Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading
Abstract
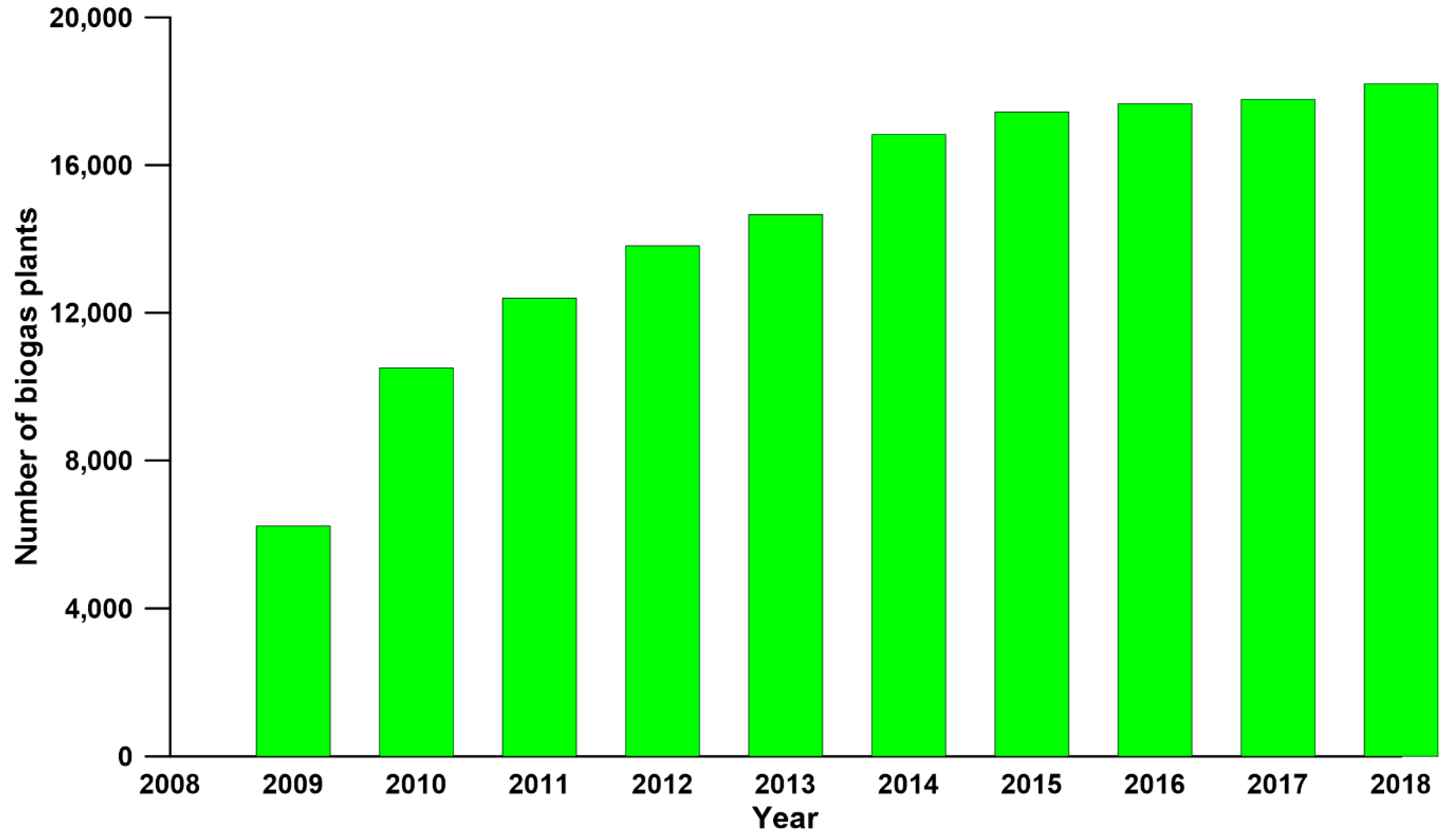
:1. Introduction
2. Materials, Experimental Equipment, and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
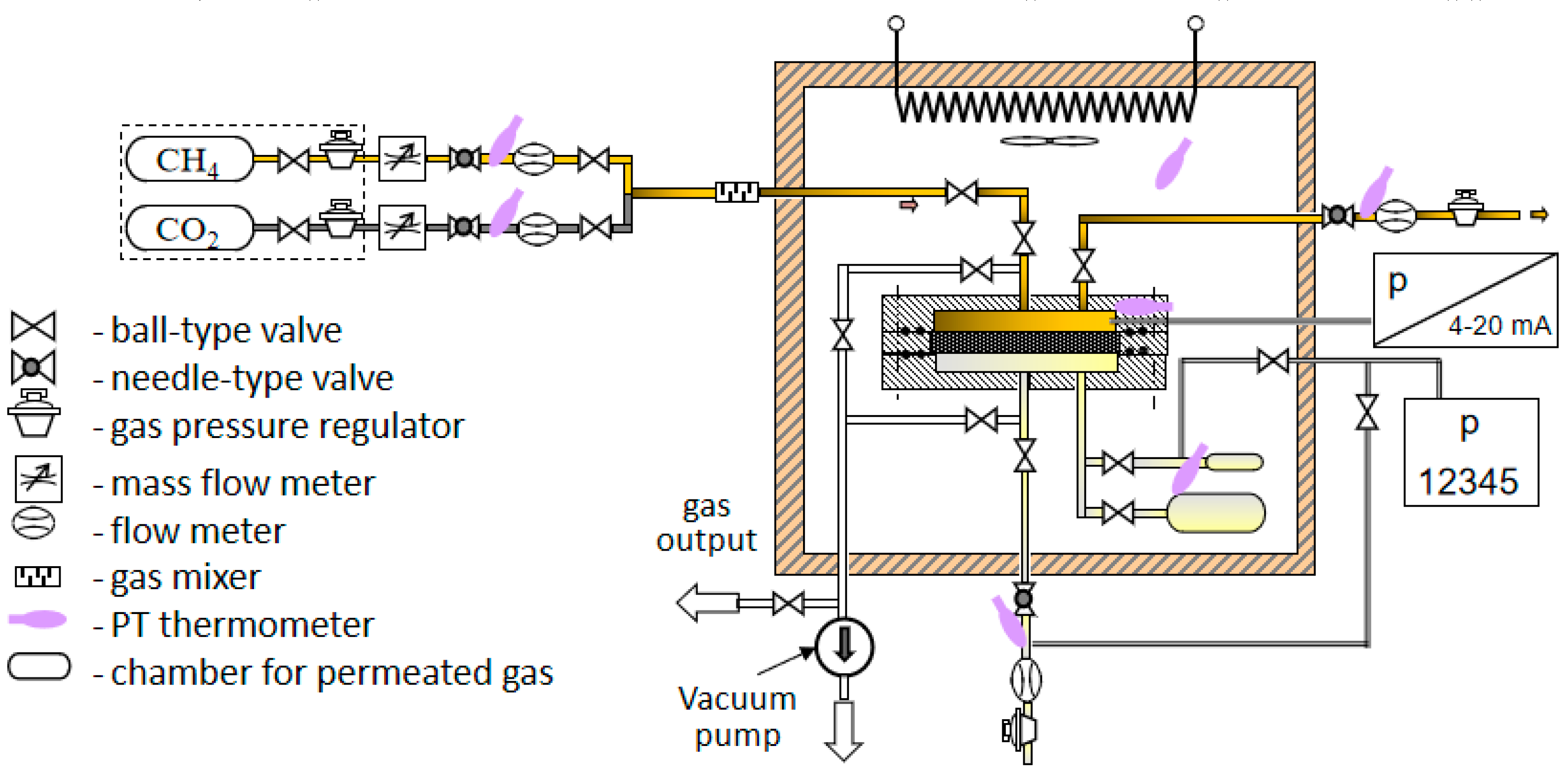
2.4. Experimental Set-Up for Gas Permeation Measurements
2.5. Gas Permeation Test
3. Results and Discussion
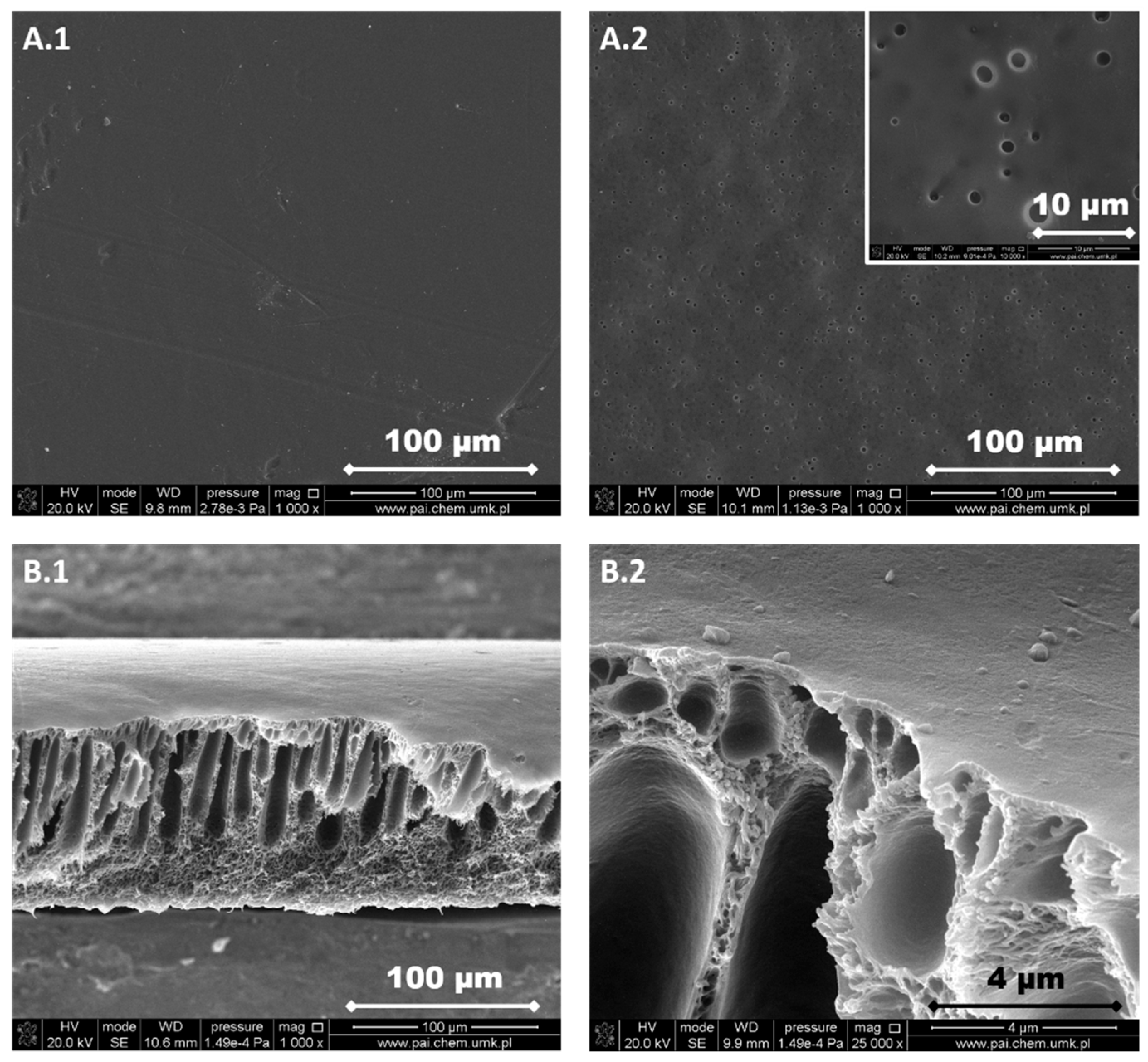
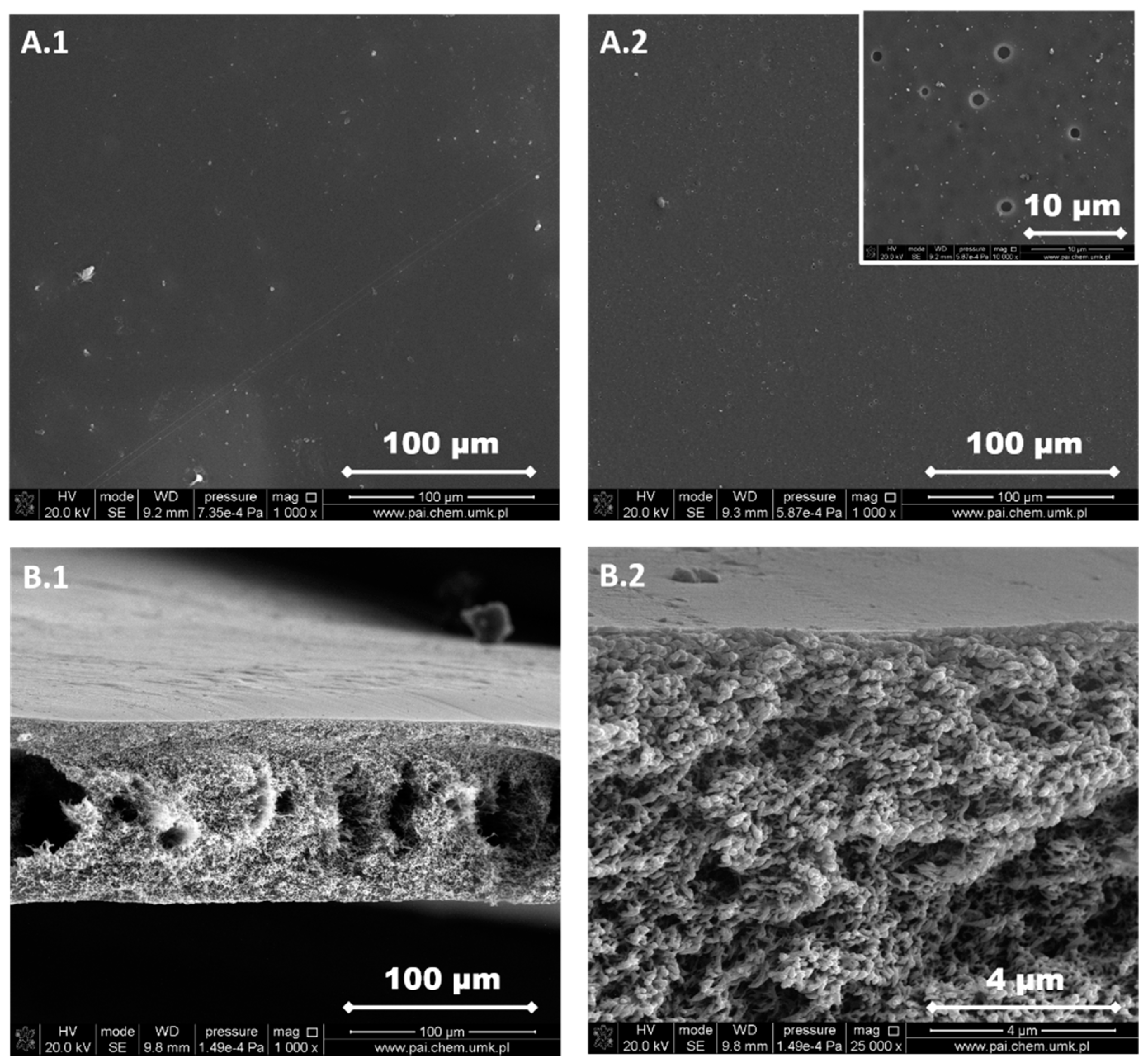
3.1. Structure and Morphology of the Investigated PPSU Membranes
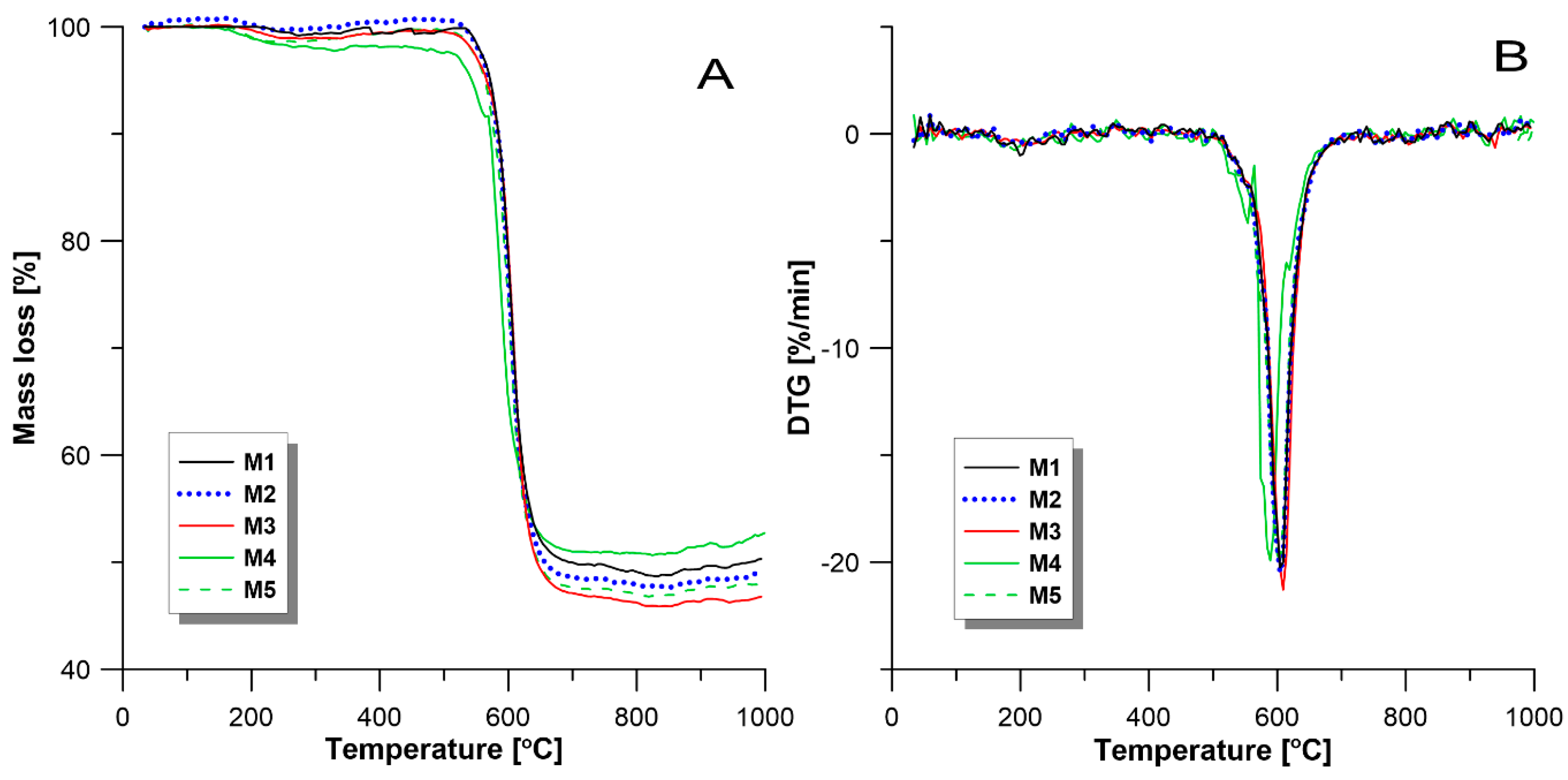
3.2. Thermal Properties
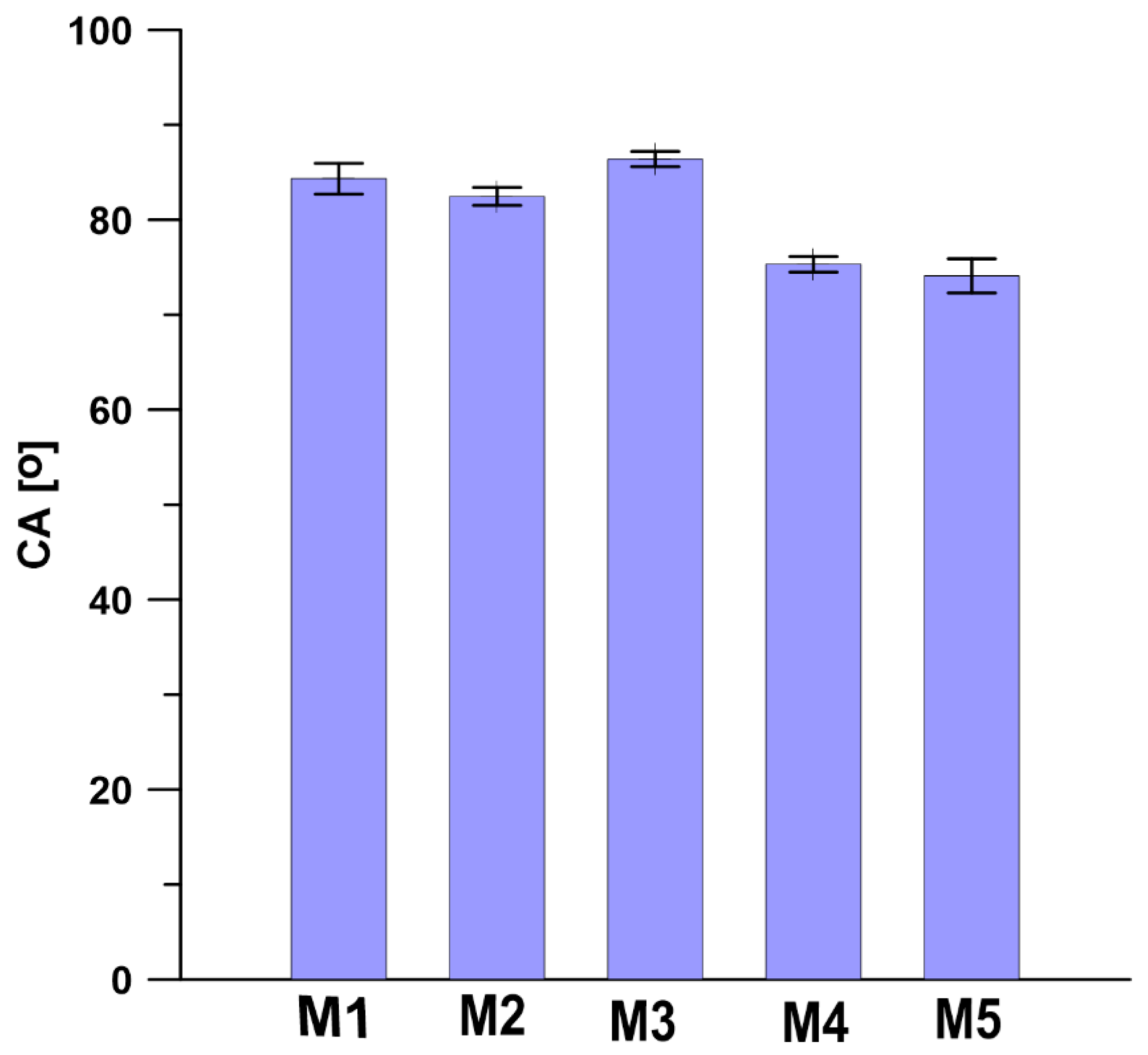
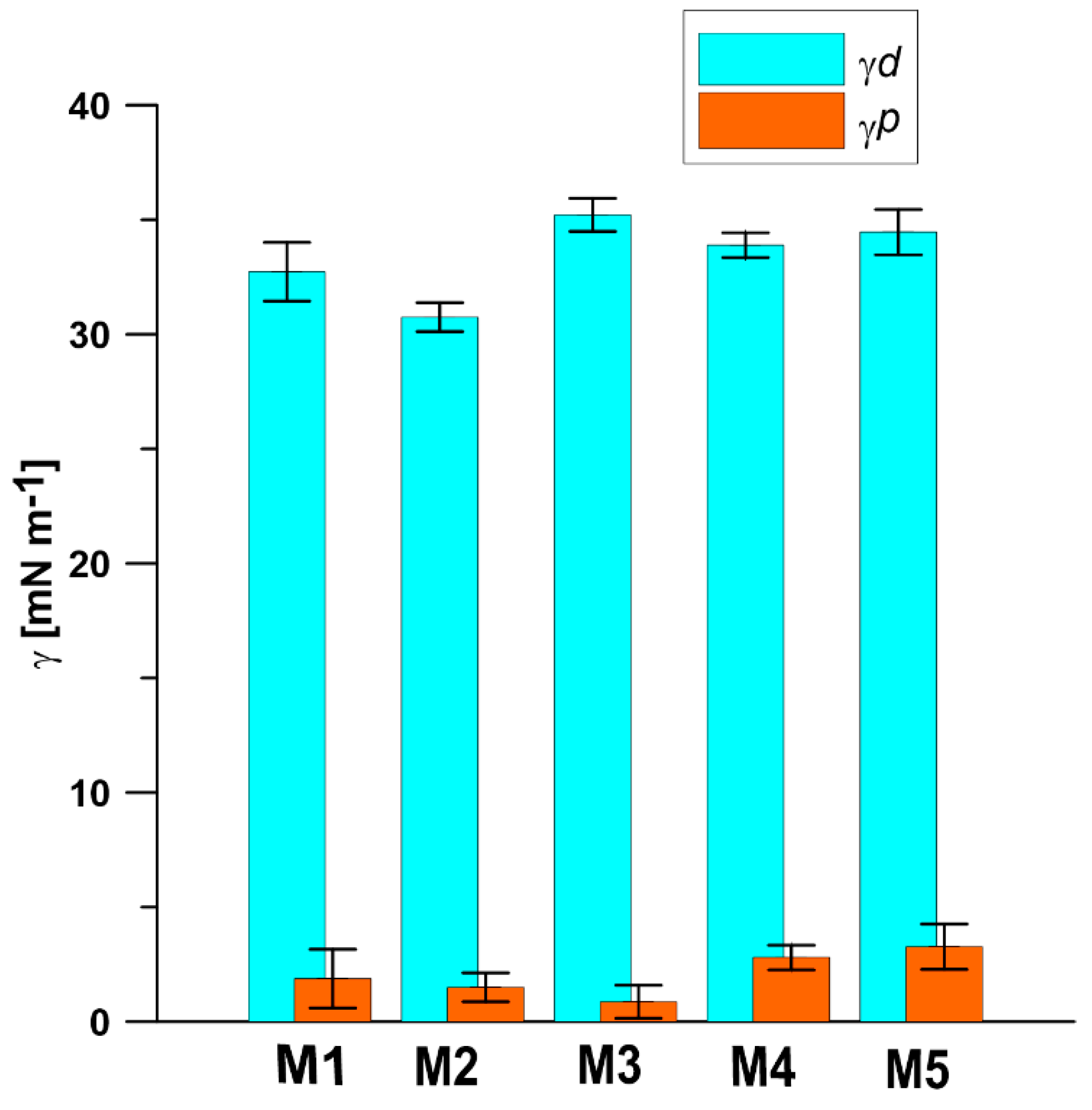
3.3. Contact Angle (CA) and Surface Free Energy (SFE)
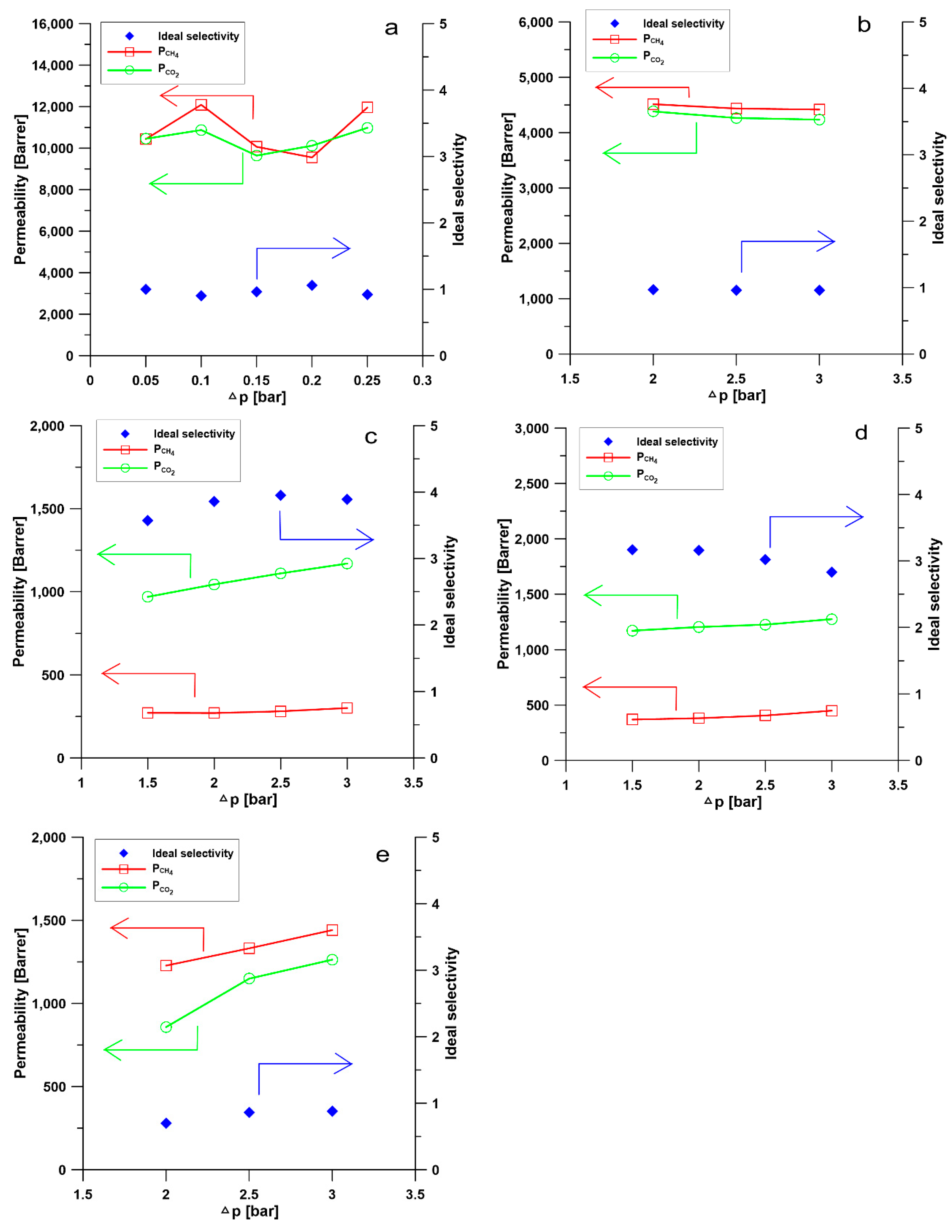
3.4. Investigation of Gas Permeability and Separation Performance of Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rongwong, W.; Boributh, S.; Assabumrungrat, S.; Laosiripojana, N.; Jiraratananon, R. Simultaneous Absorption of CO2 and H2S from Biogas by Capillary Membrane Contactor. J. Memb. Sci. 2012, 392, 38–47. [Google Scholar] [CrossRef]
- Scholz, M.; Alders, M.; Lohaus, T.; Wessling, M. Structural Optimization of Membrane-Based Biogas Upgrading Processes. J. Memb. Sci. 2015, 474, 1–10. [Google Scholar] [CrossRef]
- European Biogas Association. European Biogas Association Annual Report; European Biogas Association: Brussels, Belgium, 2019. [Google Scholar]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and Perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Lee, M.; Malde, C.; Wang, R. Development of Low Mass-Transfer-Resistance Fluorinated TiO2-SiO2/PVDF Composite Hollow Fiber Membrane Used for Biogas Upgrading in Gas-Liquid Membrane Contactor. J. Memb. Sci. 2018, 552, 253–264. [Google Scholar] [CrossRef]
- Song, C.; Fan, Z.; Li, R.; Liu, Q.; Kitamura, Y. Efficient Biogas Upgrading by a Novel Membrane-Cryogenic Hybrid Process: Experiment and Simulation Study. J. Memb. Sci. 2018, 565, 194–202. [Google Scholar] [CrossRef]
- Heile, S.; Rosenberger, S.; Parker, A.; Jefferson, B.; McAdam, E.J. Establishing the Suitability of Symmetric Ultrathin Wall Polydimethylsiloxane Hollow-Fibre Membrane Contactors for Enhanced CO2 Separation during Biogas Upgrading. J. Memb. Sci. 2014, 452, 37–45. [Google Scholar] [CrossRef]
- Lorenzo-Hernando, A.; Belaissaoui, B.; Albarracin Zaidiza, D.; Chabanon, E.; Castel, C.; Claveria-Baro, J.; Favre, E.; Roizard, D.; Rode, S. Potentialities of a Dense Skin Hollow Fiber Membrane Contactor for Biogas Purification by Pressurized Water Absorption. J. Memb. Sci. 2016, 513, 236–249. [Google Scholar]
- Abdeen, F.R.H.; Mel, M.; Jami, M.S.; Ihsan, S.I.; Ismail, A.F. A Review of Chemical Absorption of Carbon Dioxide for Biogas Upgrading. Chin. J. Chem. Eng. 2016, 24, 693–702. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Cryogenic Technology for Biogas Upgrading Combined with Carbon Capture-a Review of Systems and Property Impacts. Energy Procedia 2017, 142, 3741–3746. [Google Scholar] [CrossRef]
