Atmospheric Pressure Plasma Deposition of TiO2: A Review
Abstract
1. Introduction
2. Advantages of Atmospheric Pressure Plasma-Enhanced Deposition
3. Importance of TiO2
4. Atmospheric Pressure Plasma-Enhanced Deposition Methods
5. Atmospheric Pressure Plasma-Enhanced Deposition of TiO2
5.1. Plasma-Enhanced Chemical Vapor Deposition
5.2. Plasma-Enhanced Atomic Layer Deposition
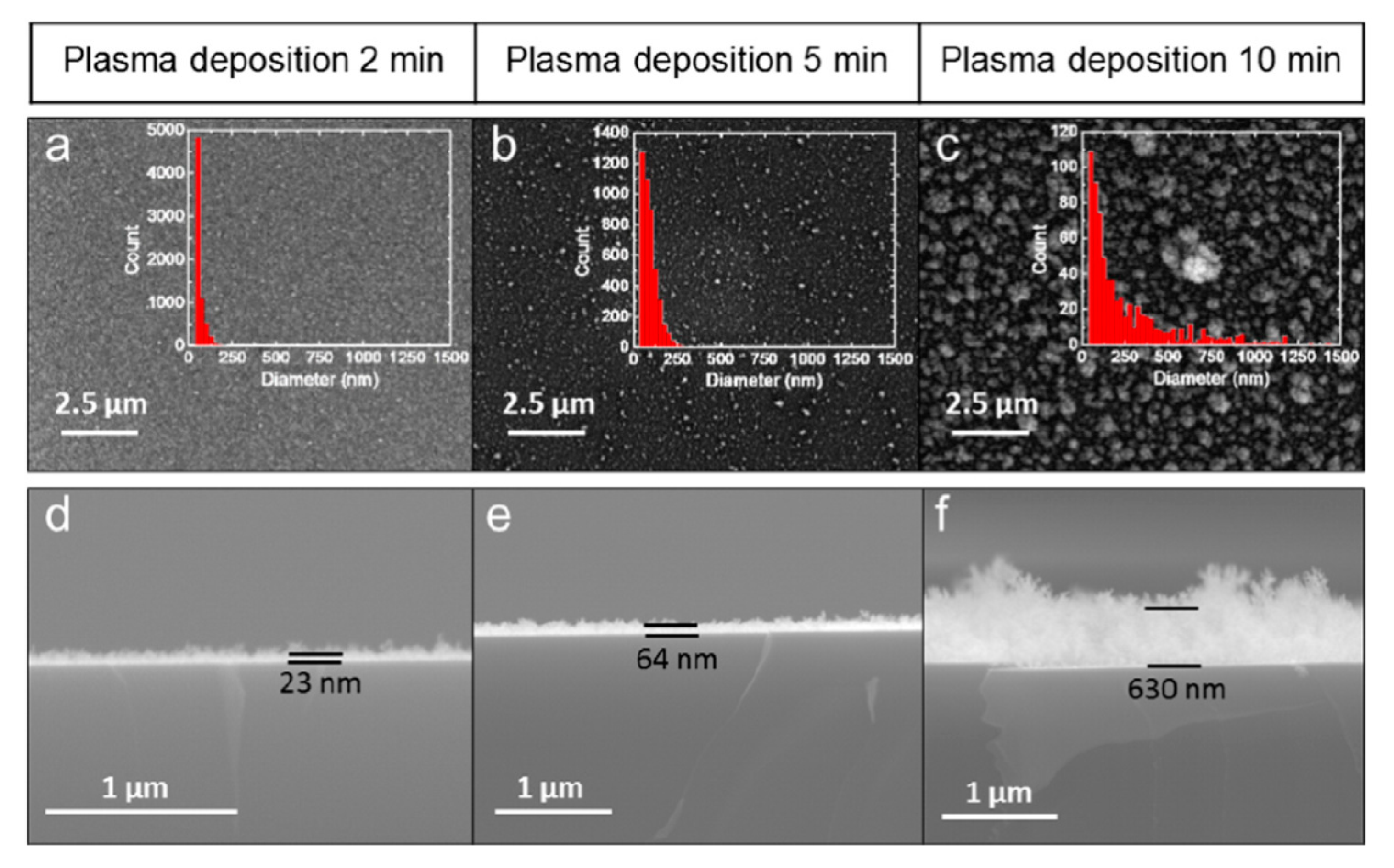
5.3. Atmospheric Pressure Dielectric Barrier Discharge Deposition
6. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Dowling, D.P.; Stallard, C.P. Achieving enhanced material finishing using cold plasma treatments. Trans. IMF 2015, 93, 119–125. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B Spectrosc. 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Chen, F.F. Introduction to Plasma Physics and Controlled Fusion, 2nd ed.; Plenum Press: New York, NY, USA, 1984. [Google Scholar]
- Park, S.; Choe, W.; Moon, S.Y.; Yoo, S.J. Electron characterization in weakly ionized collisional plasmas: From principles to techniques. Adv. Phys. X 2019, 4, 1526114. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lim, W.S.; Kim, D.E. A review of recent applications of atmospheric pressure plasma jets for materials processing. J. Coat. Technol. Res. 2015, 12, 225–235. [Google Scholar] [CrossRef]
- Kakiuchi, H.; Ohmi, H.; Yasutake, K. Atmospheric-pressure low-temperature plasma processes for thin film deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2014, 32, 030801-1–030801-16. [Google Scholar] [CrossRef]
- Schutze, A.; Jeong, J.J.Y.; Babayan, S.E.; Park, J.; Selwyn, G.S.; Hicks, R.F. The Atmospheric-Pressure Plasma Jet: A Review and Comparison to Other Plasma Sources. IEEE Trans. Plasma Sci. 1998, 26, 1685–1694. [Google Scholar] [CrossRef]
- Pappas, D. Status and potential of atmospheric plasma processing of materials. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 020801-1–020801-17. [Google Scholar] [CrossRef]
- Thornton, J.A. Plasma-assisted deposition processes: Theory, mechanisms and applications. Thin Solid Film. 1983, 107, 3–19. [Google Scholar] [CrossRef]
- Taylor, P.R.; Pirzada, S.A. Thermal plasma processing of materials: A review. Adv. Perform. Mater. 1994, 1, 35–50. [Google Scholar] [CrossRef]
- Peng, X.; Matthews, A.; Xue, S. Plasma-based processes and thin film equipment for nano-scale device fabrication. J. Mater. Sci. 2011, 46, 1–37. [Google Scholar] [CrossRef]
- Vallée, C.; Bonvalot, M.; Belahcen, S.; Yeghoyan, T.; Jaffal, M.; Vallat, R.; Chaker, A.; Lefèvre, G.; David, S.; Bsiesy, A.; et al. Plasma deposition—Impact of ions in plasma enhanced chemical vapor deposition, plasma enhanced atomic layer deposition, and applications to area selective deposition. J. Vac. Sci. Technol. A 2020, 38, 033007. [Google Scholar] [CrossRef]
- Fanelli, F.; Bosso, P.; Mastrangelo, A.M.; Fracassi, F. Thin film deposition at atmospheric pressure using dielectric barrier discharges: Advances on three-dimensional porous substrates and functional coatings. Jpn. J. Appl. Phys. 2016, 55, 07LA01. [Google Scholar] [CrossRef]
- Fauchais, P.; Vardelle, M.; Vardelle, A.; Bianchi, L. Plasma spray: Study of the coating generation. Ceram. Int. 1996, 22, 295–303. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shibata, Y.; Maeda, M.; Miyano, Y.; Murai, K.; Ohmori, A. Plasma-spraying synthesis of high-performance photocatalytic TiO2 coatings. IOP Conf. Ser. Mater. Sci. Eng. 2014, 61, 012039-1–012039-10. [Google Scholar] [CrossRef]
- Fakhouri, H.; Salem, D.B.; Carton, O.; Pulpytel, J.; Arefi-Khonsari, F. Highly efficient photocatalytic TiO2 coatings deposited by open air atmospheric pressure plasma jet with aerosolized TTIP precursor. J. Phys. D Appl. Phys. 2014, 47, 265301-1–265301-11. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M. Porous TiO2 coating using the solution precursor plasma spray process. Surf. Coat. Technol. 2008, 202, 6113–6119. [Google Scholar] [CrossRef]
- Chen, D.; Jordan, E.H.; Gell, M.; Ma, X. Dense TiO2 Coating Using the Solution Precursor Plasma Spray Process. J. Am. Ceram. Soc. 2008, 91, 865–872. [Google Scholar] [CrossRef]
- Bessergenev, V.G.; Khmelinskii, I.V.; Pereira, R.J.F.; Krisuk, V.V.; Turgambaeva, A.E.; Igumenov, I.K. Preparation of TiO2 films by CVD method and its electrical, structural and optical properties. Vacuum 2002, 64, 275–279. [Google Scholar] [CrossRef]
- O’Neill, S.A.; Parkin, I.P.; Clark, R.J.H.; Mills, A.; Elliott, N. Atmospheric pressure chemical vapour deposition of titanium dioxide coatings on glass. J. Mater. Chem. 2003, 13, 56–60. [Google Scholar] [CrossRef]
- Kanazawa, T.; Ohmori, A. Behavior of TiO2 coating formation on PET plate by plasma spraying and evaluation of coating’s photocatalytic activity. Surf. Coat. Technol. 2005, 197, 45–50. [Google Scholar] [CrossRef]
- Jung, H.; Park, J.; Yoo, E.S.; Han, G.-S.; Jung, H.S.; Ko, M.J.; Park, S.; Choe, W. Functionalization of nanomaterials by non-thermal large area atmospheric pressure plasmas: Application to flexible dye-sensitized solar cells. Nanoscale 2013, 5, 7825–7830. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, T.P. Tunneling leakage current in oxynitride: Dependence on oxygen/nitrogen content. IEEE Electron Device Lett. 1998, 19, 207–209. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Malagutti, A.R.; Mourão, H.A.J.L.; Garbin, J.R.; Ribeiro, C. Deposition of TiO2 and Ag:TiO2 thin films by the polymeric precursor method and their application in the photodegradation of textile dyes. Appl. Catal. B Environ. 2009, 90, 205–212. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 1–8. [Google Scholar] [CrossRef]
