1D Titanium Dioxide: Achievements in Chemical Sensing
Abstract
:1. Introduction
2. Crystal Structure of TiO2
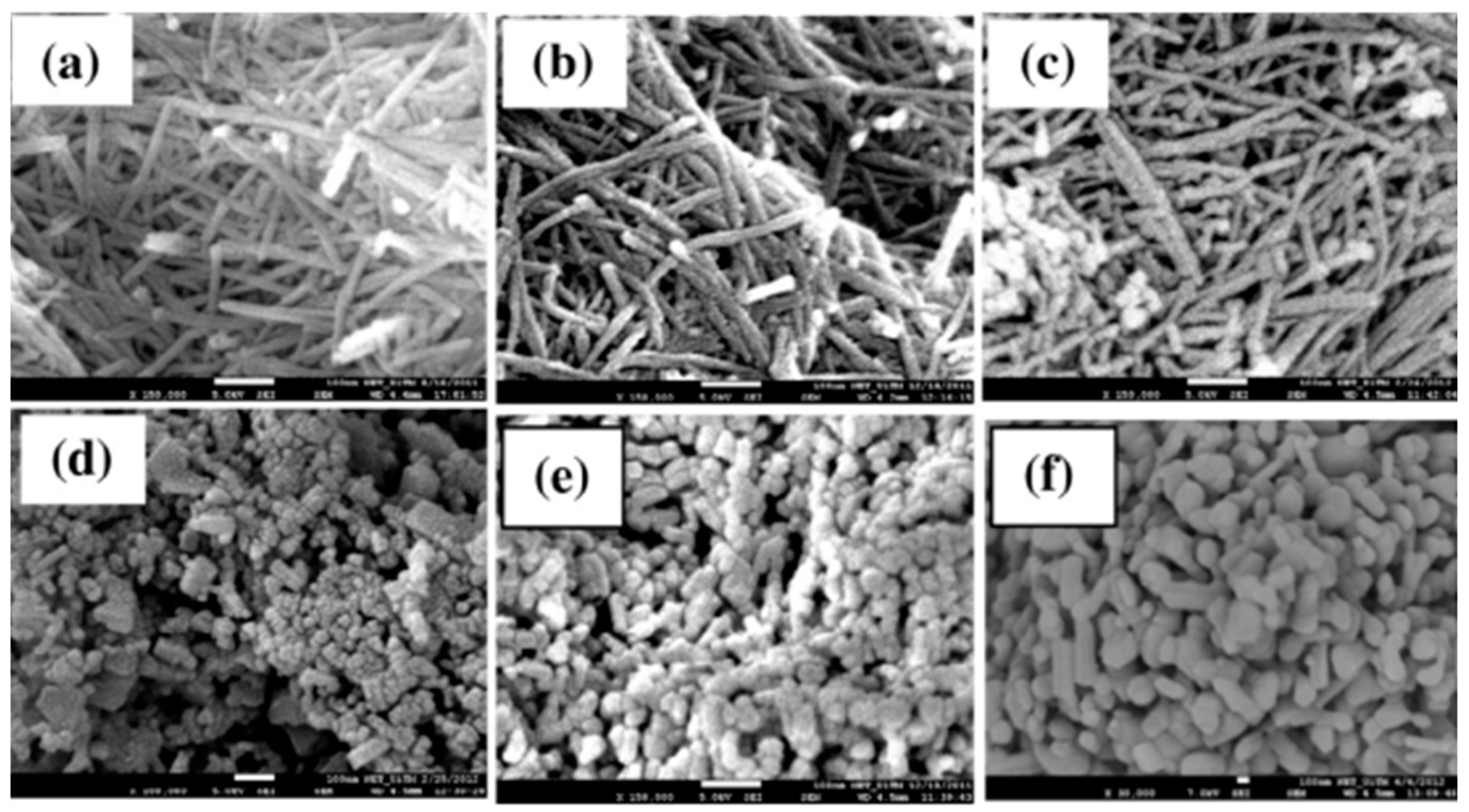
3. Synthesis of TiO2-Based 1D Nanostructures
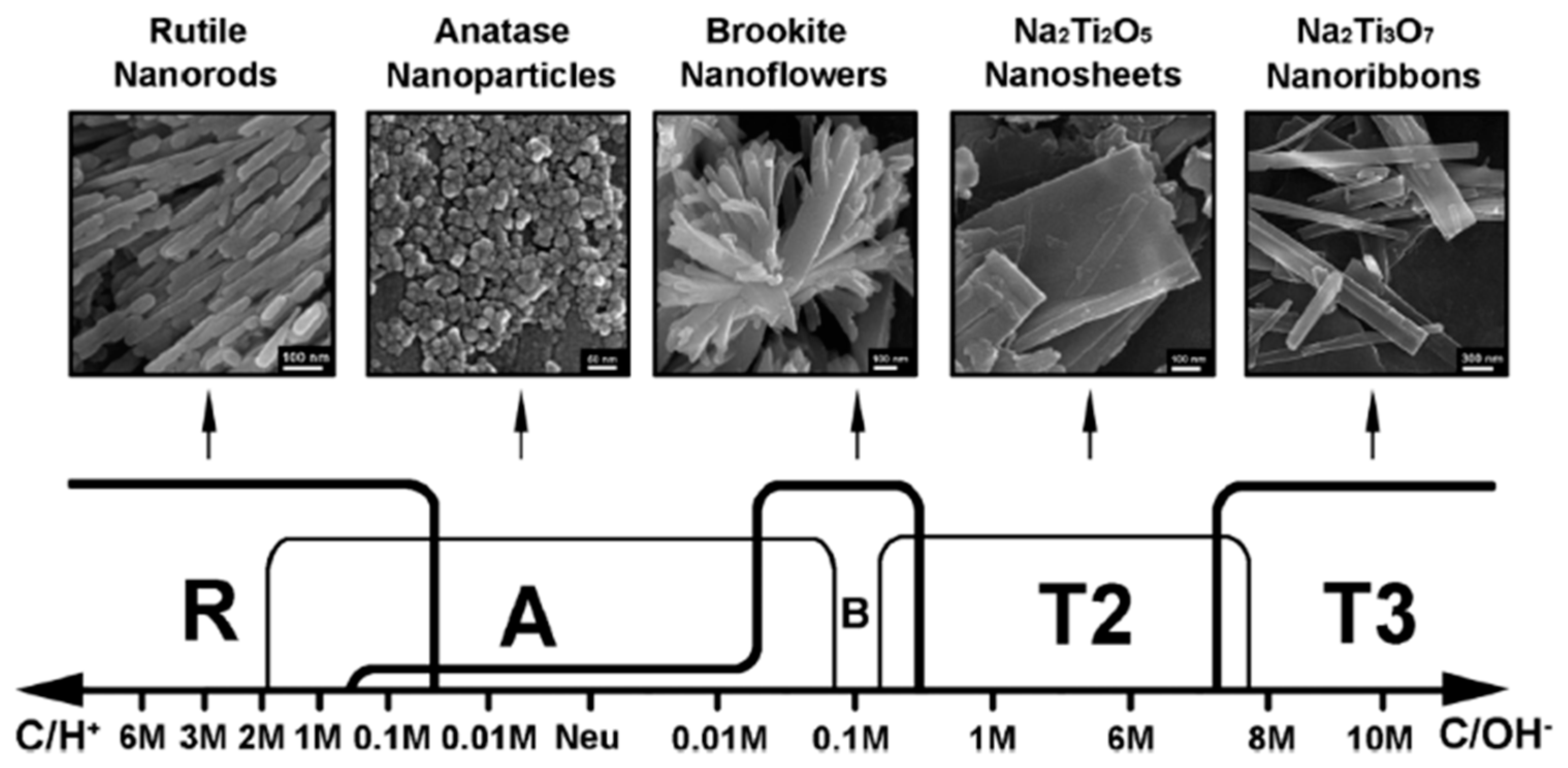
3.1. Hydrothermal Synthesis
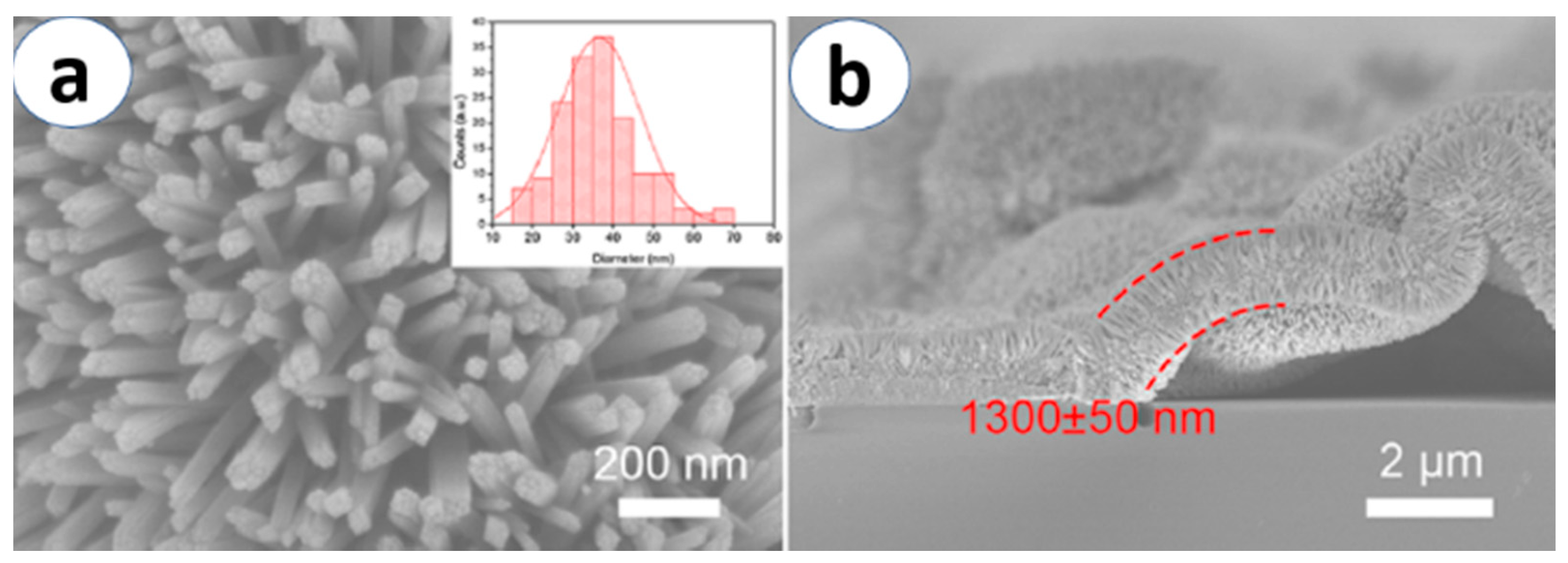
3.2. Electrochemical Anodization
3.3. Electrospinning
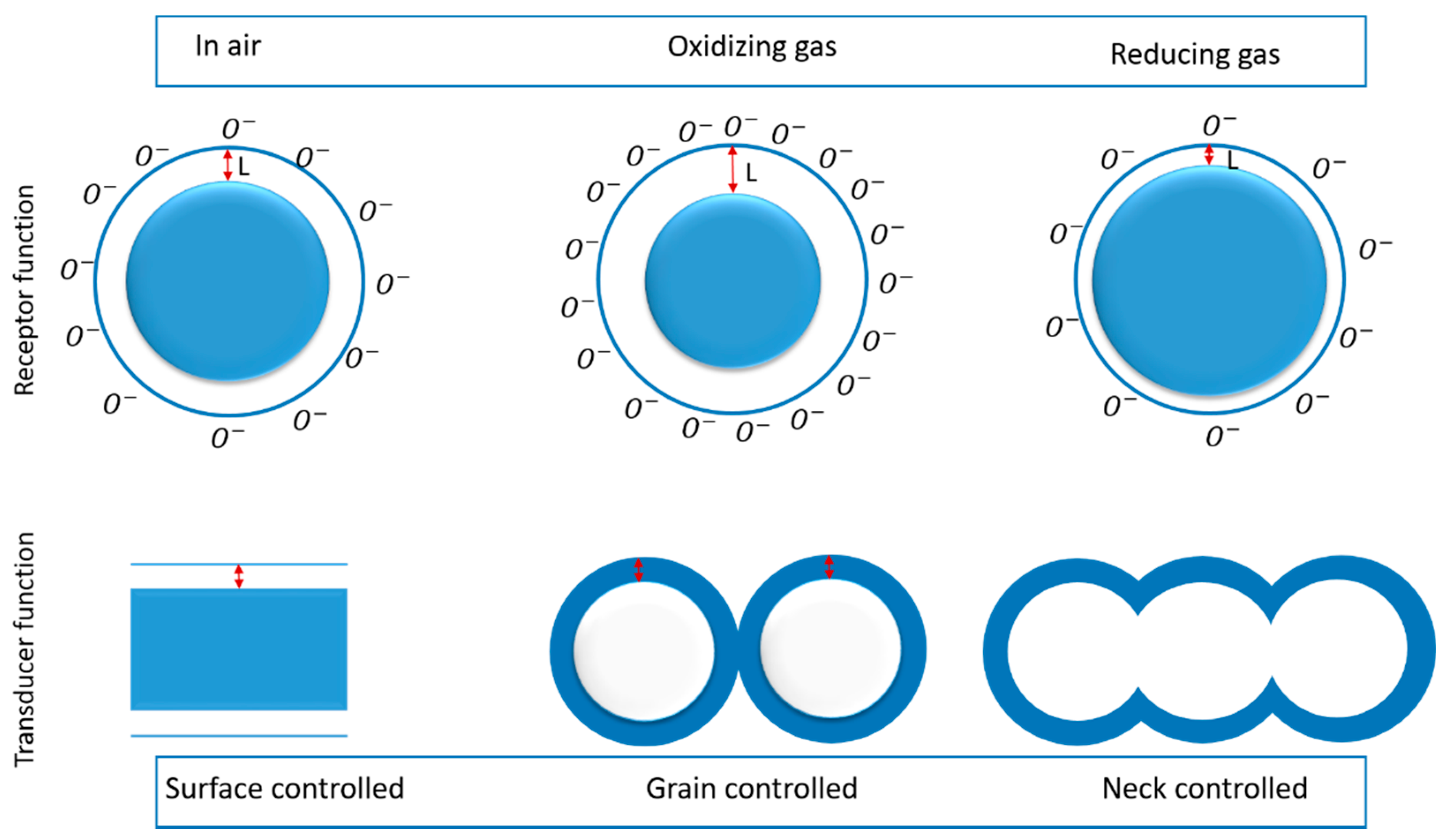
4. Working Principles of TiO2-Based Chemical Sensors
5. Chemical Sensing Properties
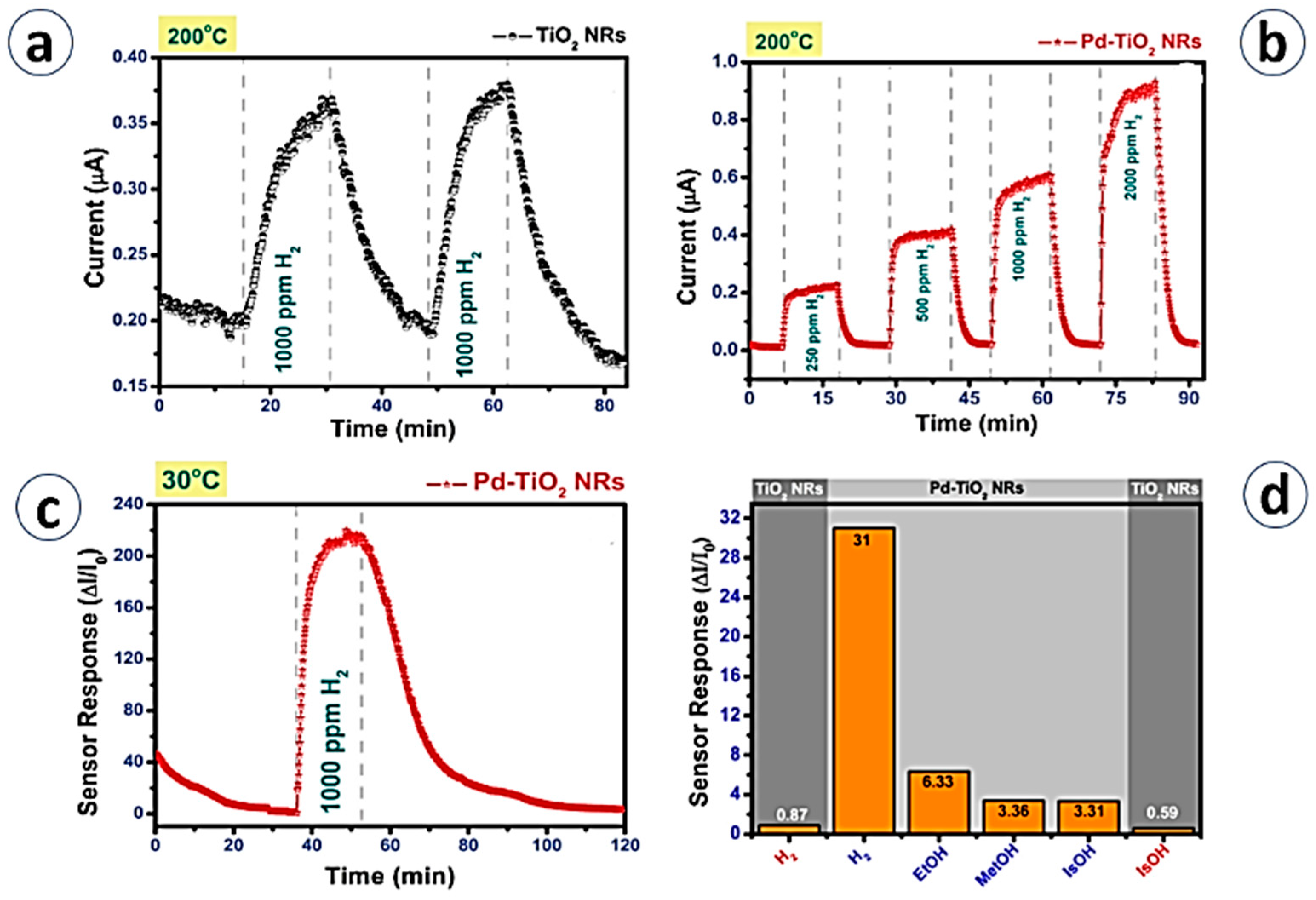
5.1. Sensing of Reducing Gases
5.2. Sensing of Oxidizing Gases
5.3. Effect of Humidity
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Comini, E. Metal oxide nanowire chemical sensors: Innovation and quality of life. Mater. Today 2016, 19, 559–567. [Google Scholar] [CrossRef]
- Zhou, Z.; Lan, C.; Wei, R.; Ho, J.C. Transparent metal-oxide nanowires and their applications in harsh electronics. J. Mater. Chem. C 2019, 7, 202–217. [Google Scholar] [CrossRef]
- Comini, E.; Bianchi, S.; Faglia, G.; Ferroni, M.; Vomiero, A.; Sberveglieri, G. Functional nanowires of tin oxide. Appl. Phys. A 2007, 89, 73–76. [Google Scholar] [CrossRef]
- Shen, Y.; Yamazaki, T.; Liu, Z.; Meng, D.; Kikuta, T.; Nakatani, N.; Saito, M.; Mori, M. Microstructure and H2 gas sensing properties of undoped and Pd-doped SnO2 nanowires. Sens. Actuators B Chem. 2009, 135, 524–529. [Google Scholar] [CrossRef]
- Suman, P.H.; Felix, A.A.; Tuller, H.L.; Varela, J.A.; Orlandi, M.O. Comparative gas sensor response of SnO2, SnO and Sn3O4 nanobelts to NO2 and potential interferents. Sens. Actuators B Chem. 2015, 208, 122–127. [Google Scholar] [CrossRef]
- Sharma, A.P.; Dhakal, P.; Pradhan, D.K.; Behera, M.K.; Xiao, B.; Baha, M. Fabrication and characterization of SnO2 nanorods for room temperature gas sensors. AIP Adv. 2018, 8, 095219. [Google Scholar] [CrossRef] [Green Version]
- Forleo, A.; Francioso, L.; Capone, S.; Casino, F.; Siciliano, P.; Tan, O.K.; Hui, H. Wafer-Level Fabrication and Gas Sensing Properties of miniaturized gas sensors based on Inductively Coupled Plasma Deposited Tin Oxide Nanorods. Procedia Chem. 2009, 1, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Qian, L.H.; Wang, K.; Li, Y.; Fang, H.T.; Lu, Q.H.; Ma, X.L. CO sensor based on Au-decorated SnO2 nanobelt. Mater. Chem. Phys. 2006, 100, 82–84. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, M.; Comini, E. One-Dimensional Nanostructured Oxide Chemoresistive Sensors. Langmuir 2020, 36, 6326–6344. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Zappa, D.; Poli, N.; Sberveglieri, G. Nickel oxide nanowires: Vapor liquid solid synthesis and integration into a gas sensing device. Nanotechnology 2016, 27, 205701. [Google Scholar] [CrossRef]