- Vivo-Vilches, J.F.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Faria, R.P.V.; Ribeiro, A.M.; Ferreira, A.F.P.; Rodrigues, A.E. Biogas Upgrading by Selective Adsorption onto CO2 activated Carbon from Wood Pellets. J. Environ. Chem. Eng. 2017, 5, 1386–1393. [Google Scholar] [CrossRef]
- Hashemi, S.E.; Sarker, S.; Lien, K.M.; Schnell, S.K.; Austbø, B. Cryogenic vs. Absorption Biogas Upgrading in Liquefied Biomethane Production–An Energy Efficiency Analysis. Fuel 2019, 245, 294–304. [Google Scholar] [CrossRef]
- Wang, Y.; Christopher, M.P.; Wang, Y.; Ying, Y.; Liu, G.; Cheng, Y.; Zhao, D.; Zhai, L.; Long, S.; Dong, J. Mixed Matrix Membranes Containing MOF@COF Hybrid Fillers for Efficient CO2/CH4 Separation. J. Memb. Sci. 2018, 573, 97–106. [Google Scholar]
- Yang, L.; Zhang, S.; Wu, H.; Ye, C.; Liang, X.; Wang, S.; Wu, X.; Wu, Y.; Ren, Y.; Liu, Y.; et al. Porous Organosilicon Nanotubes in Pebax-Based Mixed-Matrix Membranes for Biogas Purification. J. Memb. Sci. 2019, 573, 301–308. [Google Scholar] [CrossRef]
- Robeson, L.M. The Upper Bound Revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Sethunga, G.S.M.D.P.; Rongwong, W.; Wang, R.; Bae, T.H. Optimization of Hydrophobic Modification Parameters of Microporous Polyvinylidene Fluoride Hollow-Fiber Membrane for Biogas Recovery from Anaerobic Membrane Bioreactor Effluent. J. Memb. Sci. 2018, 548, 510–518. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Rongwong, W.; Chuah, C.Y.; Wang, R.; Bae, T.H. Polymer-Fluorinated Silica Composite Hollow Fiber Membranes for the Recovery of Biogas Dissolved in Anaerobic Effluent. J. Memb. Sci. 2017, 540, 146–154. [Google Scholar] [CrossRef]
- Shamsabadi, A.A.; Seidi, F.; Salehi, E.; Nozari, M.; Rahimpour, A.; Soroush, M. Efficient CO2-Removal Using Novel Mixed-Matrix Membranes with Modified TiO2 Nanoparticles. J. Mater. Chem. A 2017, 5, 4011–4025. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, W.; Yang, K.; Dai, Y.; Ruan, X.; Yan, X.; He, G. Amino-Functional ZIF-8 Nanocrystals by Microemulsion Based Mixed Linker Strategy and the Enhanced CO2/N2 Separation. Sep. Purif. Technol. 2020, 236, 116209. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. CO2/CH4 Separation by High Performance Co-Casted ZIF-8/Pebax 1657/PES Mixed Matrix Membrane. J. Nat. Gas Sci. Eng. 2016, 31, 562–574. [Google Scholar] [CrossRef]
- Sheng, L.; Guo, Y.; Zhao, D.; Ren, J.; Wang, S.; Deng, M. Enhanced CO2/CH4 Separation Performance of BTDA-TDI/MDI (P84) Copolyimide Mixed-Matrix Membranes by Incorporating Submicrometer-Sized [Ni3(HCOO)6] Framework Crystals. J. Nat. Gas Sci. Eng. 2020, 75, 103123. [Google Scholar] [CrossRef]
- Kamio, E.; Kasahara, S.; Moghadam, F.; Matsuyama, H. Development of Facilitated Transport Membranes Composed of a Dense Gel Layer Containing CO2 Carrier Formed on Porous Cylindrical Support Membranes. Chem. Eng. Res. Des. 2020, 153, 284–293. [Google Scholar] [CrossRef]
- Wang, M.; Quan, K.; Zheng, X.; Cao, Y.; Cui, X.; Xue, M.; Pan, F. Facilitated Transport Membranes by Incorporating Self-Exfoliated Covalent Organic Nanosheets for CO2/CH4 Separation. Sep. Purif. Technol. 2020, 237, 116457. [Google Scholar] [CrossRef]