- Davidsdóttir, S.; Canulescu, S.; Dirscherl, K.; Schou, J.; Ambat, R. Investigation of photocatalytic activity of titanium dioxide deposited on metallic substrates by DC magnetron sputtering. Surf. Coat. Technol. 2013, 216, 35–45. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Augugliaro, V.; Loddo, V.; Pagliaro, M.; Palmisano, G.; Palmisano, L. Clean by Light Irradiation: Practical Applications of Supported TiO2; The Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; Tokyo Bkc Inc.: Tokyo, Japan, 1999. [Google Scholar]
- Xu, J.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. UV-Protective TiO2 Thin Films with High Transparency in Visible Light Region Fabricated via Atmospheric-Pressure Plasma-Enhanced Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2018, 10, 42657–42665. [Google Scholar] [CrossRef]
- Evans, P.; Sheel, D.W. Photoactive and antibacterial TiO2 thin films on stainless steel. Surf. Coat. Technol. 2007, 201, 9319–9324. [Google Scholar] [CrossRef]
- Karches, M.; Morstein, M.; von Rohr, R.P.; Pozzo, R.L.; Giombi, J.L.; Baltanás, M.A. Plasma-CVD-coated glass beads as photocatalyst for water decontamination. Catal. Today 2002, 72, 267–279. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Thukkaram, M.; Cools, P.; Nikiforov, A.; Rigole, P.; Coenye, T.; Van Der Voort, P.; Du Laing, G.; Vercruysse, C.; Declercq, H.; Morent, R.; et al. Antibacterial activity of a porous silver doped TiO2 coating on titanium substrates synthesized by plasma electrolytic oxidation. Appl. Surf. Sci. 2020, 500, 144235. [Google Scholar] [CrossRef]
- Lee, D.G.; Lee, D.; Yoo, J.S.; Lee, S.; Jung, H.S. Effective passivation of Ag nanowire-based flexible transparent conducting electrode by TiO2 nanoshell. Nano Converg. 2016, 3. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Hovish, M.Q.; Dauskardt, R.H. Optical properties of metal oxynitride thin films grown with atmospheric plasma deposition. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Kot, M.; Łobaza, J.; Naumann, F.; Gargouri, H.; Henkel, K.; Schmeißer, D. Long-term ambient surface oxidation of titanium oxynitride films prepared by plasma-enhanced atomic layer deposition: An XPS study. J. Vac. Sci. Technol. A 2018, 36, 01A114. [Google Scholar] [CrossRef]
- Hovish, M.Q.; Hilt, F.; Rolston, N.; Xiao, Q.; Dauskardt, R.H. Open Air Plasma Deposition of Superhydrophilic Titania Coatings. Adv. Fun. Mat. 2019, 29, 1806421. [Google Scholar] [CrossRef]
- Chen, Q.; Ozkan, A.; Chattopadhyay, B.; Baert, K.; Poleunis, C.; Tromont, A.; Snyders, R.; Delcorte, A.; Terryn, H.; Delplancke-Ogletree, M.-P.; et al. N-Doped TiO2 Photocatalyst Coatings Synthesized by a Cold Atmospheric Plasma. Langmuir 2019, 35, 7161–7168. [Google Scholar] [CrossRef]
- Haidera, A.J.; Jameel, Z.N.; Al-Hussainib, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillaiab, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. App. Catal. B 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Noman, M.T.; Ashraf, M.A.; Ali, A. Synthesis and applications of nano-TiO2: A review. Environ. Sci. Pollut. Res. 2019, 26, 3262–3291. [Google Scholar] [CrossRef] [PubMed]
- Martinu, L.; Poitras, D. Plasma deposition of optical films and coatings: A review. J. Vac. Sci. Technol. A Vac. Surf. Film. 2000, 18, 2619–2645. [Google Scholar] [CrossRef]
- Yoshiki, H.; Saito, T. Preparation of TiO2 thin films on the inner surface of a quartz tube using atmospheric-pressure microplasma. J. Vac. Sci. Technol. A Vac. Surf. Film. 2008, 26, 338–343. [Google Scholar] [CrossRef]
- Filippin, A.N.; Sanchez-Valencia, J.R.; Idígoras, J.; Rojas, T.C.; Barranco, A.; Anta, J.A.; Borras, A. Plasma enhanced deposition of single and multistacked TiO2 hierarchical nanotube photoanodes. Nanoscale 2017, 9, 8133–8141. [Google Scholar] [CrossRef]
- Gazal, Y.; Dublanche-Tixier, C.; Chazelas, C.; Colas, M.; Carles, P.; Tristant, P. Multi-structural TiO2 film synthesised by an atmospheric pressure plasma-enhanced chemical vapour deposition microwave torch. Thin Solid Film. 2016, 600, 43–52. [Google Scholar] [CrossRef]
- Yamauchi, S.; Imai, Y. Plasma-Assisted Chemical Vapor Deposition of TiO2 Thin Films for Highly Hydrophilic Performance. Cryst. Struct. Theory Appl. 2013, 2, 1–7. [Google Scholar] [CrossRef][Green Version]
- Wang, S.; Wang, K.; Jehng, J.; Liu, L. Preparation of TiO2/MCM-41 by plasma enhanced chemical vapor deposition method and its photocatalytic activity. Front. Environ. Sci. Eng. 2012, 6, 304–312. [Google Scholar] [CrossRef]
- Yang, W.; Wolden, C.A. Plasma-enhanced chemical vapor deposition of TiO2 thin films for dielectric applications. Thin Solid Film. 2006, 515, 1708–1713. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Chatterjee, S.; Samanta, S.K.; Maiti, C.K. Electrical characterization of low temperature deposited TiO2 films on strained-SiGe layers. Appl. Surf. Sci. 2003, 210, 249–254. [Google Scholar] [CrossRef]
- Lee, G.W.; Woo, S.I.; Kim, J.C.; Oh, K.H. Preparation and properties of amorphous TiO2 thin films by plasma enhanced chemical vapor deposition. Thin Solid Film. 1994, 237, 105–111. [Google Scholar] [CrossRef]
- Frenck, H.J.; Kulisch, W.; Kuhr, M.; Kassing, R. Deposition of TiO2 thin films by plasma-enhanced decompostion of tetraisopropyltitanate. Thin Solid Film. 1991, 201, 327–335. [Google Scholar] [CrossRef]
