Molybdenum Oxide and Nickel Nitrate as Cooperative Sintering Aids for Yttria-Stabilized Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Control Group: Sample Set 1
2.2. Ni(NO3)2 Infiltrated Group: Sample Set 2
2.3. Mechanically Mixed MoO2 8YSZ: Sample Set 3
2.4. Mechanically Mixed MoO2 8YSZ Infiltrated with Ni(NO3)2: Sample Set 4
2.5. Electrical Impedance Spectroscopy
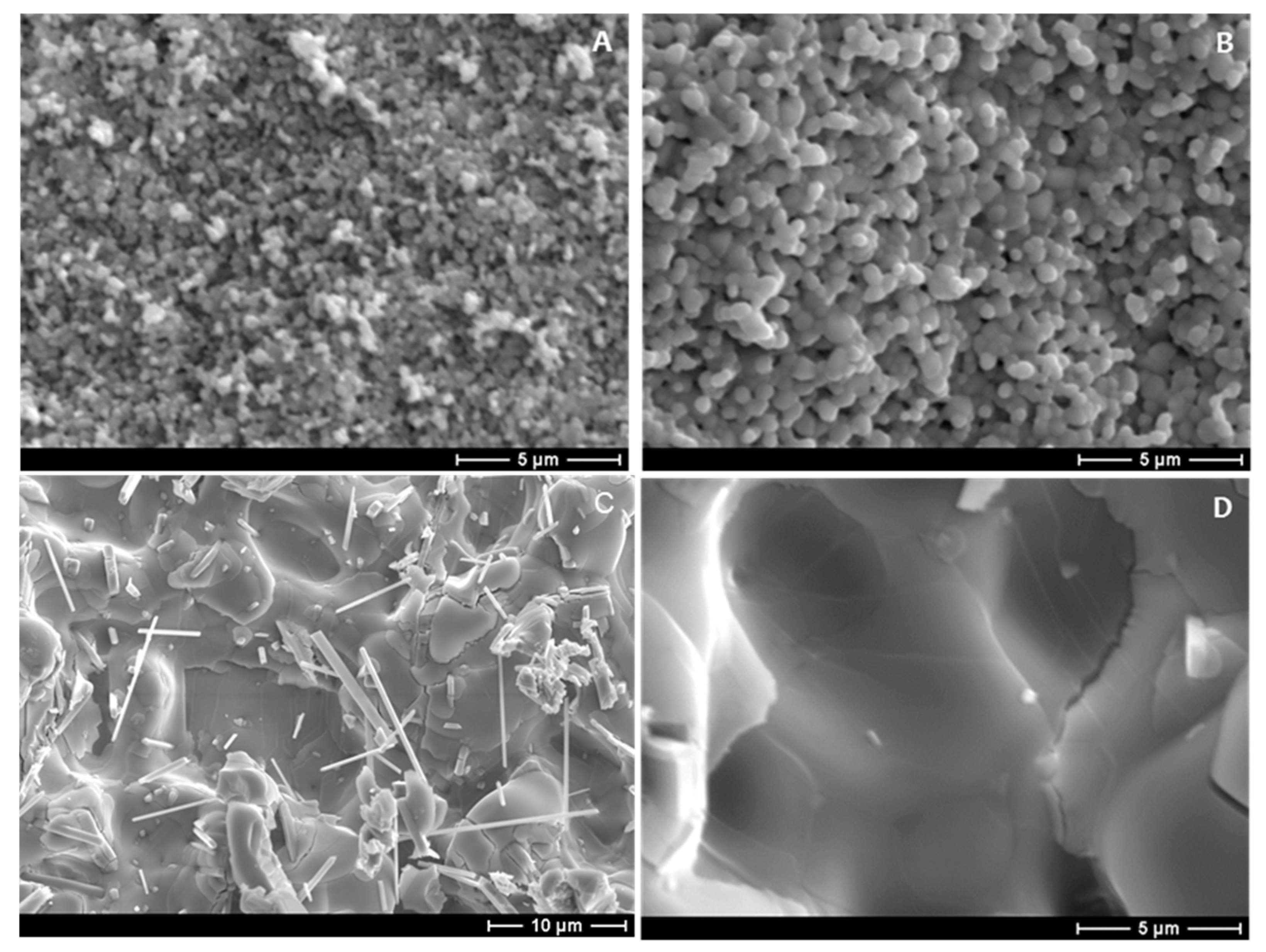
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Witz, G.; Shklover, V.; Steurer, W.; Bachegowda, S.; Bossmann, H.-P. Phase evolution in yttria stabilized zirconia thermal barrier. J. Am. Ceram. Soc. 2007, 90, 2935–2940. [Google Scholar] [CrossRef]
- Powell, M.; Meinhardt, K.; Sprenkle, V.; Chick, L.; McVay, G. Demonstration of a highly efficient solid oxide fuel cell power system using adiabatic steam reforming and anode gas recirculation. J. Power Sources 2012, 205, 377–384. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Han, M.; Tang, X.; Yin, H.; . Peng, S. Fabrication, microstructure and properties of a YSZ electrolye for SOFCs. J. Power Sources 2007, 165, 757–763. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Grain boundary resistivity in zirconia-based materials: Effect of sintering temperatures and impurities. Sold State Ion. 1995, 76, 67–80. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Zirconia-based solid electrolytes: Microstructure, stability and ionic conductivity. Solid State Ion. 1992, 52, 23–32. [Google Scholar] [CrossRef]
- Matsui, K.; Matsumoto, A.; Uehara, M.; Enomoto, N.; Hojo, J. Sintering kinetics at isothermal shrinkage: Effect of specific surface area on the initial sintering stage of fine zirconia powder. J. Am. Ceram. Soc. 2007, 90, 44–49. [Google Scholar] [CrossRef]
- Ewsuk, K.G.; Ellerby, D.T.; DiAntonio, C.B. Analysis of nanocrystalline and microcrystalline ZnO sintering using master sintering curves. J. Am. Ceram. Soc. 2006, 89, 2003–2006. [Google Scholar] [CrossRef]