- Lilleby Helberg, R.M.; Dai, Z.; Ansaloni, L.; Deng, L. PVA/PVP Blend Polymer Matrix for Hosting Carriers in Facilitated Transport Membranes: Synergistic Enhancement of CO2 Separation Performance. Green Energy Environ. 2019, 5, 59–68. [Google Scholar] [CrossRef]
- Gray, S.; Paul Chen, J.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Ismail, A.F. A Review on Polyamide Thin Film Nanocomposite (TFN) Membranes: History, Applications, Challenges and Approaches. Water Res. 2015, 80, 306–324. [Google Scholar]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.S. Recent Membrane Development for Pervaporation Processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Lipnizki, F.; Field, R.W.; Ten, P. Pervaporation-Based Hybrid Process: A Review of Process Design, Applications and Economics. J. Memb. Sci. 1999, 153, 183–210. [Google Scholar] [CrossRef]
- Heidari, M.; Hosseini, S.S.; Omidkhah Nasrin, M.; Ghadimi, A. Synthesis and Fabrication of Adsorptive Carbon Nanoparticles (ACNs)/PDMS Mixed Matrix Membranes for Efficient CO2/CH4 and C3H8/CH4 Separation. Sep. Purif. Technol. 2019, 209, 503–515. [Google Scholar] [CrossRef]
- Ye, L.; Wang, L.; Jie, X.; Yu, C.; Kang, G.; Cao, Y. The Evolution of Free Volume and Gas Transport Properties for the Thermal Rearrangement of Poly(Hydroxyamide-Co-Amide)s Membranes. J. Memb. Sci. 2019, 573, 21–35. [Google Scholar] [CrossRef]
- Mazinani, S.; Ramezani, R.; Molelekwa, G.F.; Darvishmanesh, S.; Di Felice, R.; Van der Bruggen, B. Plasticization Suppression and CO2 Separation Enhancement of Matrimid through Homogeneous Blending with a New High Performance Polymer. J. Memb. Sci. 2019, 574, 318–324. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Enhancing CO2 Separation Performance of Pebax® MH-1657 with Aromatic Carboxylic Acids. Sep. Purif. Technol. 2019, 212, 901–912. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent Progress on Fabrication Methods of Polymeric Thin Film Gas Separation Membranes for CO2 Capture. J. Memb. Sci. 2019, 572, 38–60. [Google Scholar] [CrossRef]
- Choi, O.; Ingole, P.G.; Lee, H.-K. Preparation and Characterization of Thin Film Composite Membrane for the Removal of Water Vapor from the Flue Gas at Bench Scale. Sep. Purif. Technol. 2019, 211, 401–407. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Ansaloni, L.; Janakiram, S.; Deng, L. Thin-Film-Composite Hollow Fiber Membranes Containing Amino Acid Salts as Mobile Carriers for CO2 Separation. J. Memb. Sci. 2019, 578, 61–68. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, D.; Ren, J.; Qiu, Y.; Deng, M. A Novel Pebax-C60(OH)24/PAN Thin Film Composite Membrane for Carbon Dioxide Capture. Sep. Purif. Technol. 2019, 215, 480–489. [Google Scholar] [CrossRef]
- Chao, W.; Huang, S.; An, Q.; Liaw, D.; Huang, Y. Novel Interfacially-Polymerized Polyamide Thin- Fi Lm Composite Membranes: Studies on Characterization, Pervaporation, and Positron Annihilation Spectroscopy. Polymer 2011, 52, 2414–2421. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Zuo, J.; Lai, J.; Chung, T. In-Situ Synthesis and Cross-Linking of Polyamide Thin Film Composite ( TFC ) Membranes for Bioethanol Applications. J. Memb. Sci. 2014, 458, 47–57. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Li, Q.; Liu, G.; Liu, G.; Jin, W. Mixed-Matrix Hollow Fiber Composite Membranes Comprising of PEBA and MOF for Pervaporation Separation of Ethanol/Water Mixtures. Sep. Purif. Technol. 2019, 214, 2–10. [Google Scholar] [CrossRef]