- Borras, A.; Sanchez-Valencia, J.R.; Widmer, R.; Rico, V.J.; Justo, A.; Gonzalez-Elipe, A.R. Growth of Crystalline TiO2 by Plasma Enhanced Chemical Vapor Deposition. Cryst. Growth Des. 2009, 9, 2868–2876. [Google Scholar] [CrossRef]
- Lee, Y.H. A role of energetic ions in RF-biased PECVD of TiO2. Vacuum 1998, 51, 503. [Google Scholar] [CrossRef]
- Maiti, C.K.; Samanta, S.K.; Dalapati, G.K.; Nandi, S.K.; Chatterjee, S. Electrical characterization of TiO2 gate oxides on strained-Si. Microelectron. Eng. 2004, 72, 253–256. [Google Scholar] [CrossRef]
- Hao, Q.; Fu, X.; Song, S.; Gibson, D.; Li, C.; Chu, H.O.; Shi, Y. Investigation of TiO2 Thin Film Deposited by Microwave Plasma Assisted Sputtering and Its Application in 3D Glasses. Coatings 2018, 8, 270. [Google Scholar] [CrossRef]
- Medvecká, V.; Kováčik, D.; Zahoranová, A.; Černák, M. Atmospheric pressure plasma enhanced calcination by the preparation of TiO2 fibers in submicron scale. Appl. Surf. Sci. 2018, 428, 609–615. [Google Scholar] [CrossRef]
- Mertens, J.; Hubert, J.; Vandencasteele, N.; Raes, M.; Terryn, H.; Reniers, F. Chemical and physical effect of SiO2 and TiO2 nanoparticles on highly hydrophobic fluorocarbon hybrid coatings synthesized by atmospheric plasma. Surf. Coat. Technol. 2017, 315, 274–282. [Google Scholar] [CrossRef]
- Seo, H.K.; Elliott, C.M.; Shin, H.S. Mesoporous TiO2 films fabricated using atmospheric pressure dielectric barrier discharge jet. ACS Appl. Mater. Interfaces 2010, 2, 3397–3400. [Google Scholar] [CrossRef]
- Di, L.B.; Li, X.S.; Shi, C.; Xu, Y.; Zhao, D.Z.; Zhu, A.M. Atmospheric-pressure plasma CVD of TiO2 photocatalytic films using surface dielectric barrier discharge. J. Phys. D Appl. Phys. 2009, 42, 032001–032004. [Google Scholar] [CrossRef]
- Fanelli, F.; Fracassi, F. Atmospheric pressure non-equilibrium plasma jet technology: General features, specificities and applications in surface processing of materials. Surf. Coat. Technol. 2017, 322, 174–201. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.J.; Lee, G.H.; Kim, I.S.; Shin, B.C.; Yoon, S.G. Very thin TiO2 films Prepared by Plasma Enhanced Atomic Layer Deposition (PEALD). Integr. Ferroelectr. 2004, 68, 129–137. [Google Scholar] [CrossRef]
- Choi, G.; Kim, S.K.; Won, S.; Kim, H.J.; Hwang, C.S. Plasma-Enhanced Atomic Layer Deposition of TiO2 and Al-Doped TiO2 Films Using N2O and O2 Reactants. J. Electrochem. Soc. 2009, 156, G138–G143. [Google Scholar] [CrossRef]
- Vallat, R.; Gassilloud, R.; Salicio, O.; El Hajjam, K.; Molas, G.; Pelissier, B.; Vallée, C. Area selective deposition of TiO2 by intercalation of plasma etching cycles in PEALD process: A bottom up approach for the simplification of 3D integration scheme. J. Vac. Sci. Technol. A 2019, 37, 020918. [Google Scholar] [CrossRef]
- Yuji, T.; Akatsuka, H.; Mungkung, N.; Park, B.W.; Sung, Y.M. Surface treatment of TiO2 films for dye-sensitized solar cells using atmospheric-pressure non-equilibrium DC pulse discharge plasma jet. Vacuum 2009, 83, 124–127. [Google Scholar] [CrossRef]
- Ha, H.; Yoshimoto, M.; Koinuma, H.; Moon, B.; Ishiwara, H. Open air plasma chemical vapor deposition of highly dielectric amorphous TiO2 films. Appl. Phys. Lett. 1996, 68, 2965–2967. [Google Scholar] [CrossRef]
- Jiménez, C.; De Barros, D.; Darraz, A.; Deschanvres, J.-L.; Rapenne, L.; Chaudouët, P.; Méndez, J.E.; Weiss, F.; Thomachot, M.; Sindzingre, T.; et al. Deposition of TiO2 thin films by atmospheric plasma post-discharge assisted injection MOCVD. Surf. Coat. Technol. 2007, 201, 8971–8975. [Google Scholar] [CrossRef]
- Williams, L.M.; Hess, D.W. Structural properties of titanium dioxide films deposited in an rf glow discharge. J. Vac. Sci. Technol. A 1983, 1, 1810–1819. [Google Scholar] [CrossRef]
- Williams, L.M.; Hess, D.W. Photoelectrochemical properties of plasma-deposited TiO2 thin films. Thin Solid Film. 1984, 115, 13–18. [Google Scholar] [CrossRef]
- Collette, S.; Hubert, J.; Batan, A.; Baert, K.; Raes, M.; Vandendael, I.; Daniel, A.; Archambeau, C.; Terryn, H.; Reniers, F. Photocatalytic TiO2 thin films synthesized by the post-discharge of an RF atmospheric plasma torch. Surf. Coat. Technol. 2016, 289, 172–178. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; He, T.; Ding, K.; Huang, X.; Li, H.; Shi, J.; Guo, Y.; Zhang, J. The Effects of Thermal and Atmospheric Pressure Radio Frequency Plasma Annealing in the Crystallization of TiO2 Thin Films. Coatings 2019, 9, 357. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, L.; Ding, K.; Guo, Y.; Shi, J.; Huang, X.; Zhang, J. Synergistic Effect of Plasma Discharge and Substrate Temperature in Improving the Crystallization of TiO2 Film by Atmospheric Pressure Plasma Enhanced Chemical Vapor Deposition. Plasma Chem. Plasma Process. 2019, 39, 937–947. [Google Scholar] [CrossRef]
- Mauchauffé, R.; Kang, S.; Kim, J.; Kim, J.H.; Moon, S.Y. Spectroscopic study of an atmospheric pressure plasma generated for the deposition of titanium dioxide thin films. Curr. Appl. Phys. 2019, 19, 1296–1304. [Google Scholar] [CrossRef]
- Huang, S.S.; Chen, J.S. Comparison of the characteristics of TiO2 films prepared by low-pressure and plasma-enhanced chemical vapor deposition. J. Mater. Sci. Mater. Electron. 2002, 13, 77–81. [Google Scholar] [CrossRef]
- Nakamura, M.; Kato, S.; Aoki, T.; Sirghi, L.; Hatanaka, Y. Formation mechanism for TiOx thin film obtained by remote plasma enhanced chemical vapor deposition in H2–O2 mixture gas plasma. Thin Solid Film. 2001, 401, 138–144. [Google Scholar] [CrossRef]