- Kaur, N.; Zappa, D.; Poli, N.; Comini, E. Integration of VLS-Grown WO3 Nanowires into Sensing Devices for the Detection of H2S and O3. ACS Omega 2019, 4, 16336–16343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asiah, M.N.; Mamat, M.H.; Khusaimi, Z.; Achoi, M.F.; Abdullah, S.; Rusop, M. Thermal stability and phase transformation of TiO2 nanowires at various temperatures. Microelectron. Eng. 2013, 108, 134–137. [Google Scholar] [CrossRef]
- Fàbrega, C.; Casals, O.; Hernández-Ramírez, F.; Prades, J.D. A review on efficient self-heating in nanowire sensors: Prospects for very-low power devices. Sens. Actuators B Chem. 2018, 256, 797–811. [Google Scholar] [CrossRef] [Green Version]
- Baratto, C.; Comini, E.; Ferroni, M.; Faglia, G.; Sberveglieri, G. Plasma-induced enhancement of UV photoluminescence in ZnO nanowires. CrystEngComm 2013, 15, 7981. [Google Scholar] [CrossRef]
- Kaur, N.; Comini, E.; Poli, N.; Zappa, D.; Sberveglieri, G. Nickel Oxide Nanowires Growth by VLS Technique for Gas Sensing Application. Procedia Eng. 2015, 120, 760–763. [Google Scholar] [CrossRef]
- Topcu, S.; Jodhani, G.; Gouma, P. Optimized Nanostructured TiO2 Photocatalysts. Front. Mater. 2016, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Mavrič, T.; Benčina, M.; Imani, R.; Junkar, I.; Valant, M.; Kralj-Iglič, V.; Iglič, A. Electrochemical Biosensor Based on TiO2 Nanomaterials for Cancer Diagnostics. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier B.V.: Cambridge, MA, USA, 2018; Volume 27, pp. 63–105. [Google Scholar]
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-based gas sensors with enhancedsensitivity to reducing gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Mora-Seró, I.; De Angelis, F.; Bisquert, J.; Wang, P. Titanium Dioxide Nanomaterials for Photovoltaic Applications. Chem. Rev. 2014, 114, 10095–10130. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Zhou, X.; Lin, H.; Peng, F.; Zhang, S. Designing efficient TiO2-based photoelectrocatalysis systems for chemical engineering and sensing. Chem. Eng. J. 2020, 381, 122605. [Google Scholar] [CrossRef]
- Mardare, D.; Cornei, N.; Mita, C.; Florea, D.; Stancu, A.; Tiron, V.; Manole, A.; Adomnitei, C. Low temperature TiO2 based gas sensors for CO2. Ceram. Int. 2016, 42, 7353–7359. [Google Scholar] [CrossRef]