- Irshad, M.; Ingole, P.G.; Kil, W.; Ryong, S.; Chul, E.; Keun, H. Development of Carboxylated TiO2 Incorporated Thin Fi Lm Nanocomposite Hollow Fi Ber Membranes for Fl Ue Gas Dehydration. J. Memb. Sci. 2016, 514, 622–635. [Google Scholar]
- Zulkifli, N.I.; Goh, P.S.; Wong, K.C.; Lau, W.J.; Ismail, A.F. Carbon Nanotube Incorporated Nanocomposite Membranes for CO2 Removal. J. Appl. Membr. Sci. Technol. 2017, 17. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent Developments in Zeolite Membranes for Gas Separation. J. Memb. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Kouketsu, T.; Duan, S.; Kai, T.; Kazama, S.; Yamada, K. PAMAM Dendrimer Composite Membrane for CO2 Separation: Formation of a Chitosan Gutter Layer. J. Memb. Sci. 2007, 287, 51–59. [Google Scholar] [CrossRef]
- Peter, J.; Peinemann, K.-V. Multilayer Composite Membranes for Gas Separation Based on Crosslinked PTMSP Gutter Layer and Partially Crosslinked Matrimid® 5218 Selective Layer. J. Memb. Sci. 2009, 340, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Tseng, H.-H.; Chang, S.-H.; Wey, M.-Y. A Carbon Gutter Layer-Modified α-Al2O3 Substrate for PPO Membrane Fabrication and CO2 Separation. J. Memb. Sci. 2014, 454, 51–61. [Google Scholar] [CrossRef]
- Yoo, M.J.; Kim, K.H.; Lee, J.H.; Kim, T.W.; Chung, C.W.; Cho, Y.H.; Park, H.B. Ultrathin Gutter Layer for High-Performance Thin-Film Composite Membranes for CO2 Separation. J. Memb. Sci. 2018, 566, 336–345. [Google Scholar] [CrossRef]
- Zeng, C.; Tao, J.; Lai, J.; Chung, T. High-Performance Multiple-Layer PIM Composite Hollow Fi Ber Membranes for Gas Separation. J. Memb. Sci. 2018, 563, 93–106. [Google Scholar]
- Momeni, M.; Kojabad, M.E.; Khanmohammadi, S.; Farhadi, Z.; Ghalandarzadeh, R.; Babaluo, A.; Zare, M. Impact of Support on the Fabrication of Poly (Ether-b-Amide) Composite Membrane and Economic Evaluation for Natural Gas Sweetening. J. Nat. Gas Sci. Eng. 2019, 62, 236–246. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, G.; Yu, H.; Jin, Y.; Cao, Y. Fabrication of a Highly Permeable Composite Nanofiltration Membrane via Interfacial Polymerization by Adding a Novel Acyl Chloride Monomer with an Anhydride Group. J. Memb. Sci. 2019, 570, 403–409. [Google Scholar] [CrossRef]
- Sharabati, J.-A.-D.; Guclu, S.; Erkoc-Ilter, S.; Koseoglu-Imer, D.Y.; Unal, S.; Menceloglu, Y.Z.; Ozturk, I.; Koyuncu, I. Interfacially Polymerized Thin-Film Composite Membranes: Impact of Support Layer Pore Size on Active Layer Polymerization and Seawater Desalination Performance. Sep. Purif. Technol. 2019, 212, 438–448. [Google Scholar] [CrossRef]
- Kwon, H.-E.; Kwon, S.J.; Park, S.-J.; Shin, M.G.; Park, S.-H.; Park, M.S.; Park, H.; Lee, J.-H. High Performance Polyacrylonitrile-Supported Forward Osmosis Membranes Prepared via Aromatic Solvent-Based Interfacial Polymerization. Sep. Purif. Technol. 2019, 212, 449–457. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Mao, Y.; Liu, G.; Jin, W. Effect of Substrate on Formation and Nanofiltration Performance of Graphene Oxide Membranes. J. Memb. Sci. 2019, 574, 196–204. [Google Scholar] [CrossRef]