- Mathur, S.; Kuhn, P. CVD of titanium oxide coatings: Comparative evaluation of thermal and plasma enhanced processes. Surf. Coat. Technol. 2006, 201, 807–814. [Google Scholar] [CrossRef]
- Rie, K.T.; Gebauer, A.; Wohle, J. Plasma assisted CVD for low temperature coatings to improve the wear and corrosion resistance. Surf. Coat. Technol. 1996, 86–87, 498–506. [Google Scholar] [CrossRef]
- Kang, S.; Mauchauffé, R.; You, Y.S.; Moon, S.Y. Insights into the Role of Plasma in Atmospheric Pressure Chemical Vapor Deposition of Titanium Dioxide Thin Films. Sci. Rep. 2018, 8, 16684-1–16684-13. [Google Scholar] [CrossRef]
- Baba, K.; Bulou, S.; Choquet, P.; Boscher, N.D. Photocatalytic Anatase TiO2 Thin Films on Polymer Optical Fiber Using Atmospheric-Pressure Plasma. ACS Appl. Mater. Interfaces 2017, 9, 13733–13741. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.H.; Park, Y.B.; Park, D.W. Kinetic and mechanistic study on the chemical vapor deposition of titanium dioxide thin films by in situ FT-IR using TTIP. Surf. Coat. Technol. 2003, 171, 198–204. [Google Scholar] [CrossRef]
- Rong, W.; Kazuhito, H.; Akira, F.; Makoto, C.; Eiichi, K.; Astushi, K.; Mitsuhide, S.; Toshiya, W. Light-induced amphiphilic surfaces. Nature 1997, 338, 431–432. [Google Scholar] [CrossRef]
- Battistona, G.A.; Gerbasia, R.; Gregoria, A.; Porchiaa, M.; Cattarinb, S.; Rizzic, G.A. PECVD of amorphous TiO2 thin films: Effect of growth temperature and plasma gas composition. Thin Solid Film. 2000, 371, 126–131. [Google Scholar] [CrossRef]
- Zhou, M.; Roualdès, S.; Zhao, J.; Autès, V.; Ayral, A. Nanocrystalline TiO2 thin film prepared by low-temperature plasma-enhanced chemical vapor deposition for photocatalytic applications. Thin Solid Film. 2015, 589, 770–777. [Google Scholar] [CrossRef]
- Perraudeau, A.; Dublanche-Tixier, C.; Tristant, P.; Chazelas, C.; Vedraine, S.; Ratier, B. Low-temperature deposition of TiO2 by atmospheric pressure PECVD towards photoanode elaboration for perovskite and solid-state dye-sensitized solar cells. EPJ Photovolt. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Gazal, Y.; Chazelas, C.; Dublanche-Tixier, C.; Tristant, P. Contribution of optical emission spectroscopy measurements to the understanding of TiO2 growth by chemical vapor deposition using an atmospheric-pressure plasma torch. J. Appl. Phys. 2017, 121. [Google Scholar] [CrossRef]
- Perraudeau, A.; Dublanche-Tixier, C.; Tristant, P.; Chazelas, C. Dynamic mode optimization for the deposition of homogeneous TiO2 thin film by atmospheric pressure PECVD using a microwave plasma torch. Appl. Surf. Sci. 2019, 493, 703–709. [Google Scholar] [CrossRef]
- Zhou, M.; Roualdès, S.; Ayral, A. New photocatalytic contactors obtained by PECVD deposition of TiO2 thin layers on the surface of macroporous supports: PECVD TiO2-based membranes as photocatalytic contactors. Eur. Phys. J. Spec. Top. 2015, 224, 1871–1882. [Google Scholar] [CrossRef]
- Aghaee, M.; Verheyen, J.; Stevens, A.A.E.; Kessels, W.M.M.; Creatore, M. TiO2 thin film patterns prepared by chemical vapor deposition and atomic layer deposition using an atmospheric pressure microplasma printer. Plasma Process. Polym. 2019, 16, 12. [Google Scholar] [CrossRef]
- Boscher, N.D.; Olivier, S.; Maurau, R.; Bulou, S.; Sindzingre, T.; Belmonte, T.; Choquet, P. Photocatalytic anatase titanium dioxide thin films deposition by an atmospheric pressure blown arc discharge. Appl. Surf. Sci. 2014, 311, 721–728. [Google Scholar] [CrossRef]
- Profijt, H.B.; van de Sanden, M.C.M.; Kessels, W.M.M. Substrate-biasing during plasma-assisted atomic layer deposition to tailor metal-oxide thin film growth. J. Vac. Sci. Technol. A Vac. Surf. Film. 2012, 31, 01A106-1–01A106-9. [Google Scholar] [CrossRef]
- Ratzsch, S.; Kley, E.B.; Tünnermann, A.; Szeghalmi, A. Influence of the oxygen plasma parameters on the atomic layer deposition of titanium dioxide. Nanotechnology 2015, 26, 024003. [Google Scholar] [CrossRef] [PubMed]
- Chiappim, W.; Testoni, G.E.; Doria, A.C.O.C.; Pessoa, R.S.; Fraga, M.A.; Galvão, N.K.A.M.; Grigorov, K.G.; Vieira, L.; Maciel, H.S. Relationships among growth mechanism, structure and morphology of PEALD TiO2 films: The influence of O2 plasma power, precursor chemistry and plasma exposure mode. Nanotechnology 2016, 27, 305701. [Google Scholar] [CrossRef]
- Strobel, A.; Schnabel, H.-D.; Reinhold, U.; Rauer, S.; Neidhardt, A. Room temperature plasma enhanced atomic layer deposition for TiO2 and WO3 films. J. Vac. Sci. Technol. A 2016, 34, 01A118. [Google Scholar] [CrossRef]
- Rai, V.R.; Agarwal, S. In situ diagnostics for studying gas-surface reactions during thermal and plasma-assisted atomic layer deposition. J. Vac. Sci. Technol. A 2012, 30, 01A158. [Google Scholar] [CrossRef]
- Rai, V.R.; Agarwal, S. Surface reaction mechanisms during plasma-assisted atomic layer deposition of titanium dioxide. J. Phys. Chem. C 2009, 113, 12962–12965. [Google Scholar] [CrossRef]
- Theirich, D.; Müller, R.; Zilberberg, K.; Trost, S.; Behrendt, A.; Riedl, T. Atmospheric pressure plasma ALD of titanium oxide. Chem. Vap. Depos. 2013, 19, 167–173. [Google Scholar] [CrossRef]
- Lisovskiy, V.A.; Yegorenkov, V.D. Alpha–gamma transition in RF capacitive discharge in low-pressure oxygen. Vacuum 2004, 74, 19–28. [Google Scholar] [CrossRef][Green Version]