- Cui, H.-F.; Wu, W.-W.; Li, M.-M.; Song, X.; Lv, Y.; Zhang, T.-T. A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Lee, K.H.; Sasaki, S.; Hashimoto, K.; Ikebukuro, K.; Karube, I. Photocatalytic sensor for chemical oxygen demand determination based on oxygen electrode. Anal. Chem. 2000, 72, 3379–3382. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yin, W.; Li, J.; Bai, J.; Huang, K.; Li, J.; Zhou, B. TiO2 Nanotube Sensor for Online Chemical Oxygen Demand Determination in Conjunction with Flow Injection Technique. Water Environ. Res. 2014, 86, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef] [PubMed]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Polymorphic phase transition among the titania crystal structures using a solution-based approach: From precursor chemistry to nucleation process. Nanoscale 2014, 6, 11574–11632. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.; Jin, J.; Peng, T. Preparation of brookite TiO2 nanoparticles with small sizes and the improved photovoltaic performance of brookite-based dye-sensitized solar cells. Nanoscale 2016, 8, 18771–18781. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, D.; Liu, Z.; Wu, N.-L.; Wei, M. Brookite TiO2 mesocrystals with enhanced lithium-ion intercalation properties. Chem. Commun. 2018, 54, 11491–11494. [Google Scholar] [CrossRef]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chinese Sci. Bull. 2011, 56, 1639. [Google Scholar] [CrossRef] [Green Version]
- El Goresy, A.; Chen, M.; Gillet, P.; Dubrovinsky, L.; Graup, G.; Ahuja, R. A natural shock-induced dense polymorph of rutile with α-PbO2 structure in the suevite from the Ries crater in Germany. Earth Planet. Sci. Lett. 2001, 192, 485–495. [Google Scholar] [CrossRef]
- Karthick, S.N.; Hemalatha, K.V.; Balasingam, S.K.; Manik Clinton, F.; Akshaya, S.; Kim, H. Dye-Sensitized Solar Cells: History, Components, Configuration, and Working Principle. In Interfacial Engineering in Functional Materials for Dye-Sensitized Solar Cells; Wiley: Hoboken, NJ, USA, 2019; pp. 1–16. [Google Scholar]
- Van de Krol, R. Erratum: “Mott-Schottky Analysis of Nanometer-Scale Thin-Film Anatase TiO2” [J. Electrochem. Soc., 144, 1723 (1997)]. J. Electrochem. Soc. 1998, 145, 3697. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Shi, J.; Wang, X. Growth of rutile titanium dioxide nanowires by pulsed chemical vapor deposition. Cryst. Growth Des. 2011, 11, 949–954. [Google Scholar] [CrossRef]
- Kemell, M.; Pore, V.; Tupala, J.; Ritala, M.; Leskelä, M. Atomic layer deposition of nanostructured TiO2 photocatalysts via template approach. Chem. Mater. 2007, 19, 1816–1820. [Google Scholar] [CrossRef]
