstructure and mechanical properties of 5.8 GHz microwave-sintered ZrO2/Al2O3 ceramics. Ceram. Int. 2019, 45, 18059–18064. [Google Scholar] [CrossRef]
- Flegler, A.; Burye, T.; Yang, Q.; Nicholas, J. Cubic yttria stabilized zirconia sintering additive impacts: A comparative study. Ceram. Int. 2014, 40, 16323–16335. [Google Scholar] [CrossRef]
- Obolkina, T.; Goldberg, M.; Smirnov, V.; Smirnov, S.; Titov, D.; Konovalov, A.; Kudryavtsev, E.; Antonova, O.; Barinov, S.; Komlev, V. Increasing the Sintering Rate and Strength of ZrO2–Al2O3 Ceramic Materials by Iron Oxide Additions. Inorg. Mater. 2020, 56, 182–189. [Google Scholar] [CrossRef]
- He, R.; Liu, W.; Wu, Z.; An, D.; Huang, M.; Wu, H.; Jiang, Q.; Ji, X.; Wu, S.; Xie, Z. Fabrication of complex-shaped zirconia ceramic parts via a DLP- stereolithography-based 3D printing method. Ceram. Int. 2018, 44, 3412–3416. [Google Scholar] [CrossRef]
- Zhang, K.; He, R.; Ding, G.; Feng, C.; Song, W.; Fang, D. Digital light processing of 3Y-TZP strengthened ZrO2 ceramics. Mater. Sci. Eng. A 2020, 774, 138768. [Google Scholar] [CrossRef]
- Osman, R.; van der Veen, A.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; a dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav. Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef]
- Ding, G.; He, R.; Zhang, K.; Xie, C.; Wang, M.; Yang, Y.; Fang, D. Stereolithography-based additive manufacturing of gray-colored SiC ceramic green body. J. Am. Ceram. Soc. 2019, 102, 7198–7209. [Google Scholar] [CrossRef]
- Llusar, M.; Forés, A.; Badenes, J.; Calbo, J.; Tena, M.; Monrós, G. Colour analysis of some cobalt-based blue pigments. J. Eur. Ceram. Soc. 2001, 21, 1121–1130. [Google Scholar] [CrossRef]
- Hartmanová, M.; Hanic, F.; Tunega, D. Structural and electro-optical properties of Co-doped yttria-stabilized zirconia. Chem. Pap. 1998, 52, 12–15. [Google Scholar]
- Lewis, G.; Atkinson, A.; Steele, B. Journal search results—Cite This for Me. J. Mater. Sci. Lett. 2001, 20, 1155–1157. [Google Scholar] [CrossRef]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A. Review paper Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 2, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Aherwar, A.; Singh, A.K.; Patnaik, A. Cobalt Based Alloy: A Better Choice Biomaterial for Hip Implants. Trends Biomater. Artif. Organs 2016, 30, 50–55. [Google Scholar] [CrossRef]
- Rittidech, A.; Somrit, R.; Tunkasiri, T. Effect of adding Y2O3 on structural and mechanical properties of Al2O3–ZrO2 ceramics. Ceram. Int. 2013, 39, S433–S436. [Google Scholar] [CrossRef]
- Borlaf, M.; Serra-Capdevila, A.; Colominas, C.; Graule, T. Development of UV-curable ZrO2 slurries for additive manufacturing (LCM-DLP) technology. J. Eur. Ceram. Soc. 2019, 39, 3797–3803. [Google Scholar] [CrossRef]
- de l’Eclairage, Commision Internationale. Recommendations on Uniform Color Spaces, Color-Difference Equations, Psychometric Color Terms; CIE: Paris, France, 1978. [Google Scholar]
- Borrell, A.; Salvador, M.D.; Rayón, E.; Penaranda-Foix, F.L. Improvement of microstructural properties of 3Y-TZP materials by conventional and non-conventional sintering techniques. Ceram. Int. 2012, 38, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Sumita, S. Influence of oxide additives, firing temperature, and dispersing media on sintered Al2O3. J. Ceram. Soc. Jpn. 1991, 99, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Maca, K.; Trunec, M.; Chmelik, R. Processing and properties of fine-grained transparent MgAl2O4 ceramics. Ceram. Silik. 2007, 51, 94. [Google Scholar]