- Napari, M.; Tarvainen, O.; Kinnunen, S.; Arstila, K.; Julin, J.; Fjellvåg, Ø.S.; Weibye, K.; Nilsen, O.; Sajavaara, T. The α and γ plasma modes in plasma-enhanced atomic layer deposition with O2–N2 capacitive discharges. J. Phys. D 2017, 50, 095201. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A 2011, 29, 050801. [Google Scholar] [CrossRef]
- Knoops, H.C.M.; Faraz, T.; Arts, K.; Kessels, W.M.M. Status and prospects of plasma-assisted atomic layer deposition featured. J. Vac. Sci. Technol. A 2019, 37, 030902. [Google Scholar] [CrossRef]
- Niemelä, J.P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Q.; Ozkan, A.; Chattopadhyay, B.; Wallaert, G.; Baert, K.; Terryn, H.; Delplancke-Ogletree, M.P.; Geerts, Y.; Reniers, F. Atmospheric pressure dielectric barrier discharge synthesis of morphology-controllable TiO2 films with enhanced photocatalytic activity. Thin Solid Film. 2018, 664, 90–99. [Google Scholar] [CrossRef]
- Homola, T.; Dzik, P.; Veselý, M.; Kelar, J.; Černák, M.; Weiter, M. Fast and Low-Temperature (70 °C) Mineralization of Inkjet Printed Mesoporous TiO2 Photoanodes Using Ambient Air Plasma. ACS Appl. Mater. Interfaces 2016, 8, 33562–33571. [Google Scholar] [CrossRef] [PubMed]
- Pandiyaraj, K.N.; Vasu, D.; Padmanabhan, P.V.A.; Ghobeira, R.; Tabaei, P.S.E.; Coolsb, P.; De Geyter, N.; Morent, R.; Deshmukh, R.R.; Pichumanid, M. Synergetic effect of the catalytic action of plasma jet deposited TiOx coatings and atmospheric pressure plasma treatment on the degradation of RYRR. Surf. Coat. Technol. 2020, 389, 125642. [Google Scholar] [CrossRef]
- Chen, C.; Bai, H.; Chein, H.M.; Chen, T.M. Continuous generation of TiO2 nanoparticles by an atmospheric pressure plasma-enhanced process. Aerosol Sci. Technol. 2007, 41, 1018–1028. [Google Scholar] [CrossRef]
- Profili, J.; Gherardi, N.; Naudé, N.; Stafford, L. Deposition of TiO2-SiO2 nanocomposite coatings using atmospheric-pressure plasmas. In Proceedings of the IEEE Nanotechnology Materials and Devices Conference, Toulouse, France, 9–12 October 2016; IEEE: Piscataway, NJ, USA, 2016; p. 3. [Google Scholar] [CrossRef]
- Peng, T.; Pulpytel, J.; Horovitz, I.; Jaiswal, A.K.; Avisar, D.; Mamane, H.; Lalman, J.A.; Arefi-Khonsari, F. One-step deposition of nano-Ag-TiO2 coatings by atmospheric pressure plasma jet for water treatment: Application to trace pharmaceutical removal using solar photocatalysis. Plasma Process. Polym. 2019, 16, 1800213. [Google Scholar] [CrossRef]
- Liao, W.Y.; Yang, Y.J.; Hsu, C.M.; Hsu, C.C.; Cheng, I.C.; Chen, J.Z. Atmospheric-pressure-plasma-jet sintered dual-scale porous TiO2 using an economically favorable NaCl solution. J. Power Sources 2015, 281, 252–257. [Google Scholar] [CrossRef]
- Chang, H.; Yang, Y.-J.; Hsu, C.-M.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-Pressure-Plasma-Jet Particulate TiO2 Scattering Layer Deposition Processes for Dye-Sensitized Solar Cells. ECS J. Solid State Sci. Technol. 2014, 3, Q177–Q181. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Chang, H.; Liu, H.-W.; Yang, Y.-J.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-pressure-plasma-jet processed nanoporous TiO2 photoanodes and Pt counter-electrodes for dye-sensitized solar cells. RSC Adv. 2015, 5, 45662. [Google Scholar] [CrossRef]
- Chen, J.-Z.; Hsu, C.-C.; Wang, C.; Liao, W.-Y.; Wu, C.-H.; Wu, T.-J.; Liu, H.-W.; Chang, H.; Lien, S.-T.; Li, H.-C.; et al. Rapid Atmospheric-Pressure-Plasma-Jet Processed Porous Materials for Energy Harvesting and Storage Devices. Coatings 2015, 5, 26–38. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Wang, Z.; Yang, L.; Wang, C. Production of titanium dioxide powders by atmospheric pressure plasma jet. Phys. Procedia 2011, 18, 168–173. [Google Scholar] [CrossRef][Green Version]
- Krüger, F.; Gergs, T.; Trieschmann, J. Machine learning plasma-surface interface for coupling sputtering and gas phase transport simulations. Plasma Sources Sci. Technol. 2019, 28, 035002. [Google Scholar] [CrossRef]
- Gidon, D.; Pei, X.; Bonzanini, A.D.; Graves, D.B.; Mesbah, A. Machine Learning for Real-Time Diagnostics of Cold Atmospheric Plasma Sources. IEEE Trans. Radiat. Plasma Med. Sci. 2019, 3, 597–605. [Google Scholar] [CrossRef]
- Pakseresht, A.H.; Ghasali, E.; Nejati, M.; Shirvanimoghaddam, K.; Javadi, A.H.; Teimouri, R. Development empirical-intelligent relationship between plasma spray parameters and coating performance of yttria-stabilized zirconia. Int. J. Adv. Manuf. Technol. 2015, 76, 1031–1045. [Google Scholar] [CrossRef]








| Precursor | Carrier Gas (Flow Rate) | Feed Gas (Flow Rate) | Plasma Power f | Frequency | Substrate c (Temperature) | Deposition Rate, nm/min | Film Crystallinity (Conditions, Crystallite Size)/Morphology (Conditions, Particle Size) | PD Treatment (TPD, Time) d | Effect of PD Treatment | Scope of Study/Applications | Year [Ref] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TTIP | N2 (glass: 25 SCCM, Si: 53 SCCM) | O2 (glass: 0 SCCM, Si: 0–10 SCCM) | 1–9 kV | 60–70 kHz | silicate glass; Si b | glass: 4700–5500 Si: 3250 | Amorphous/rough with particle-like features, macroporous (glass); smooth (Si) | ----- | ----- | Fabrication; Optics | 2019 [93] |
| TiCl4 | Ar (25 SCCM) | Ar (1000 SCCM) + O2 (10 SCCM) | 11 kV | 10 kHz | quartz (no heating or 100 °C–400 °C) | 1000–3000 | Amorphous (no heating); anatase (Tsub ≥ 300 °C)/dense and fluffy (≤200 °C); dense cauliflower-like aggregates (≥300 °C) | Annealed in Ar (275 °C, 2 hrs) e | No change in crystallinity | Photocatalysis | 2019 [77] |