- Gibson, I.R.; Dransfield, G.P.; Irvine, J.T.S. Sinterability of commercial 8 mol.% yttria-stabilized zirconia powders and the effect of sintered density on the ionic conductivity. J. Mater. Sci. 1998, 33, 4297–4305. [Google Scholar] [CrossRef]
- Mazaheri, M.; Simchi, A.; Dourandish, M.; Golestani-Fard, F. Master sintering curves of a nanoscale 3Y-TZP powder compacts. Ceram. Int. 2009, 35, 547–554. [Google Scholar] [CrossRef]
- German, R. Sintering Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Gessinger, G.H.; Fischmeister, H.F. A modified model for the sintering of tungsten with nickel additions. J. Less Common Met. 1972, 27, 129–141. [Google Scholar] [CrossRef]
- Matsui, K.; Yoshida, H.; Ikuhara, Y. Sintering kinetics at constant rates of heating: Effect of Al2O3 on the initial sintering stage of fine zirconia powder. J. Am. Ceram. Soc. 2005, 88. [Google Scholar] [CrossRef]
- Wang, J.; Raj, R. Activation energy for the sintering of two-phase alumina/zirconia ceramics. J. Am. Ceram. Soc. 1991, 74, 1959–1963. [Google Scholar] [CrossRef]
- Feighery, A.J.; Irvine, J.T.S. Effect of alumina additions upon electrical properties of 8 mol.% yttria-stabilised zirconia. Solid State Ion. 1999, 121, 209–216. [Google Scholar] [CrossRef]
- Knibbe, R.; Drennan, J.; Dicks, A.; Love, J. Effect of alumina additions on the anode | electrolyte enterface in solid oxide fuel cells. J. Power Sources 2008, 179, 511–519. [Google Scholar] [CrossRef]
- Knibbe, R.; Drennan, J.; Love, J.G. Effect of alumina additions in YSZ on the microstructure and degradation of the LSM-YSZ interface. Solid State Ion. 2009, 180, 984–989. [Google Scholar] [CrossRef]
- Chen, S.; Deng, W.; Shen, P. Stability of cubic ZrO2 (10 mol.% Y2O3) when alloyed with NiO, Al2O3, or TiO2: Implications to solid electrolytes and cermets. Mater. Sci. Eng. 1994, 22, 247–255. [Google Scholar] [CrossRef]
- Kowalski, K.; Bernasik, A.; Camra, J.; Radecka, M.; Jedliński, J. Diffusion of niobium in yttria-stabilized zirconia and in tatania-doped yttria-stabilized zirconia polycrystalling materials. J. Eur. Ceram. Soc. 2006, 26, 3139–3143. [Google Scholar] [CrossRef]