- Maijenburg, A.W.; Veerbeek, J.; De Putter, R.; Veldhuis, S.A.; Zoontjes, M.G.C.; Mul, G.; Montero-Moreno, J.M.; Nielsch, K.; Schäfer, H.; Steinhart, M.; et al. Electrochemical synthesis of coaxial TiO2-Ag nanowires and their application in photocatalytic water splitting. J. Mater. Chem. A 2014, 2, 2648–2656. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Lin, L.; He, D. Phase and morphological transitions of titania/titanate nanostructures from an acid to an alkali hydrothermal environment. J. Mater. Chem. A 2013, 1, 1659–1668. [Google Scholar] [CrossRef]
- Rodríguez-Reyes, M.; Dorantes-Rosales, H.J. A simple route to obtain TiO2 nanowires by the sol-gel method. J. Sol Gel Sci. Technol. 2011, 59, 658–661. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Salehifar, N. Improvement in gas-sensing properties of TiO2 nanofiber sensor by UV irradiation. Sens. Actuators B Chem. 2015, 211, 146–156. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolesnichenko, V.L.; O’Connor, C.J. Recent advances in the liquid-phase syntheses of inorganic nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef]
- Alev, O.; Şennik, E.; Kilinç, N.; Öztürk, Z.Z. Gas sensor application of hydrothermally growth TiO2 nanorods. Procedia Eng. 2015, 120, 1162–1165. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.Y.; Cheng, X.L.; Zhang, X.F.; Zheng, Z.K.; Tang, X.F.; Xu, Y.M.; Gao, S.; Zhao, H.; Huo, L.H. A novel sensor for fast detection of triethylamine based on rutile TiO2 nanorod arrays. Sens. Actuators B Chem. 2014, 205, 322–328. [Google Scholar] [CrossRef]
- Zhao, G.; Xuan, J.; Gong, Q.; Wang, L.; Ren, J.; Sun, M.; Jia, F.; Yin, G.; Liu, B. In Situ Growing Double-Layer TiO2 Nanorod Arrays on New-Type FTO Electrodes for Low-Concentration NH3 Detection at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 8573–8582. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Liu, W. One-step photochemical deposition of PdAu alloyed nanoparticles on TiO2 nanowires for ultra-sensitive H2 detection. J. Mater. Chem. A 2016, 4, 2236–2245. [Google Scholar] [CrossRef]
- Alam, U.; Fleisch, M.; Kretschmer, I.; Bahnemann, D.; Muneer, M. One-step hydrothermal synthesis of Bi-TiO2 nanotube/graphene composites: An efficient photocatalyst for spectacular degradation of organic pollutants under visible light irradiation. Appl. Catal. B Environ. 2017, 218, 758–769. [Google Scholar] [CrossRef]
- Ng, S.; Kuberský, P.; Krbal, M.; Prikryl, J.; Gärtnerová, V.; Moravcová, D.; Sopha, H.; Zazpe, R.; Yam, F.K.; Jäger, A.; et al. ZnO Coated Anodic 1D TiO2 Nanotube Layers: Efficient Photo-Electrochemical and Gas Sensing Heterojunction. Adv. Eng. Mater. 2018, 20, 1700589. [Google Scholar] [CrossRef] [Green Version]
- Alev, O.; Kılıç, A.; Çakırlar, Ç.; Büyükköse, S.; Öztürk, Z. Gas Sensing Properties of p-Co3O4/n-TiO2 Nanotube Heterostructures. Sensors 2018, 18, 956. [Google Scholar] [CrossRef] [Green Version]