| TEOT | He (20 SCCM) | He (300 SCCM) + H2 (0–25 SCCM) | 105 W | 13.56 MHz | Si (200°C–500 °C) | 900 (Tsub = 500 °C) | Amorphous (Tsubs < 350 °C), Crystalline (anatase phase, >350 °C) | ---- | ---- | Synthesis; Electronics | 1996 [71] |
| TiCl4 + methanol/ethanol/propanol/H2O | N2 (0.1–0.4 dm3/min) | N2 (7.1–11 dm3/min) | ----- | ----- | SiCO coated glass (500°C− 650 °C) | 300 | Crystalline anatase phase (40 nm)/uniform | ---- | ---- | Photocatalysis; Hydrophilicity | 2002 [20] |
| TTIP | Ar (PAr + TTIP = 15.5–22.5 Pa) g | O2 (PO2 = 17.7 Pa) g | 50 W | ----- | Si; anatase seeded Si; quartz (150 °C) | 50–300 | Amorphous/micro-columnar, porous | Annealed in air (300 °C, 5 hrs) | Crystalization (anatase, 20 nm) | Photocatalysis; Hydrophilicity | 2015 [88] |
| TiCl4 | ----- | O2 (0.9 SCCM) | 10 W | 13.56 MHz | Glass; Si; Ti foil; sapphire; NaCl plate (25–700 °C) | 10–300 | Glass: amorphous (Tsub < 300 °C); anatase (up to 400 °C; rutile & anatase (500 °C);rutile (600 °C)/Si: smooth (200 °C), crystallite (≥400 °C) | ---- | ---- | Synthesis; Plasma diagnostics | 1983 [73] |
| TEOT a (10 µL/min) | Ar; Ar/O2: 80%/20–0%/100% (1 SLM) | Ar (10 SLM) | 200 W | 2.45 GHz | polymer | 75 | Crystalline anatase (19 nm)/homogeneous with spherical particles (30–150 nm) | ---- | ---- | Photocatalysis | 2017 [84] |
| TTIP a | N2 (10 SCCM) | O2 (2 SCCM) | 20–50 W | 13.56 MHz | Si (240 °C–420 °C) | 58 (Tsub = 320 °C) | Amorphous (Tsub < 240 °C); crystalline anatase ( >240 °C) | ----- | ----- | Plasma diagnostics; Optical films | 2003 [85] |
| TTIP a | Ar (0.2–0.5 L/min) | Ar (5 L/min) | 6 kV | 50 Hz | Si; quartz (25°C–90 °C) | 44 | Amorphous/rough, granular | ----- | ----- | Coatings | 2018 [32] |
| TTIP | Ar | H2 (70) + N2 (0–35) + Ar (20 SCCM) | 58–73 W | 1.548 kHz | Steel | 40 | Amorphous/smooth, dense and columnar surface | Annealed in air (400 °C) | Crystalization (anatase) | Hydrophilicity; Mechanics | 2006 [81] |
| TTIP | N2 or Ar | N2 or Ar (15–20) + O2 (0–5 SCCM) | 100–500 W | 13.56 MHz | Glass, ITO (120 °C–250 °C) | 10–25 (N2); 17–37 (N2 + O2 plasma) | Amorphous/smooth (N2, Tsub = 120 °C); rough and granular (N2, 250 °C or N2 + O2 plasma) | ----- | ----- | PEC cells | 2000 [87] |
| TTIP | Ar (30 SCCM) | O2 (15–30 SCCM) | 5–20 W | ----- | Si (150 °C–450 °C) | 3–30 | Amorphous (Tsub < 450 °C) | ----- | ----- | Electronics | 2006 [54] |
| TTIP | ----- | O2 | 1400 W | 2.45 GHz | Strained Si/SiGe | 24 | ----- | ----- | ----- | Microelectronics | 2004 [60] |
| TTIP | ----- | O2 | 1400 W | 2.45 GHz | strained SiGe/Si | 24 | Amorphous (5 min deposition), partially crystalline (anatase, 12 min deposition) | ----- | ----- | Microelectronics | 2003 [55] |
| TiCl4 | Ar (14 SCCM) | O2 (9 SCCM) + Ar (900 SCCM) | 4.2 W | 15 kHz | Glass | 22 | Amorphous | Annealed in air (350 °C–450 °C) | Crystalization (anatase) | Photocatalysis | 2009 [65] |
| TTIP | Ar | Ar (150 SCCM) | 65 W | ----- | Si (150 °C–310 °C) | 15 | Amorphous/Homogeneous | Annealed in N2 (400–900 °C) | Anatase: 400 °C, rutile: 700 °C; n↗ and dfilm↘ h | Optical and electrical films | 1991 [57] |
| TTIP | He (480–500 SCCM) | O2 (0–20 SCCM) | 14 W | 13.56 MHz | Quartz | 13 (He + O2, 2% of O2 | Amorphous: rough surface with craters and wrinkles only He; smooth and transparent (2% of O2; micro-nanoparticles (0.3–3 µm) 4% of O2 | ----- | ----- | Synthesis | 2008 [49] |
| TTIP a | He (5 SCCM) | H2 (48)+ O2 (12) or H2 (60) or O2 (60 SCCM) | 500 W | 2.45 GHz | Si; quartz (100 °C) | 0.16/0.19/10.8: O2/H2/H2 + O2 plasma | ----- | ----- | ----- | Optical films; Plasma diagnostics | 2001 [80] |
| TTIP | ----- | O2 | 10 W | 13.56 MHz | Quartz (150 °C–400 °C) | ≈9 | Crystalline anatase (Tsub > 300 °C)/dense plate-like nanograins (45 nm) | ----- | ----- | Hydrophilicity | 2013 [52] |
| TOAA i; TIPO a | N2 (80%) + O2 (20%) (3.5–5 L/min) | N2 (80%) + O2 (20%) (15 L/min) | 600 W | 100 kHz | Si (200 °C–300 °C) | 6.7 | Amorphous (Tsub < 270 °C), crystalline (anatase, Tsub > 270 °C)/smooth, granular (20 nm) | ----- | ----- | Low-temperature synthesis | 2008 [72] |
| TTIP | O2 | Ar (90%) + O2 (10%) | 400 W | ----- | Si, quartz (250 °C) | 1.9–5 | Crystalline (dominantly anatase)/microstructural domains | ----- | ----- | Crystal growth model | 2009 [58] |
| TTIP | Ar (80 SCCM) | O2 (2–16 SCCM) | 20–150 W | 13.56 MHz | Si (100 °C–400 °C) | 0.916 (Tsub = 250 °C, 100 W, O2 = 8 SCCM) | Amorphous/smooth (1 hr treatment); partially crystalline/coarse (treatment time ≥ 3 hrs) | N2/O2 plasma (150 W; Tsub = 250 °C; 30 mins) | Increased dielectric constant | Electronics | 1994 [56] |
| TTIP | N2 (50 SCCM) | O2 (100 SCCM) | 100 W | 13.56 MHz | Si (450 °C) | 0.33/0.63 (30/60 min treatment) | Amorphous/sparse grains (30 min treatment), smooth surface (60 min treatment) | ----- | ----- | Synthesis process | 2002 [79] |
| TTIP | He (1 SLM) | He (9 SLM) + O2 (0 or 0.005 SLM) | 70 W | 13.56 MHz | Si (dynamic: 5 mm/s) | 2.2 nm/pass k | Amorphous/smooth with uniformly dispersed nanoagglomerates (He plasma); smooth, crack-free and smaller agglomerates (He + O2 plasma) | Annealed in air (450 °C, 2 hrs) | Reduced carbon content and film thickness | Plasma diagnostics | 2018 [83] |
| TTIP | Ar (1 SLM) | Ar (18 SLM) | 370 W | 2.45 GHz | Si | ----- | Crystalline (center and intermediate), amorphous (periphery)/smooth, porous, columnar grains (center) rough and cylindrical grains (intermediate), cauliflower-like agglomerate (periphery) | ----- | ----- | Synthesis; Crystal growth mechanism | 2016 [51] |
| TiCl4 | ----- | O2 | 10–200 W | 13.56 MHz | Ti foil (200 °C–600 °C) | ----- | Crystalline rutile phase (Tsub = 600 °C, O2:TiCl4 = 9:1, 50–300 nm) | ----- | ----- | PEC cells | 1984 [74] |