- Traqueia, L.S.M.; Pagnier, T.; Marques, F.M.B. Structural and electrical characterization of titania-doped YSZ. J. Eur. Ceram. Soc. 1997, 17, 1019–1026. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.G. The Effect of Bi2O3 on the microstructure and electrical conductivity of ZrO2-Y2O3 ceramics. J. Mater. Sci. Mater. Electron. 1994, 5, 260–266. [Google Scholar] [CrossRef]
- Batista, R.M.; Muccillo, E.N. Densification and electrical conductivity of yttria-stabilized zirconia containing NiO additions. ECS Trans. 2008, 13, 47–54. [Google Scholar] [CrossRef]
- Jan, V.H.; Ruben, V. Conductivity of Mn and Ni-doped stabilized zirconia electrolyte. J. Eur. Ceram. Soc. 2004, 24, 1177–1180. [Google Scholar]
- Coors, W.G.; O’Brien, J.R.; White, J.T. Conductivity degradation of NiO-containing 8YSZ and 10YSZ electrolyte during reduction. Solid State Ion. 2009, 180, 246–251. [Google Scholar] [CrossRef]
- Linderoth, S.; Bonanos, N.; Hansen, K.V.; Bilde-Sørensen, J.B. Effect of NiO-to-Ni transformation on conductivity and structure of yttria-stabilized ZrO2. J. Am. Ceram. Soc. 2001, 84, 2652–2656. [Google Scholar] [CrossRef]
- Appel, C.C.; Botton, G.A.; Horsewell, A.; Stobbs, W.M. Chemical and structural changes in manganese-doped yttria-stabilized zirconia wtudied by electron energy loss spectroscopy combined with electron diffraction. J. Am. Ceram. Soc. 1999, 82, 429–455. [Google Scholar] [CrossRef]
- Lewis, G.S.; Atkinson, A.; Steele, B.C.H. Cobalt additive for lowering the sintering temperature of yttria-stabilized zirconia. J. Mater. Sci. Lett. 2001, 20, 1155–1157. [Google Scholar] [CrossRef]
- Gentile, P.S.; Sofie, S.W.; Key, C.F.; Smith, R.J. Silicon volatility from alumina and aluminosilicates under solid oxide fuel cell operating conditions. Int. J. Appl. Ceram. Technol. 2012, 9, 1035–1048. [Google Scholar] [CrossRef]
- Zhang, T.S.; Chan, S.H.; Kong, L.B.; Sheng, P.T. Synergetic effect of NiO and SiO2 on the sintering and porperties of 8 mol.% yttria-stabilized zirconia electrolytes. Electrochim. Acta 2009, 54, 927–934. [Google Scholar] [CrossRef]