- Teplyakov, V.V.; Shalygin, M.G.; Kozlova, A.A.; Netrusov, A.I. Composite Membranes with a Polyvinyltrimethylsilane Skin Layer for Separation of Water–Alcohol Mixtures. Pet. Chem. 2018, 58, 949–957. [Google Scholar] [CrossRef]
- Gonzales, R.; Park, M.; Tijing, L.; Han, D.; Phuntsho, S.; Shon, H. Modification of Nanofiber Support Layer for Thin Film Composite Forward Osmosis Membranes via Layer-by-Layer Polyelectrolyte Deposition. Membranes 2018, 8, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Ying, Y.; Peng, X. High Efficient Thin-Film Composite Membrane: Ultrathin Hydrophilic Polyamide Film on Macroporous Superhydrophobic Polytetrafluoroethylene Substrate. Appl. Mater. Today 2017, 8, 54–59. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, J.; Wang, S. Recent Developments in Membranes for Efficient Hydrogen Purification. J. Memb. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.; Zhang, J.; Li, X. Facilitating CO2 Transport Across Mixed Matrix Membranes Containing Multifunctional Nanocapsules. ACS Appl. Mater. Interfaces 2018, 10, 43031–43039. [Google Scholar] [CrossRef]
- Akhmetshina, A.; Yanbikov, N.; Atlaskin, A.; Trubyanov, M.; Mechergui, A.; Otvagina, K.; Razov, E.; Mochalova, A.; Vorotyntsev, I. Acidic Gases Separation from Gas Mixtures on the Supported Ionic Liquid Membranes Providing the Facilitated and Solution-Diffusion Transport Mechanisms. Membranes 2019, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Sadrzadeh, M.; Rezakazemi, M.; Mohammadi, T. Fundamentals and Measurement Techniques for Gas Transport in Polymers; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Kentish, S.; Scholes, C.; Stevens, G. Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications. Recent Patents Chem. Eng. 2012, 1, 52–66. [Google Scholar]
- Rea, R.; De Angelis, M.G.; Baschetti, M.G. Models for Facilitated Transport Membranes: A Review. Membranes 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Sundell, B.J.; Harrigan, D.J.; Hayden, S.C.; Vaughn, J.T.; Guzan, K.A.; Lawrence III, J.A.; Ostraat, M.L. Improved Gas Transport Properties of Cellulose Acetate via Sub-Tg Acid-Catalyzed Silanation. J. Memb. Sci. 2019, 573, 448–454. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L. Effect of Spinning Conditions on the Fabrication of Cellulose Acetate Hollow Fiber Membrane for CO2 Separation from N2 and CH4. Polym. Test. 2019, 73, 1–11. [Google Scholar] [CrossRef]
- Moghadassi, A.R.; Rajabi, Z.; Hosseini, S.M.; Mohammadi, M. Preparation and Characterization of Polycarbonate-Blend-Raw/Functionalized Multi-Walled Carbon Nano Tubes Mixed Matrix Membrane for CO2 Separation. Sep. Sci. Technol. 2013, 48, 1261–1271. [Google Scholar] [CrossRef]
- González-Díaz, M.O.; Sulub-Sulub, R.; Herrera-Kao, W.; Vázquez-Torres, H.; Zolotukhin, M.G.; Aguilar-Vega, M. Enhanced Gas Transport Performance of Polyamide Membranes by Postpolymerization Modification. Ind. Eng. Chem. Res. 2018, 57, 8989–8996. [Google Scholar] [CrossRef]