- Alev, O.; Şennik, E.; Öztürk, Z.Z. Improved gas sensing performance of p-copper oxide thin film/n-TiO2 nanotubes heterostructure. J. Alloys Compd. 2018, 749, 221–228. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.X.; Song, H.; Wang, J.W.; Song, Y.; Liang, Y.Q.; Zhang, X.M. One-Dimensional MoS2-Decorated TiO2 nanotube gas sensors for efficient alcohol sensing. J. Alloys Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Sopha, H.; Hromadko, L.; Nechvilova, K.; Macak, J.M. Effect of electrolyte age and potential changes on the morphology of TiO2 nanotubes. J. Electroanal. Chem. 2015, 759, 122–128. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; Grimes, C.A. Long vertically aligned titania nanotubes on transparent conducting oxide for highly efficient solar cells. Nat. Nanotechnol. 2009, 4, 592–597. [Google Scholar] [CrossRef]
- Munirathinam, B.; Pydimukkala, H.; Ramaswamy, N.; Neelakantan, L. Influence of crystallite size and surface morphology on electrochemical properties of annealed TiO2 nanotubes. Appl. Surf. Sci. 2015, 355, 1245–1253. [Google Scholar] [CrossRef]
- Mazierski, P.; Nischk, M.; Gołkowska, M.; Lisowski, W.; Gazda, M.; Winiarski, M.J.; Klimczuk, T.; Zaleska-Medynska, A. Photocatalytic activity of nitrogen doped TiO2 nanotubes prepared by anodic oxidation: The effect of applied voltage, anodization time and amount of nitrogen dopant. Appl. Catal. B Environ. 2016, 196, 77–88. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Y.; Wu, B.; Zhang, X. Different crystalline phases of aligned TiO2 nanowires and their ethanol gas sensing properties. Phys. E Low Dimens. Syst. Nanostruct. 2019, 114, 113601. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Z.X.; Wang, W.H.; Kuo, C.L.; Hwang, K.C.; Hong, C.C. Self-regenerating photocatalytic sensor based on dielectrophoretically assembled TiO2 nanowires for chemical vapor sensing. Sens. Actuators B Chem. 2014, 194, 1–9. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Lu, H.; Yan, C.; Chen, K.; Lu, H.; Gao, J.; Yang, Z.; Zhu, G.; Wang, C.; et al. Ethanol sensing properties and reduced sensor resistance using porous Nb2O5-TiO2 n-n junction nanofibers. Sens. Actuators B Chem. 2019, 283, 602–612. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B Chem. 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Wang, L.; Gao, J.; Wu, B.; Kan, K.; Xu, S.; Xie, Y.; Li, L.; Shi, K. Designed Synthesis of In2O3 Beads@TiO2-In2O3 Composite Nanofibers for High Performance NO2 Sensor at Room Temperature. ACS Appl. Mater. Interfaces 2015, 7, 27152–27159. [Google Scholar] [CrossRef]