| TTIP | ----- | ----- | 400 W | 2.45 GHz | FTO (250 °C) | ----- | Crystalline anatase/hierarchical nanotube and multistacked nanotrees | ----- | ----- | Nanoarchitecture; Photovoltaics | 2017 [50] |
| TTIP | N2 (0–50 mL/min) | O2 (50–100 mL/min) | 0–150 W | 13.56 MHz | MCM-41/Silica (200 °C–500 °C) | ----- | Amorphous | Annealed (0–800 °C) | Crystalization (anatase + rutile; particle size: 30.3–60 nm | Photocatalysis | 2012 [53] |
| TTIP | Ar (0.7 SLM) | Ar (17 SLM) | 420 W | 2.45 GHz | Si | ----- | Crystalline anatase (dominant)+ rutile/columnar, cauliflower-like structure composed of nanoparticles (10–20 nm) | ----- | ----- | Synthesis | 2019 [91] |
| TTIP | Ar (0.2 SLM) | Ar (17 SLM) | 420 W | 2.45 GHz | Si, FTO | ----- | Si: crystalline anatase/columnar, faceted grains with intercolumnar porosity; FTO: amorphous/columnar, cauliflower-like assembly | Ultrasound cleaning | Improved optical transmission of films | Synthesis; Solar cells | 2019 [89] |
| TTIP | Ar (PAr+TTIP = 0.225 mbar) g | O2 (partial pressure, 0.177 mbar) g | 50 W | ----- | Si; porous (100 nm; 800 nm) Al2O3 (150 °C) | ----- | Amorphous/micro-columnar porous | Annealed in air (300 °C, 5 h) | Crystallization (anatase, particle size: 20 nm) | Photocatalysis; Water treatment | 2015 [92] |
| TTIP | He (1 SLM) | He (9 SLM) + O2 (0–0.01 SLM) | 70–300 W | 13.56 MHz | Si (dynamic: 5 mm/s) | ----- | He plasma: homogeneous, dense; He + O2 plasma: aggregates | ----- | ----- | Plasma diagnostics | 2019 [78] |
| TIPO a (0.5 gm/hr) | N2 (5 L/min) | N2 (30–50 L/min) | 600–1000 W | 100 kHz | Si (dynamic: 2.5 mm/s) | ----- | Crystalline anatase/Agglomerated, hierarchical with cauliflower-like structure | ----- | ----- | Photocatalysis | 2014 [94] |
| Ti(OtBu)4 j | Ar (4–5.9 mol/hr) | Ar (4–5.9) + O2 (0.12–0.8 mol/hr) | 200–500 W | 13.56 MHz or 2.45 GHz | Glass | ----- | Amorphous/uniform, dense, well-adherent | Annealed (440 °C, 2.5 h) | Crystallization (anatase, particle size = 15 nm) | Photocatalysis; Water purification | 2002 [34] |
| TTIP | Ar (1 SLM) | Ar (18 SLM) | 370 W | 2.45 GHz | Si | ----- | Center: crystalline/columnar; Periphery: amorphous/cauliflower-like structure | ----- | ----- | Plasma diagnostics; Growth mechanism | 2017 [90] |
| Precursor | Carrier Gas (Flow Rate) | Feed Gas (Flow Rate) | Plasma Power e | Frequency | Precursor/Plasma Pulse Time (sec) | Substrate c (Temperature) | Deposition Rate, nm/Cycle (Conditions) | Film Crystallinity (Conditions, Crystallite Size)/Morphology (Conditions, Particle Size) | PD Treatment (TPD, Time) d | Effect of PD Treatment | Scope of Study/Applications | Year [Ref] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTIP a | N2 | H2O; O2; O3 | 160 W | 13.56 MHz | 15/80: O2; 15/30: O3 | Si (150 °C : O2 or O3; 275 °C: H2O | 0.08 (O2 plasma) | Crystalline rutile + anatase (dominant) | ----- | ----- | Instrumentation | 2012 [99] |
| TTIP a | N2 | O2 + Ar (1:1) | 100–160 W | 13.56 MHz | 15/180: O2; 15/30 : O3 | ZnSe; Si (50 °C–150 °C) | 0.083 | Crystalline rutile + anatase (dominant) | ----- | ----- | Deposition mechanism | 2009 [100] |
| TDMAT h | N2 (60 SCCM) | N2 (265 SCCM) | 200 W | ----- | 0.5/30 | Si (350 °C) | ----- | ----- | ---- | ---- | Surface modification | 2018 [41] |
| TiCl4 a | Ar (50 SCCM) | H2O or O2 (5–35) + Ar (50 SCCM) | 300 W | 13.56 MHz | 0.3/3–12 | Si (30 °C–180 °C) | 0.07–0.08 (300 W, Tsub = 90 °C) | Anatase (Tsub = 180 °C (H2O); ≥90 °C (O2))/rough surface | ---- | ---- | Synthesis | 2016 [98] |
| TTIP | Ar (150 SCCM) | O2 (10–100 SCCM) + H2O (as an oxidizer) | 100 and 300 W | 13.56 MHz | 1.5/8 H2O pulse: 0.03g | Si, fused silica (70 °C–200 °C) | ----- | Anatase (low O2 flow; 19–30 nm) in amorphous matrix/scattered hillock-like feature with rough and cliffy surface | ---- | ---- | Plasma diagnostics | 2015 [96] |
| TTIP | Ar (1000 SCCM) | Ar (1000 SCCM) + O2 (0 or 10 SCCM) | 2.5–6.5 kV | 3–8 kHz | 5/7 | ----- | 0.16 | -----/Smooth (roughness ~ 0.25 nm) | ---- | ---- | Solar cells | 2013 [101] |
| TTIP | ----- | O2 or N2O (30 SCCM) | 300 W | ----- | 1/1 : O2 1/5 : N2O | Ru/Ta2O5/SiO2/Si (250 °C) | 0.035 (O2), 0.059 (N2O) | Crystalline rutile/-----spherical particles (30–150 nm) | Annealed in 5% O2 + 95% N2 (400 °C, 30 mins) | Decrease dielectric constant | DRAM; Electronics | 2009 [68] |
| TiCl4 a | N2 (250 SCCM) | O2 (50 SCCM) | 150 W | 13.56 MHz | 0.4/3 | Si (150 °C) | 0.057 (LCM); f 0.062–0.08 (HCM) | Amorphous/Smooth (LCM); round or conical crystallites in amorphous matrix (HCM) | ----- | ----- | Plasma diagnostics; Synthesis | 2017 [103] |
| TDEAT i | He | O2 (250 SCCM) + Ar (2500 SCCM) | 75 W | 13.56 MHz | 4/2 | Si, SiO2, TiN (110 °C–350 °C) | 0.045 (Si, Tsub ≤ 300 °C) | ----- | O2/Ar/NF3 (250/2500/5 SCCM) plasma | Etching (etching rate = 0.19 nm/s) | Synthesis; Electronics | 2019 [69] |
| TTIP; TiCl4 a | N2 (250 SCM) | O2 (50 SCCM) + N2 (250 SCCM) | 50–200 W | 13.56 MHz | 0.1–2/0.1–2 | Si (250 °C) | 0.092 : TiCl4; 0.059 : TTIP (150 W) | Crystalline anatase/granular | ----- | ----- | Plasma diagnostics | 2016 [97] |
| TDMAT h | Ar (100 SCCM) | O2 | 60 W | ----- | 0.1/0.5 | Si (150 °C–250 °C) | 0.036 (200 °C) | -----/Smooth, uniform, continuous (200 °C) | Annealed in O2 (500 °C–700 °C, 10 mins) | Increase roughness | Electronics | 2004 [67] |
| TTIP a | N2 (25 SCM) | O2 (10 SCCM) + N2 | 1–9 kV | 60–70 kHz | 0.315–1.9/0.0004 | Si b | 0.15 | Amorphous/smooth | ----- | ----- | Fabrication; Optics | 2019 [93] |