- Awad, A.; Aljundi, I.H. Layer-by-Layer Assembly of Carbide Derived Carbon-Polyamide Membrane for CO2 Separation from Natural Gas. Energy 2018, 157, 188–199. [Google Scholar] [CrossRef]
- Karimi, S.; Firouzfar, E.; Khoshchehreh, M.R. Assessment of Gas Separation Properties and CO2 Plasticization of Polysulfone/Polyethylene Glycol Membranes. J. Pet. Sci. Eng. 2019, 173, 13–19. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, D.; Pang, J. Preparation of High-Performance Antifouling Polyphenylsulfone Ultrafiltration Membrane by the Addition of Sulfonated Polyaniline. J. Polym. Res. 2018, 25, 66. [Google Scholar] [CrossRef]
- Jansen, J.C.; Darvishmanesh, S.; Tasselli, F.; Bazzarelli, F.; Bernardo, P.; Tocci, E.; Friess, K.; Randova, A.; Drioli, E.; Van der Bruggen, B. Influence of the Blend Composition on the Properties and Separation Performance of Novel Solvent Resistant Polyphenylsulfone/Polyimide Nanofiltration Membranes. J. Memb. Sci. 2013, 447, 107–118. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of High Flux Ultrafiltration Polyphenylsulfone Membranes Applying the Systems with Upper and Lower Critical Solution Temperatures: Effect of Polyethylene Glycol Molecular Weight and Coagulation Bath Temperature. J. Memb. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Yong, W.F.; Lee, Z.K.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Blends of a Polymer of Intrinsic Microporosity and Partially Sulfonated Polyphenylenesulfone for Gas Separation. ChemSusChem 2016, 9, 1953–1962. [Google Scholar] [CrossRef]
- Jomekian, A.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Innovative Layer by Layer and Continuous Growth Methods for Synthesis of ZIF-8 Membrane on Porous Polymeric Support Using Poly(Ether- Block -Amide) as Structure Directing Agent for Gas Separation. Microporous Mesoporous Mater. 2016, 234, 43–54. [Google Scholar] [CrossRef]
- Yousef, S.; Sarwar, Z.; Šereika, J.; Striūgas, N.; Krugly, E.; Danilovas, P.P.; Martuzevicius, D. A New Industrial Technology for Mass Production of Graphene/PEBA Membranes for CO2/CH4 Selectivity with High Dispersion, Thermal and Mechanical Performance. Polymers 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, T.H.; Tseng, H.H.; Wey, M.Y. Preparation and Characterization of PPSU/PBNPI Blend Membrane for Hydrogen Separation. Int. J. Hydrogen Energy 2008, 33, 4178–4182. [Google Scholar] [CrossRef]
- Jullok, N.; Van Hooghten, R.; Luis, P.; Volodin, A.; Van Haesendonck, C.; Vermant, J.; Van der Bruggen, B. Effect of Silica Nanoparticles in Mixed Matrix Membranes for Pervaporation Dehydration of Acetic Acid Aqueous Solution: Plant-Inspired Dewatering Systems. J. Clean. Prod. 2016, 112, 4879–4889. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of Polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Kujawa, J.; Rozicka, A.; Cerneaux, S.; Kujawski, W. The Influence of Surface Modification on the Physicochemical Properties of Ceramic Membranes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 567–575. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Li, G.; Kujawski, W. Fabrication of PDMS Based Membranes with Improved Separation Efficiency in Hydrophobic Pervaporation. Sep. Purif. Technol. 2020, 234, 116092. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Zadhoush, A. Investigation of the Relation between Viscoelastic Properties of Polysulfone Solutions, Phase Inversion Process and Membrane Morphology: The Effect of Solvent Power. J. Memb. Sci. 2017, 532, 47–57. [Google Scholar] [CrossRef]