- Arafat, M.M.; Haseeb, A.S.M.A.; Akbar, S.A.; Quadir, M.Z. In-situ fabricated gas sensors based on one dimensional core-shell TiO2-Al2O3 nanostructures. Sens. Actuators B Chem. 2017, 238, 972–984. [Google Scholar] [CrossRef]
- Wang, H.; Yao, Y.; Wu, G.; Sun, Q.; Wang, M.; Chen, X.; Wang, J. A room temperature oxygen gas sensor based on hierarchical TiO2. In Proceedings of the IEEE 3M-NANO 2016–6th IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, Chongqing, China, 18–22 July 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA; pp. 199–202. [Google Scholar]
- Lee, J.S.; Katoch, A.; Kim, J.H.; Kim, S.S. Growth of networked TiO2 nanowires for gas-sensing applications. J. Nanosci. Nanotechnol. 2016, 16, 11580–11585. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Shingange, K.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Fabrication of ultra-high sensitive and selective CH4 room temperature gas sensing of TiO2 nanorods: Detailed study on the annealing temperature. Sens. Actuators B Chem. 2017, 238, 402–419. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, Y.C. Design of nanoscaled surface morphology of TiO2-Ag2O composite nanorods through sputtering decoration process and their low-concentration NO2 gas-sensing behaviors. Nanomaterials 2019, 9, 1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Lin, S.J.; Wu, C.H.; Wu, R.J. Synthesis of TiO2 nanowires for rapid NO2 detection. Sens. Actuators A Phys. 2018, 272, 288–294. [Google Scholar] [CrossRef]
- Enachi, M.; Lupan, O.; Braniste, T.; Sarua, A.; Chow, L.; Mishra, Y.K.; Gedamu, D.; Adelung, R.; Tiginyanu, I. Integration of individual TiO 2 nanotube on the chip: Nanodevice for hydrogen sensing. Phys. Status Solidi—Rapid Res. Lett. 2015, 9, 171–174. [Google Scholar] [CrossRef]
- Kumar, K.G.; Avinash, B.S.; Rahimi-Gorji, M.; Majdoubi, J. Photocatalytic activity and smartness of TiO2 nanotube arrays for room temperature acetone sensing. J. Mol. Liq. 2020, 300, 112418. [Google Scholar] [CrossRef]
- Moon, J.; Hedman, H.P.; Kemell, M.; Tuominen, A.; Punkkinen, R. Hydrogen sensor of Pd-decorated tubular TiO2 layer prepared by anodization with patterned electrodes on SiO2/Si substrate. Sens. Actuators B Chem. 2016, 222, 190–197. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Wang, H.; Xiong, M.; Yang, T.; Zakharova, G.S. Highly sensitive and selective ammonia gas sensors based on PbS quantum dots/TiO2 nanotube arrays at room temperature. Sens. Actuators B Chem. 2016, 236, 529–536. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Bhowmik, B.; Fecht, H.J. Operating Temperature, Repeatability, and Selectivity of TiO2 Nanotube-Based Acetone Sensor: Influence of Pd and Ni Nanoparticle Modifications. IEEE Trans. Device Mater. Reliab. 2015, 15, 376–383. [Google Scholar] [CrossRef]