- Zhang, T.S.; Chan, S.H.; Wang, W.; Hbaieb, K.; Kong, L.B.; Ma, J. Effect of Mn addition on the densification, grain growth and ionic conductivity of pure and SiO2-containing 8YSZ electrolytes. Solid State Ion. 2009, 180, 82–89. [Google Scholar] [CrossRef]
- Hunt, C.; Driscoll, D.; Weisenstein, A.; Sofie, S. Nickel Nitrate and Molybdenum Oxide as a Yttria-stabilized Zirconia Synergistic Sintering Aid. In Processing, Properties, and Design of Advanced Ceramics and Composites; American Ceramic Society: Columbus, OH, USA, 4–8 October 2015. [Google Scholar]
- Zhang, T.S.; Du, Z.H.; Li, S.; Kong, L.B.; Song, X.C.; Lu, J.; Ma, J. Transitional metal-doped 8 mol.% yttria-stabilized zirconia electrolytes. Solid State Ion. 2009, 180, 1311–1317. [Google Scholar] [CrossRef]
- Patakangas, J.; Ma, Y.; Jing, Y.; Lund, P. Review and analysis of characterization methods and ionic conductivities for low-temperature solid oxide fuel cells (LT-SOFC). J. Power Sources 2014, 263, 315–331. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Sundararaman, D.; Ramasamy, S. Ionic conductivity studies of ultrafine-grained yttria stabilized zirconia polymorphs. Solid State Ion. 1999, 123, 271–278. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison-Wesley Publishing Company: Boston, MA, USA, 1956; pp. 297–304. [Google Scholar]
- White, J.; Reimanis, I.E.; Menzer, S.; Coors, G. The enhanced stabilization of the cubic phase in yttria-stabilized zirconia with the addition of nickel oxide. J. Am. Ceram. Soc. 2011, 94, 2030–2036. [Google Scholar] [CrossRef]
- White, J.T.; Reimanis, I.E.; O’Brien, J.R. Solubility of NiO in pechini-derived ZrO2 examined with SQUID magnetometry. J. Mater. Sci. 2012, 47, 1690–1696. [Google Scholar] [CrossRef]
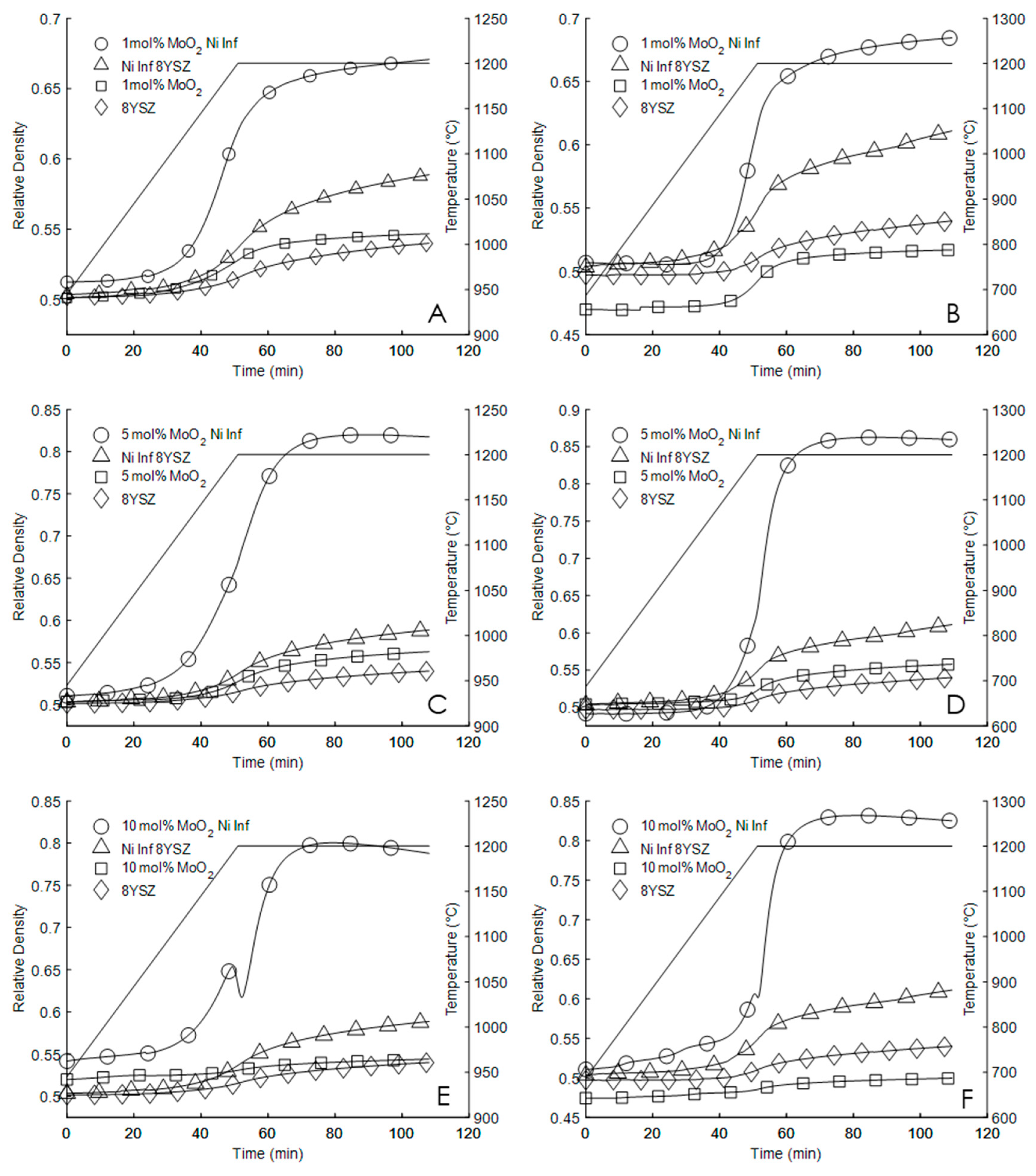



| Heating Rate (°C per Min) | Control | Ni(No3)2 | 1% MoO2 | 1% MoO2 Ni(No3)2 | 5% MoO2 | 5% MoO2 Ni(No3)2 | 10% MoO2 | 10% MoO2 Ni(No3)2 | |
|---|---|---|---|---|---|---|---|---|---|