- Pereira, V.R.; Isloor, A.M.; Bhat, U.K.; Ismail, A.F. Preparation and Antifouling Properties of PVDF Ultrafiltration Membranes with Polyaniline (PANI) Nanofibers and Hydrolysed PSMA (H-PSMA) as Additives. Desalination 2014, 351, 220–227. [Google Scholar] [CrossRef]
- Karlsson, L.E.; Jannasch, P. Polysulfone Ionomers for Proton-Conducting Fuel Cell Membranes: 2. Sulfophenylated Polysulfones and Polyphenylsulfones. Electrochim. Acta 2005, 50, 1939–1946. [Google Scholar] [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane Engineering: Latest Advancements in Gas Separation and Pre-Treatment Processes, Petrochemical Industry and Refinery, and Future Perspectives in Emerging Applications. Fuel Process. Technol. 2020, 206, 106464. [Google Scholar] [CrossRef]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. High Permeation and Antifouling Polysulfone Ultrafiltration Membranes with in Situ Synthesized Silica Nanoparticles. Mater. Today Commun. 2020, 22, 100784. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Pereira, J.M.; Trilles, C.A.; Aquino, R.R.; Huang, S.H.; Lee, K.R.; Lai, J.Y. Performance and Antifouling Behavior of Thin-Film Nanocomposite Nanofiltration Membranes with Embedded Silica Spheres. Sep. Purif. Technol. 2019, 210, 521–529. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Raisi, A.; Aroujalian, A. Modeling of Gas Solubility and Permeability in Glassy and Rubbery Membranes Using Lattice Fluid Theory. Polymer 2017, 115, 184–196. [Google Scholar] [CrossRef]
- Naderi, A.; Yong, W.F.; Xiao, Y.; Chung, T.S.; Weber, M.; Maletzko, C. Effects of Chemical Structure on Gas Transport Properties of Polyethersulfone Polymers. Polymer 2018, 135, 76–84. [Google Scholar] [CrossRef]
- Minelli, M.; Oradei, S.; Fiorini, M.; Sarti, G.C. CO2 Plasticization Effect on Glassy Polymeric Membranes. Polymer 2019, 163, 29–35. [Google Scholar] [CrossRef]
- Saedi, S.; Madaeni, S.S.; Shamsabadi, A.A. Fabrication of Asymmetric Polyethersulfone Membranes for Separation of Carbon Dioxide from Methane Using Polyetherimide as Polymeric Additive. Chem. Eng. Res. Des. 2014, 92, 2431–2438. [Google Scholar] [CrossRef]
- Hu, C.-C.; Cheng, P.-H.; Chou, S.-C.; Lai, C.-L.; Huang, S.-H.; Tsai, H.-A.; Hung, W.-S.; Lee, K.-R. Separation Behavior of Amorphous Amino-Modified Silica Nanoparticle/Polyimide Mixed Matrix Membranes for Gas Separation. J. Memb. Sci. 2020, 595, 117542. [Google Scholar] [CrossRef]
- Azizi, N.; Mohammadi, T.; Mosayebi Behbahani, R. Comparison of Permeability Performance of PEBAX-1074/TiO2, PEBAX-1074/SiO2 and PEBAX-1074/Al2O3 Nanocomposite Membranes for CO2/CH4 Separation. Chem. Eng. Res. Des. 2017, 117, 177–189. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. Asymmetric Membrane Based on Matrimid® and Polysulphone Blends for Enhanced Permeance and Stability in Binary Gas (CO2/CH4) Mixture Separations. Sep. Purif. Technol. 2010, 75, 15–21. [Google Scholar] [CrossRef]