- Perillo, P.M.; Rodríguez, D.F. TiO2 nanotubes membrane flexible sensor for low-temperature H2S detection. Chemosensors 2016, 4, 15. [Google Scholar] [CrossRef] [Green Version]
- Tong, X.; Shen, W.; Chen, X. Enhanced H2S sensing performance of cobalt doped free-standing TiO2 nanotube array film and theoretical simulation based on density functional theory. Appl. Surf. Sci. 2019, 469, 414–422. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.; Meng, C.; Gao, Z.; Cao, X.; Li, X.; Xu, L.; Zhu, W.; Peng, X.; Zhang, B.; et al. A high-response ethanol gas sensor based on one-dimensional TiO2/V2O5 branched nanoheterostructures. Nanotechnology 2016, 27, 425503. [Google Scholar] [CrossRef] [PubMed]
- Şennik, E.; Alev, O.; Öztürk, Z.Z. The effect of Pd on the H2 and VOC sensing properties of TiO2 nanorods. Sens. Actuators B Chem. 2016, 229, 692–700. [Google Scholar] [CrossRef]
- Kusior, A.; Radecka, M.; Zych, L.; Zakrzewska, K.; Reszka, A.; Kowalski, B.J. Sensitization of TiO2/SnO2 nanocomposites for gas detection. Sens. Actuators B Chem. 2013, 189, 251–259. [Google Scholar] [CrossRef]
- Zakrzewska, K. Gas sensing mechanism of TiO2-based thin films. Pergamon 2004, 74, 335–338. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Faglia, G.; Sberveglieri, G. TiO2 Nanotubes: Recent Advances in Synthesis and Gas Sensing Properties. Sensors 2013, 13, 14813–14838. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horizons 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Göpel, W.; Schierbaum, K.D. SnO2 sensors: Current status and future prospects. Sens. Actuators B Chem. 1995, 26, 1–12. [Google Scholar] [CrossRef]
- Williams, D.E. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Sabri, Y.M.; Kandjani, A.E.; Rashid, S.S.A.A.H.; Harrison, C.J.; Ippolito, S.J.; Bhargava, S.K. Soot template TiO2 fractals as a photoactive gas sensor for acetone detection. Sens. Actuators B Chem. 2018, 275, 215–222. [Google Scholar] [CrossRef]
- Avinash, B.S.; Chaturmukha, V.S.; Harish, B.M.; Jayanna, H.S.; Rajeeva, M.P.; Naveen, C.S.; Lamani, A.R. Synthesis, Characterization and Room Temperature Acetone Sensing of TiO2 Nanotubes. Sens. Lett. 2018, 16, 105–109. [Google Scholar] [CrossRef]
- Hazra, A.; Bhowmik, B.; Dutta, K.; Chattopadhyay, P.P.; Bhattacharyya, P. Stoichiometry, length, and wall thickness optimization of TiO2 nanotube array for efficient alcohol sensing. ACS Appl. Mater. Interfaces 2015, 7, 9336–9348. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Ma, S.; Sun, A.; Xu, X.; Yang, G.; Gao, J.; Zhang, Z.; Zhu, H. Characterization and acetone gas sensing properties of electrospun TiO2 nanorods. Superlattices Microstruct. 2015, 81, 107–113. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2−x/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2020, 20, 74. [Google Scholar] [CrossRef] [Green Version]