- Viswanath, B.; Ravishankar, N.; Nayar, S.; Sinha, A. Synthesis, Sintering and Microstructural Characterization of Nanocrystalline Hydroxyapatite Composites. MRS Proc. 2004, 845. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Lakusta, M.; Danilenko, I.; Konstantinova, T.; Volkova, G.; Nosolev, I.; Gorban, O.; Syniakina, S.; Burkhovetskiy, V. The Effect of a Small Amount SiO2 on Sintering Kinetics of Tetragonal Zirconia Nanopowders. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Li, J.; Zhou, H.; Chen, J. Microstructure and mechanical properties of yttria-stabilized ZrO2/Al2O3 nanocomposite ceramics. Ceram. Int. 2008, 34, 1797–1803. [Google Scholar] [CrossRef]
- Chiou, Y.; Lin, S. Influence of CoO and Al2O3 on the phase partitioning of ZrO2-3 mol% Y2O3. Ceram. Int. 1996, 22, 249–256. [Google Scholar] [CrossRef]
- Cava, S.; Tebcherani, S.M.; Pianaro, S.A.; Paskocimas, C.A.; Longo, E.; Varela, J.A. Structural and spectroscopic analysis of -Al2O2 to α-Al2O3-CoAl2O4 phase transition. Mater. Chem. Phys. 2006, 102–108. [Google Scholar] [CrossRef]
- Tsigara, A.; Mountrichas, G.; Gatsouli, K.; Nichelatti, A.; Pispas, S.; Madamopoulos, N.; Vainos, N.A.; Du, H.L.; Roubani-Kalantzopoulou, F. Hybrid polymer/cobalt chloride humidity sensors based on optical diffraction. Sens. Actuators B Chem. 2007, 120, 481–486. [Google Scholar] [CrossRef]
- You, M.H.; Yan, X.; Zhang, J.; Wang, X.X.; He, X.X.; Yu, M.; Ning, X.; Long, Y.Z. Colorimetric humidity sensors based on electrospun polyamide/CoCl2 nanofibrous membranes. Nanoscale Res. Lett. 2017, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Zou, J. Synthesis and characterization of blue TiO2/CoAl2O4 complex pigments with good colour and enhanced near-infrared reflectance properties. RSC Adv. 2015, 5, 87932–87939. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, M.H.; Lee, D.Y.; Han, J.S. Mechanical properties, phase stability, and biocompatibility of (Y, Nb)-TZP/Al2O3 composite abutments for dental implant. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 53, 438–443. [Google Scholar] [CrossRef]
- Podzorova, L.I.; Shvorneva, L.I.; Il’icheva, A.A.; Alad’ev, N.A.; Pen’kova, O.I. Microstructure and phase composition of ZrO2-CeO2-Al2O3 materials modified with MgO and Y2O3. Inorg. Mater. 2013, 49, 376–381. [Google Scholar] [CrossRef]
- Tsukrenko, V.; Dudnik, E.; Shevchenko, A. Nanocrystalline zirconia based powders synthesized by hydrothermal method. Process. Appl. Ceram. 2012, 6, 151–157. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Virkar, A.; Clarke, D. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar] [CrossRef]
- Pavia, A.; Laurent, C.; Weibel, A.; Peigney, A.; Chevallier, G.; Estournès, C. Hardness and friction behavior of bulk CoAl2O4 and Co–Al2O3 composite layers formed during Spark Plasma Sintering of CoAl2O4 powders. Ceram. Int. 2012, 38, 5209–5217. [Google Scholar] [CrossRef]
- Trunec, M. Effect of grain size on mechanical properties of 3Y-TZP ceramics. Ceramics–Silikáty 2008, 52, 165–171. [Google Scholar]
- Santos, C.; Teixeira, L.H.P.; Daguano, J.K.M.F.; Rogero, S.O.; Strecker, K.; Elias, C.N. Mechanical properties and cytotoxicity of 3Y-TZP bioceramics reinforced with Al2O3 particles. Ceram. Int. 2009, 35, 709–718. [Google Scholar] [CrossRef]
- Lison, D.; Van den Brule, S.; Van Maele-Fabry, G. Cobalt and its compounds: Update on genotoxic and carcinogenic activities. Crit. Rev. Toxicol. 2018, 48, 522–539. [Google Scholar] [CrossRef]
- Fleury, C.; Petit, A.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Tabrizian, M.; Huk, O.L. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: Morphology, cytotoxicity, and oxidative stress. Biomaterial 2006, 27, 3351–3360. [Google Scholar] [CrossRef]
- Stopford, W.; Turner, J.; Cappellini, D.; Brock, T. Bioaccessibility testing of cobalt compounds. J. Environ. Monit. 2003, 5, 675–680. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Reinosa, J.J.; Del Campo, A.; Fernández, J.F. 2D particles forming a nanostructured shell: A step forward cool NIR reflectivity for CoAl2O4 pigments. Dye. Pigment. 2017, 137, 1–11. [Google Scholar] [CrossRef]