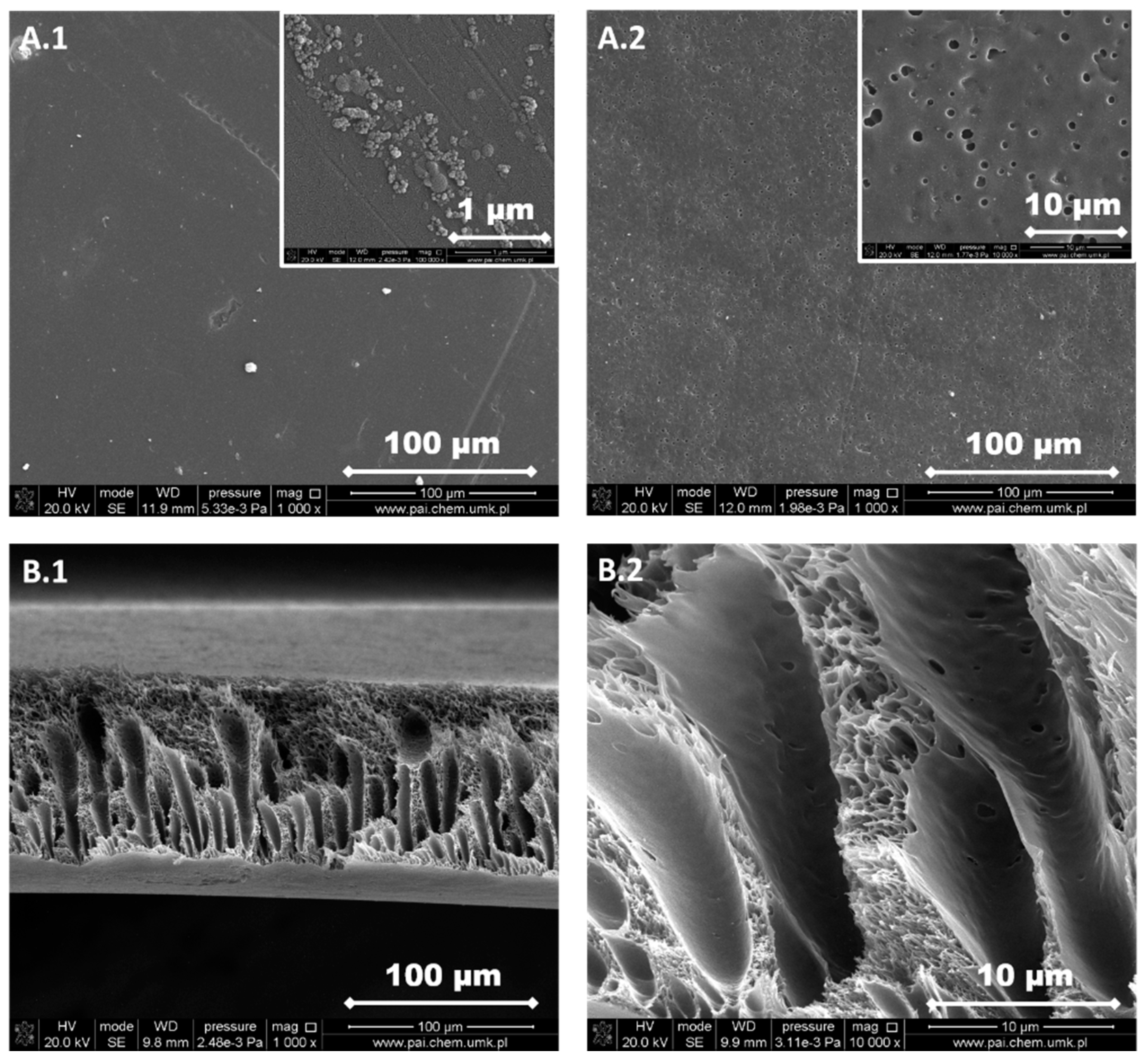
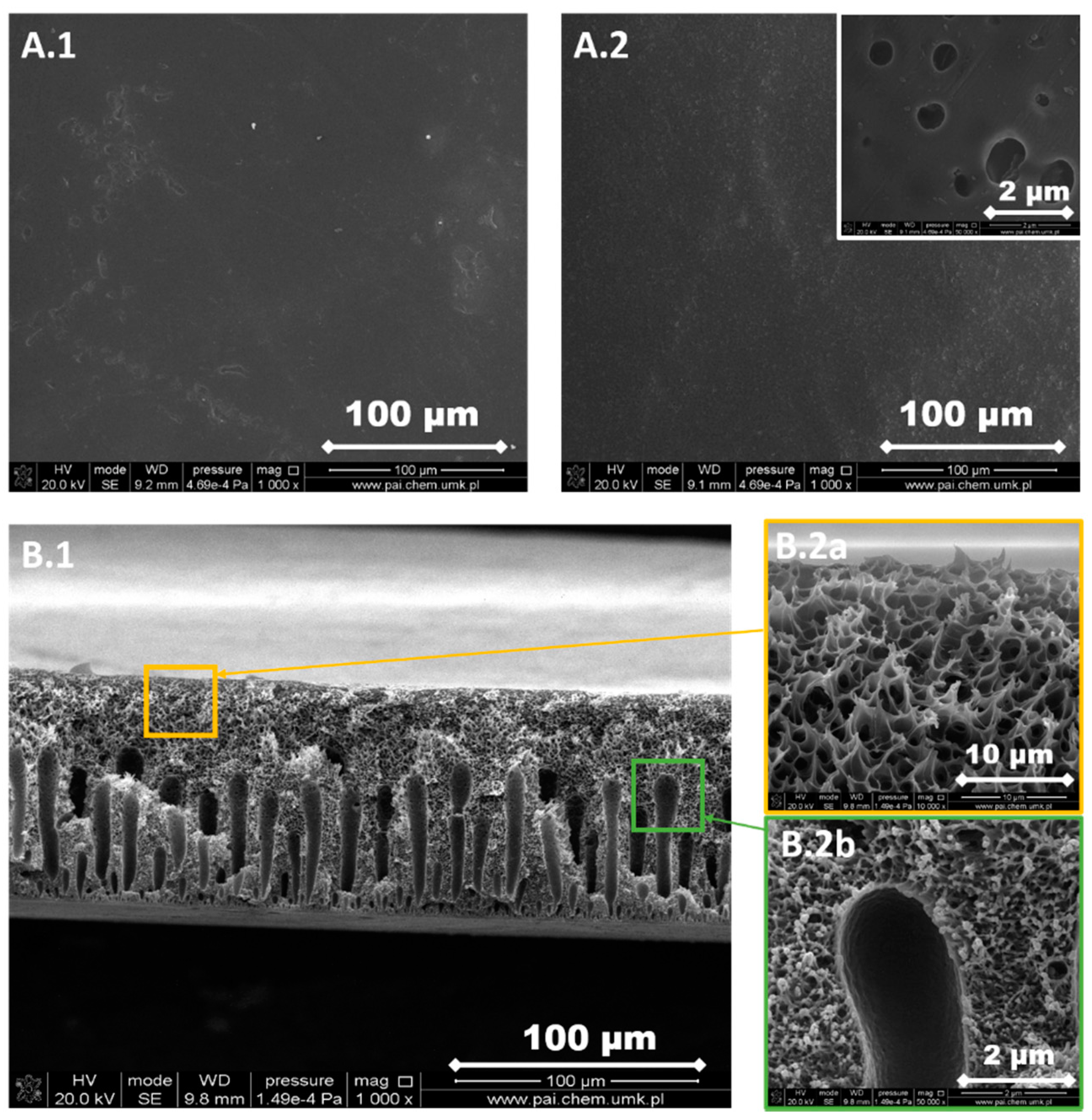
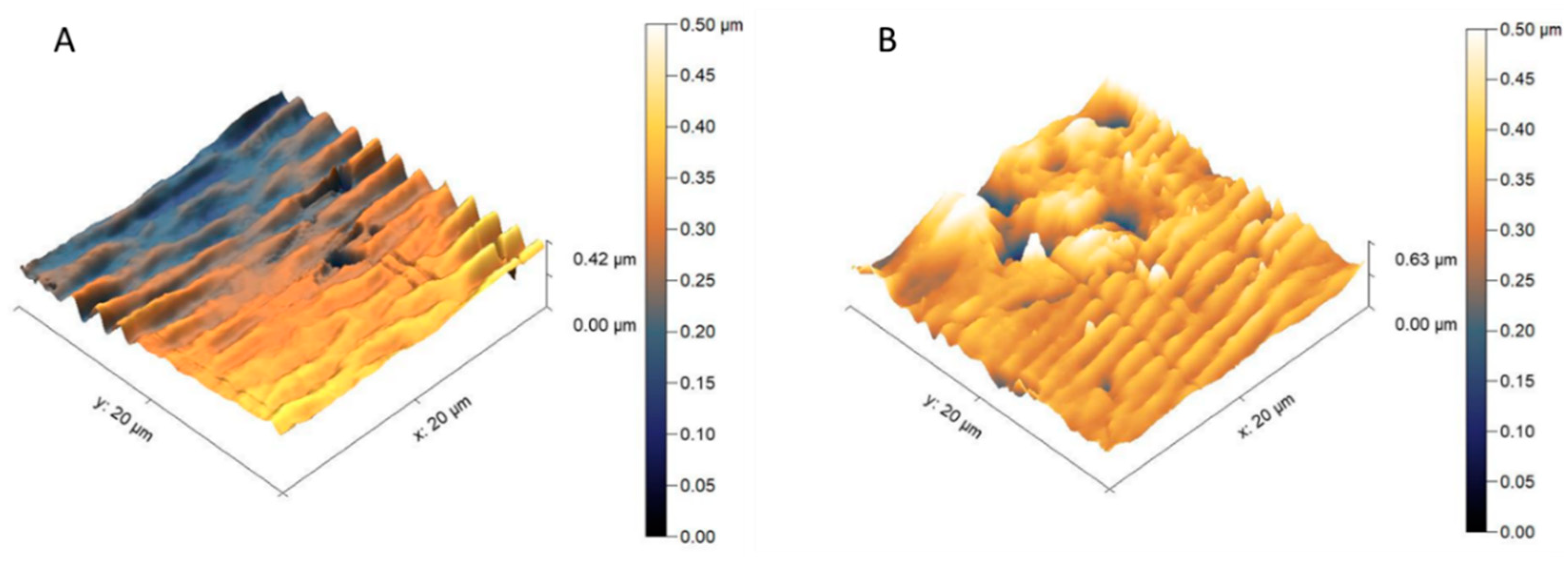
| Type of Membranes | Composition of Polymer Solution | |||
|---|---|---|---|---|
| PPSU [wt.%] | NMP [wt.%] | Glycerin [wt.%] | SN [wt.%] | |
| M1 | 30.0 | 70.0 | - | - |
| M2 | 27.5 | 64.5 | 8 | - |
| M3 | 27.5 | 62.5 | 10 | - |
| M4 | 27.5 | 72.4 | - | 0.1 |
| M5 | 27.5 | 72.2 | - | 0.3 |
| Membranes | Membrane Thickness [μm] | Layer Function | Layer Thickness [μm] |
|---|---|---|---|
| M1 | 101 ± 3.9 | Selective | 0.33 ± 0.03 |
| Supporting | 100 ± 3.8 | ||
| M2 | 135 ± 1.0 | Selective | 3.8 ± 0.63 |
| Supporting | 131 ± 0.86 | ||
| M3 | 98 ± 1.0 | Selective | 1.2 ± 0.13 |
| Supporting | 96 ± 0.89 | ||
| M4 | 90 ± 4.5 | Selective | 0.43 ± 0.07 |
| Supporting | 89 ± 4.6 | ||
| M5 | 110 ± 0.35 | Selective | 1.7 ± 0.14 |
| Supporting | 108 ± 0.30 |
| Membranes | Mass Loss [%] | The Temperature of Decomposition [°C] | |
|---|---|---|---|
| M1 | 51.87 | 603.8 | |
| M2 | 52.62 | 605.3 | |
| M3 | 53.94 | 609.8 | |
| M4 | 48.37 | 589.3 | |
| M5 | 52.49 | 605.4 |
| Membranes | CH4 Permeability [Barrer] | CO2 Permeability [Barrer] | Selectivity CO2/CH4 |
|---|---|---|---|
| M1 | 9553.51 | 10,118.37 | 1.06 |
| M2 | 4514.3 | 4383.27 | 0.97 |
| M3 | 270.42 | 1044.01 | 3.86 |
| M4 | 381.06 | 1202.77 | 3.16 |
| M5 | 1227.69 | 857.90 | 0.70 |
| Membranes | Permeability [Barrer] | Selectivity (α) | PSI* [Barrer] | Ref. | |
|---|---|---|---|---|---|
| CO2 | CH4 | CO2/CH4 | |||
| PPSU | 8.0 | 0.3 | 25.00 | 192.0 | [71] |
| PPSU | 4.5 | 1.8 | 2.50 | 6.8 | [74] |
| 50PPSU/50PBNPI | 34.1 | 8.0 | 4.30 | 112.5 | |
| M1 | 10,118.4 | 9553.5 | 1.06 | 607.1 | This work |
| M3 | 1044.0 | 270.4 | 3.86 | 2985.8 | |
| M4 | 1202.8 | 381.1 | 3.16 | 2598.0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kujawski, W.; Li, G.; Van der Bruggen, B.; Pedišius, N.; Tonkonogij, J.; Tonkonogovas, A.; Stankevičius, A.; Šereika, J.; Jullok, N.; Kujawa, J. Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading. Materials 2020, 13, 2847. https://doi.org/10.3390/ma13122847
Kujawski W, Li G, Van der Bruggen B, Pedišius N, Tonkonogij J, Tonkonogovas A, Stankevičius A, Šereika J, Jullok N, Kujawa J. Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading. Materials. 2020; 13(12):2847. https://doi.org/10.3390/ma13122847
Chicago/Turabian StyleKujawski, Wojciech, Guoqiang Li, Bart Van der Bruggen, Nerijus Pedišius, Jurij Tonkonogij, Andrius Tonkonogovas, Arūnas Stankevičius, Justas Šereika, Nora Jullok, and Joanna Kujawa. 2020. "Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading" Materials 13, no. 12: 2847. https://doi.org/10.3390/ma13122847
APA StyleKujawski, W., Li, G., Van der Bruggen, B., Pedišius, N., Tonkonogij, J., Tonkonogovas, A., Stankevičius, A., Šereika, J., Jullok, N., & Kujawa, J. (2020). Preparation and Characterization of Polyphenylsulfone (PPSU) Membranes for Biogas Upgrading. Materials, 13(12), 2847. https://doi.org/10.3390/ma13122847