| Precursor | Carrier Gas (Flow Rate) | Feed Gas (Flow Rate) | Plasma Power f | Frequency | Substrate c (Temperature) | Deposition Rate, nm/min | Film Crystallinity (Conditions, Crystallite Size)/Morphology (Conditions, Particle Size) | PD Treatment (TPD, Time) d | Effect of PD Treatment | Scope of Study/Applications | Year [Ref] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atmospheric Pressure Dielectric Barrier Discharge | |||||||||||
| TTIP | Ar (2 SLM) | O2 (0.5 SLM); Ar (2 SLM) +NH3 (NH3/Ar = 0.5–5%): N-doping | 30 W; 30–100 | 2.7 kHz | Si (150 °C) | ----- | Amorphous/----- | Annealed in air (450 °C, 2 hrs) | Crystallization (anatase) | Photocatalysis; Doping | 2019 [43] |
| TTIP | Ar (0–30 SLM) | O2 (0–7.5 SLM) + Ar (0–9.5 SLM) | 6–30 W (source) | 2.7 kHz | Si (150 °C) | 107 (Ar/O2: 7.5/0.5 SLM; 15 W) | Amorphous/granular (low flow rate or high power); Dense (high flow rate or low power) | Annealed in air (400 °C, 2 hrs) | Crystallization (anatase + rutile) | Photocatalysis | 2018 [107] |
| ----- | ----- | Ar (500 SCCM) | 40–80 W | 13.56 MHz | TiO2/quartz | ----- | Anatase (power ≥ 60 W)/ columnar (power = 80 W) | ---- | ---- | Calcination; Photocatalysis | 2019 [76] |
| TTIP | He (480–500 SCCM) | O2 (0–20 SCCM) | 14 W | 13.56 MHz | Quartz | 13 (He + O2), 2% of O2 | Amorphous/He: rough with craters and wrinkles; 2% of O2: smooth and transparent; 4% of O2: micro-nanoparticles (0.3–3 µm) | ---- | ---- | Synthesis | 2008 [49] |
| TiCl4 | Ar (25 SCCM) | Ar (1000 SCCM) + O2 (10 SCCM) | 11 KV | 10 kHz | Quartz (no heating or 100 °C–400 °C) | 1000–3000 | Amorphous (no heating); anatase (Tsub ≥ 300 °C)/dense and fluffy (Tsub ≤ 200 °C); dense cauliflower-like aggregate (Tsub ≥ 300 °C) | Annealed in Ar (275 °C, 2 hrs) e | No change in crystallinity | Photocatalysis | 2019 [77] |
| TTIP + H2O (10 µL/min) | N2 (137.4 SCCM) 0%/100% (1 SLM) | ----- | 0–10.2 kV | 60–240 Hz | Pyrex glass | ----- | Anatase (22.2 nm)/nanoparticles (372–27.9 nm at 0–8.58 kV; 60 kHz; TTIP/H2O = 11.9); film (240 Hz; 10.2 kV, TTIP/H2O = 11.9) spherical particles (30–150 nm) | ---- | ---- | Synthesis | 2007 [110] |
| TiO2 NPs + HMDSO f | ----- | N2 (1.2 SLM) + N2O (240 SCCM) | ----- | 0.3–5 kHz | Si | ----- | ----- | ----- | ----- | Nanocomposite synthesis | 2016 [111] |
| TTIP | N2 (Glass: 25 SCCM) (Si: 53 SCCM) | O2 (glass: 0 SCCM) (Si: 0–10 SCCM) | 1–9 kV | 60–70 kHz | silicate glass; Si b | Glass: 4700–5500 Si: 3250 | Amorphous/rough with particle-like features, macroporous (glass); smooth (Si) | ----- | ----- | Fabrication; Optics | 2019 [93] |
| Atmospheric Pressure Plasma Jet/Torch | |||||||||||
| ----- | ----- | Ar (10 L/min) + N2 (0.5 L/min) | 150 W | 10 kHz | TiO2/FTO glass | ----- | Rutile + anatase (10–50 nm)/porous (25 nm) | ----- | ----- | DSSCs | 2008 [70] |
| TEOT | He (20 SCCM) | He (300 SCCM) + H2 (0–25 SCCM) | 105 W | 13.56 MHz | Si (200 °C–500 °C) | 900 (Tsub = 500 °C) | Amorphous (Tsub < 350 °C), Crystalline (anatase, >350 °C) | ----- | ----- | Synthesis; Electronics | 1996 [71] |
| TIOT g | Ar (3000 SCCM) | Ar (4000 SCCM) | 18–26 kV | 50 kHz | ----- | ----- | Anatase (dominant) + rutile/cauliflower-like structure with densely aggregated spherical particles with a diameter < 100 nm | ----- | ----- | Photocatalysis; Hydrophilicity | 2020 [109] |
| TTIP a | Ar (0.2–0.5 L/min) | Ar (5 L/min) | 6 kV | 50 kHz | Si; Quartz (25 °C–90 °C) | 44 | Amorphous/rough, granular | ----- | ----- | Protective coating | 2018 [32] |
| TiCl4 | O2 (25 SCCM) | Ar (6000 SCCM) | 30 W | 13.56 MHz | FTO glass | 9000 | Amorphous (25 nm)/porous | Annealed in air (450–500 °C, 1 hr) | Crystallization (anatase) | DSSCs | 2010 [64] |
| Pulsed Injection Metallorganic Chemical Vapor Deposition | |||||||||||
| TIPO; TOAA a | N2 (80%) + O2 (20%) (3.5–5 L/min) | N2 (80%) + O2 (20%) (15 L/min) | 600 W | 100 kHz | Si (200 °C–300 °C) | 6.67 | Amorphous (Tsub <270 °C), anatase (>270 °C)/smooth, granular (20 nm) | ----- | ----- | Low temperature synthesis | 2008 [72] |
| Surface Dielectric Barrier Discharge and Diffuse Coplanar Surface Barrier Discharge | |||||||||||
| TiCl4 | Ar (14 SCCM) | O2 (9 SCCM) + Ar (900 SCCM) | 4.2 W | 15 kHz | Glass | 22 | Amorphous/----- | Annealed in air (350–450 °C) | Crystallization (anatase) | Photocatalysis | 2009 [65] |
| ----- | ----- | Ambient air | 400 W | 14 kHz | Ti(Bu)/PVP fiber h | ----- | -----/Granular | ----- | ----- | Submicron fiber fabrication | 2018 [62] |
| ----- | ----- | ----- | ----- | ----- | TiO2 + methyl silica binder/FTO | ----- | ----- | ----- | ----- | Surface processing; Photocatalysis | 2016 [108] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Adhikari, E.; Sapkota, P.; Sebastian, A.; Ptasinska, S. Atmospheric Pressure Plasma Deposition of TiO2: A Review. Materials 2020, 13, 2931. https://doi.org/10.3390/ma13132931
Banerjee S, Adhikari E, Sapkota P, Sebastian A, Ptasinska S. Atmospheric Pressure Plasma Deposition of TiO2: A Review. Materials. 2020; 13(13):2931. https://doi.org/10.3390/ma13132931
Chicago/Turabian StyleBanerjee, Soumya, Ek Adhikari, Pitambar Sapkota, Amal Sebastian, and Sylwia Ptasinska. 2020. "Atmospheric Pressure Plasma Deposition of TiO2: A Review" Materials 13, no. 13: 2931. https://doi.org/10.3390/ma13132931
APA StyleBanerjee, S., Adhikari, E., Sapkota, P., Sebastian, A., & Ptasinska, S. (2020). Atmospheric Pressure Plasma Deposition of TiO2: A Review. Materials, 13(13), 2931. https://doi.org/10.3390/ma13132931