- Barrioni, B.R.; de Laia, A.G.S.; Valverde, T.M.; da Mata Martins, T.M.; Caliari, M.V.; de Sa, M.A.; de Goes, A.M.; de Magalhães Pereira, M. Evaluation of in vitro and in vivo biocompatibility and structure of cobalt-releasing sol-gel bioactive glass. Ceram. Int. 2018, 44, 20337–20347. [Google Scholar] [CrossRef]
- Quinlan, E.; Partap, S.; Azevedo, M.M.; Jell, G.; Stevens, M.M.; O’Brien, F.J. Hypoxia-mimicking bioactive glass/collagen glycosaminoglycan composite scaffolds to enhance angiogenesis and bone repair. Biomaterials 2015, 52, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, C.; Li, H.; Yuen, J.; Chang, J.; Xiao, Y. Preparation, characterization and in vitro angiogenic capacity of cobalt substituted β-tricalcium phosphate ceramics. J. Mater. Chem. 2012, 22, 21686–21694. [Google Scholar] [CrossRef]
- Ciapetti, G.; Cenni, E.; Pratelli, L.; Pizzoferrato, A. In vitro evaluation of cell/biomaterial interaction by MTT assay. Biomaterials 1993, 14, 359–364. [Google Scholar] [CrossRef]
- Promakhov, V.; Zhukov, A.; Dubkova, Y.; Zhukov, I.; Kovalchuk, S.; Zhukova, T.; Olisov, A.; Klimenko, V.; Savkina, N. Structure and properties of ZrO2–20% Al2O3 ceramic composites obtained using additive technologies. Materials 2018, 11, 2361. [Google Scholar] [CrossRef] [Green Version]


| Materials | Sintering Temperature, °C | ||||
|---|---|---|---|---|---|
| 1350 | 1400 | 1450 | 1500 | 1550 | |
| Composition I | 9.09 | 4.07 | 0.06 | 0.12 | 0 |
| Composition II | 2.40 | 0.06 | 0.12 | 0.08 | 0 |
| Composition III | 2.74 | 0.06 | 0.22 | 0.08 | 0 |
| Composition IV | 2.27 | 0.24 | 0.16 | 0.09 | 0.05 |
| Materials | pH Value of Extract, CGM | Time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 48 | 72 | ||||||||
| OD, a.u. (M ± m) | PVC, % | TI, % | OD, a.u. (M ± m) | PVC, % | TI, % | OD, a.u. (M ± m) | PVC, % | TI, % | ||
| Composition I | 7.4 | 0.219 ± 0.002 | 89.4 | 10.6 | 0.440 ± 0.010 | 90.9 | 9.1 | 0.633 ± 0.016 | 80.8 | 19.2 |
| Composition II | 7.4 | 0.242 ± 0.002 | 98.8 | 1.2 | 0.446 ± 0.001 | 92.1 | 7.9 | 0.632 ± 0.005 | 80.7 | 19.3 |
| Composition III | 7.4 | 0.252 ± 0.04 | 102.9 | 0.0 | 0.454 ± 0.006 | 93.8 | 6.2 | 0.651 ± 0.006 | 83.1 | 16.9 |
| Composition IV | 7.4 | 0.241 ± 0.007 | 98.4 | 1.6 | 0.457 ± 0.003 | 94.4 | 5.6 | 0.678 ± 0.002 | 86.6 | 13.4 |
| Control (CGM) | 7.3 | 0.245 ± 0.009 | 100.0 | 0.0 | 0.484 ± 0.003 | 100.0 | 0.0 | 0.783 ± 0.007 | 100.0 | 0.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldberg, M.; Obolkina, T.; Smirnov, S.; Protsenko, P.; Titov, D.; Antonova, O.; Konovalov, A.; Kudryavtsev, E.; Sviridova, I.; Kirsanova, V.; et al. The Influence of Co Additive on the Sintering, Mechanical Properties, Cytocompatibility, and Digital Light Processing Based Stereolithography of 3Y-TZP-5Al2O3 Ceramics. Materials 2020, 13, 2789. https://doi.org/10.3390/ma13122789
Goldberg M, Obolkina T, Smirnov S, Protsenko P, Titov D, Antonova O, Konovalov A, Kudryavtsev E, Sviridova I, Kirsanova V, et al. The Influence of Co Additive on the Sintering, Mechanical Properties, Cytocompatibility, and Digital Light Processing Based Stereolithography of 3Y-TZP-5Al2O3 Ceramics. Materials. 2020; 13(12):2789. https://doi.org/10.3390/ma13122789
Chicago/Turabian StyleGoldberg, Margarita, Tatiana Obolkina, Sergey Smirnov, Pavel Protsenko, Dmitriy Titov, Olga Antonova, Anatoliy Konovalov, Egor Kudryavtsev, Irina Sviridova, Valentina Kirsanova, and et al. 2020. "The Influence of Co Additive on the Sintering, Mechanical Properties, Cytocompatibility, and Digital Light Processing Based Stereolithography of 3Y-TZP-5Al2O3 Ceramics" Materials 13, no. 12: 2789. https://doi.org/10.3390/ma13122789