| 5 | Initial Density | 50.7 | 50.3 | 49.9 | 51.3 | 50.4 | 50.4 | 52.1 | 52.1 |
| Final Density | 54.3 | 59.3 | 54.9 | 67.3 | 56.5 | 82 | 54.5 | 77.7 | |
| Max Sintering Rate | 0.101 | 0.249 | 0.154 | 0.718 | 0.204 | 1.287 | 0.068 | 2.227 | |
| 10 | Initial Density | 50 | 50.4 | 47.2 | 50.7 | 49.6 | 49.2 | 47.7 | 51.6 |
| Final Density | 54 | 61.2 | 51.9 | 69 | 55.9 | 85.9 | 50 | 82.4 | |
| Max Sintering Rate | 0.156 | 0.293 | 0.277 | 1.208 | 0.317 | 3.696 | 0.103 | 4.003 |
| Peak Location (°2θ) | Lattice (Å) | ||||||
|---|---|---|---|---|---|---|---|
| 111 | 200 | 220 | 311 | 222 | 400 | ||
| YSZ | 30.0632 | 34.8520 | 50.1140 | 59.5534 | 62.4907 | 73.5891 | 5.144 |
| Ni YSZ | 30.1260 | 34.9254 | 50.2234 | 59.6873 | 62.6327 | 73.7642 | 5.134 |
| 1%Mo YSZ | 30.1106 | 34.9074 | 50.1965 | 59.6544 | 62.5978 | 73.7212 | 5.136 |
| 1%Mo Ni YSZ | 30.1937 | 35.0046 | 50.3413 | 59.8317 | 62.7858 | 73.9530 | 5.136 |
| 5%Mo YSZ | 30.1236 | 34.9226 | 50.2192 | 59.6822 | 62.6272 | 73.7575 | 5.134 |
| 5%Mo Ni YSZ | 30.0991 | 34.8939 | 50.1764 | 59.6298 | 62.5717 | 73.6890 | 5.138 |
| 10%Mo YSZ | 30.2407 | 35.0595 | 50.4232 | 59.9319 | 62.8921 | 74.0842 | 5.115 |
| 10%Mo Ni YSZ | 30.1347 | 34.9356 | 50.2386 | 59.7058 | 62.6523 | 73.7884 | 5.132 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, C.; Allemeier, J.K.; Driscoll, D.; Weisenstein, A.; Sofie, S. Molybdenum Oxide and Nickel Nitrate as Cooperative Sintering Aids for Yttria-Stabilized Zirconia. Materials 2020, 13, 2875. https://doi.org/10.3390/ma13122875
Hunt C, Allemeier JK, Driscoll D, Weisenstein A, Sofie S. Molybdenum Oxide and Nickel Nitrate as Cooperative Sintering Aids for Yttria-Stabilized Zirconia. Materials. 2020; 13(12):2875. https://doi.org/10.3390/ma13122875
Chicago/Turabian StyleHunt, Clay, John Kyle Allemeier, David Driscoll, Adam Weisenstein, and Stephen Sofie. 2020. "Molybdenum Oxide and Nickel Nitrate as Cooperative Sintering Aids for Yttria-Stabilized Zirconia" Materials 13, no. 12: 2875. https://doi.org/10.3390/ma13122875