- Pandey, S.K.; Kim, K.H.; Tang, K.T. A review of sensor-based methods for monitoring hydrogen sulfide. TrAC Trends Anal. Chem. 2012, 32, 87–99. [Google Scholar] [CrossRef]
- Moon, C.H.; Zhang, M.; Myung, N.V.; Haberer, E.D. Highly sensitive hydrogen sulfide (H2S) gas sensors from viral-templated nanocrystalline gold nanowires. Nanotechnology 2014, 25, 135205. [Google Scholar] [CrossRef]
- Carslaw, D.C.; Farren, N.J.; Vaughan, A.R.; Drysdale, W.S.; Young, S.; Lee, J.D. The diminishing importance of nitrogen dioxide emissions from road vehicle exhaust. Atmos. Environ. X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Predic, B.; Yan, Z.; Eberle, J.; Stojanovic, D.; Aberer, K. ExposureSense: Integrating daily activities with air quality using mobile participatory sensing. In Proceedings of the 2013 IEEE International Conference on Pervasive Computing and Communications Workshops, PerCom Workshops, San Diego, CA, USA, 18–22 March 2013; pp. 303–305. [Google Scholar]
- Kaur, N.; Zappa, D.; Comini, E. Shelf Life Study of NiO Nanowire Sensors for NO2 Detection. Electron. Mater. Lett. 2019, 15, 743–749. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.J.; Iba, K.; Schroeder, J.I. CO2 Sensing and CO2 Regulation of Stomatal Conductance: Advances and Open Questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef] [Green Version]
- Ponnuvelu, D.V.; Pullithadathil, B.; Prasad, A.K.; Dhara, S.; Mohamed, K.; Tyagi, A.K.; Raj, B. Highly sensitive, atmospheric pressure operatable sensor based on Au nanoclusters decorated TiO2@Au heterojunction nanorods for trace level NO2 gas detection. J. Mater. Sci. Mater. Electron. 2017, 28, 9738–9748. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Q.; Yao, Y.; Li, Y.; Wang, J.; Chen, L. A micro sensor based on TiO2 nanorod arrays for the detection of oxygen at room temperature. Ceram. Int. 2016, 42, 8565–8571. [Google Scholar] [CrossRef]
- Raza, M.H.; Kaur, N.; Comini, E.; Pinna, N. Toward Optimized Radial Modulation of the Space-Charge Region in One-Dimensional SnO2-NiO Core-Shell Nanowires for Hydrogen Sensing. ACS Appl. Mater. Interfaces 2020, 12, 4594–4606. [Google Scholar] [CrossRef] [PubMed]
- Fomekong, R.L.; Saruhan, B. Influence of humidity on NO2-sensing and selectivity of spray-CVD grown ZnO thin film above 400 °C. Chemosensors 2019, 7, 42. [Google Scholar] [CrossRef] [Green Version]
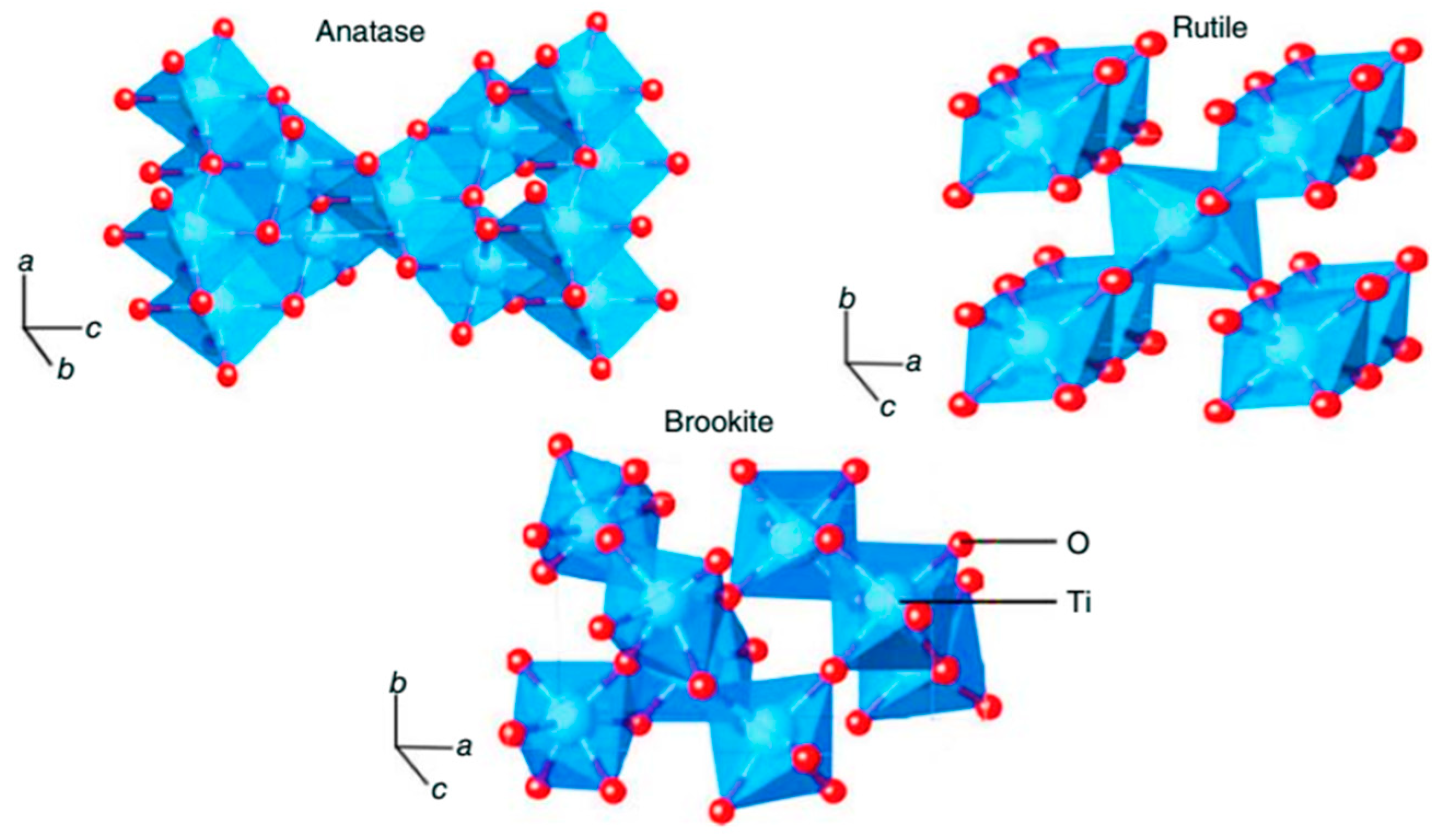
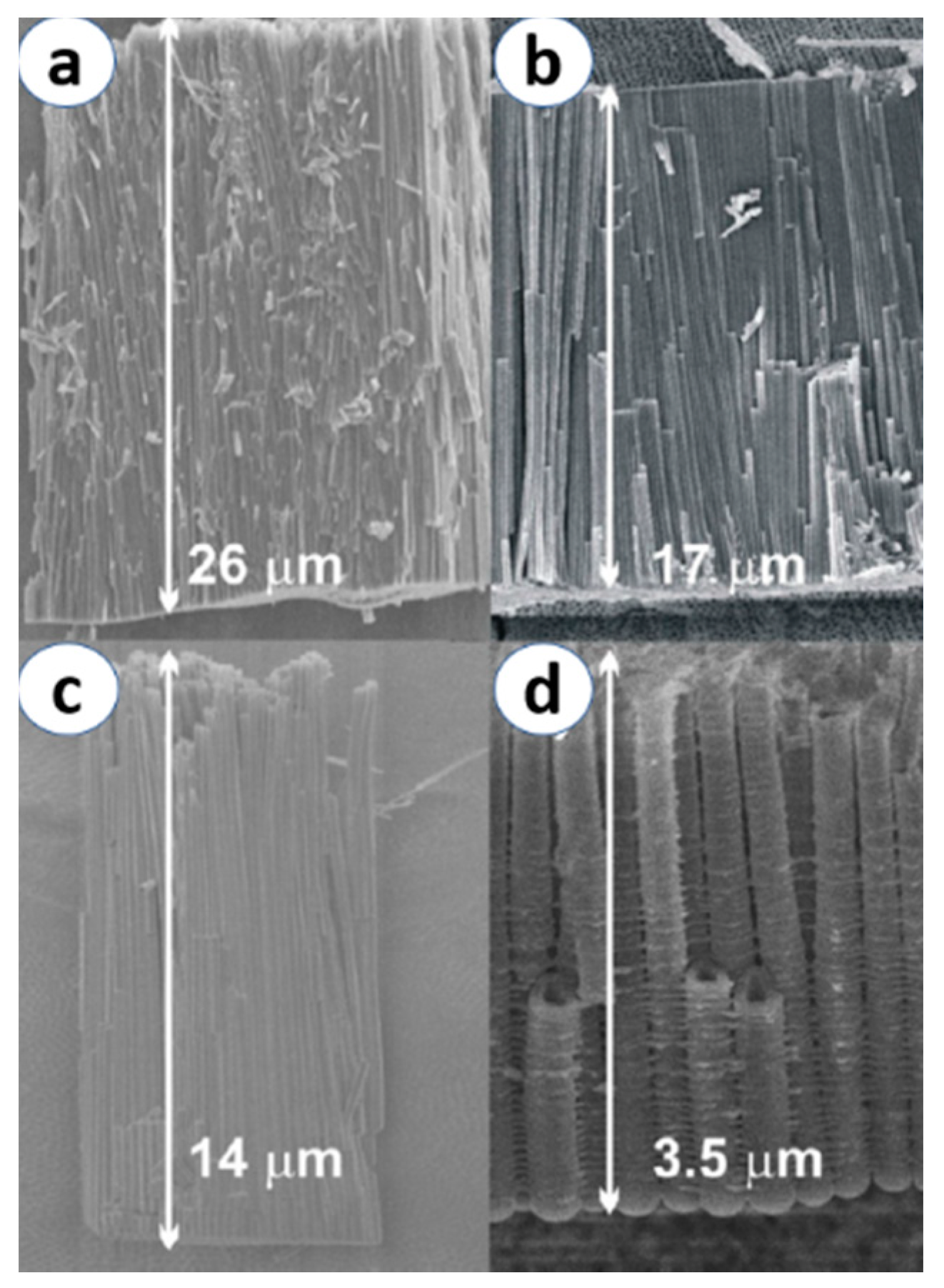

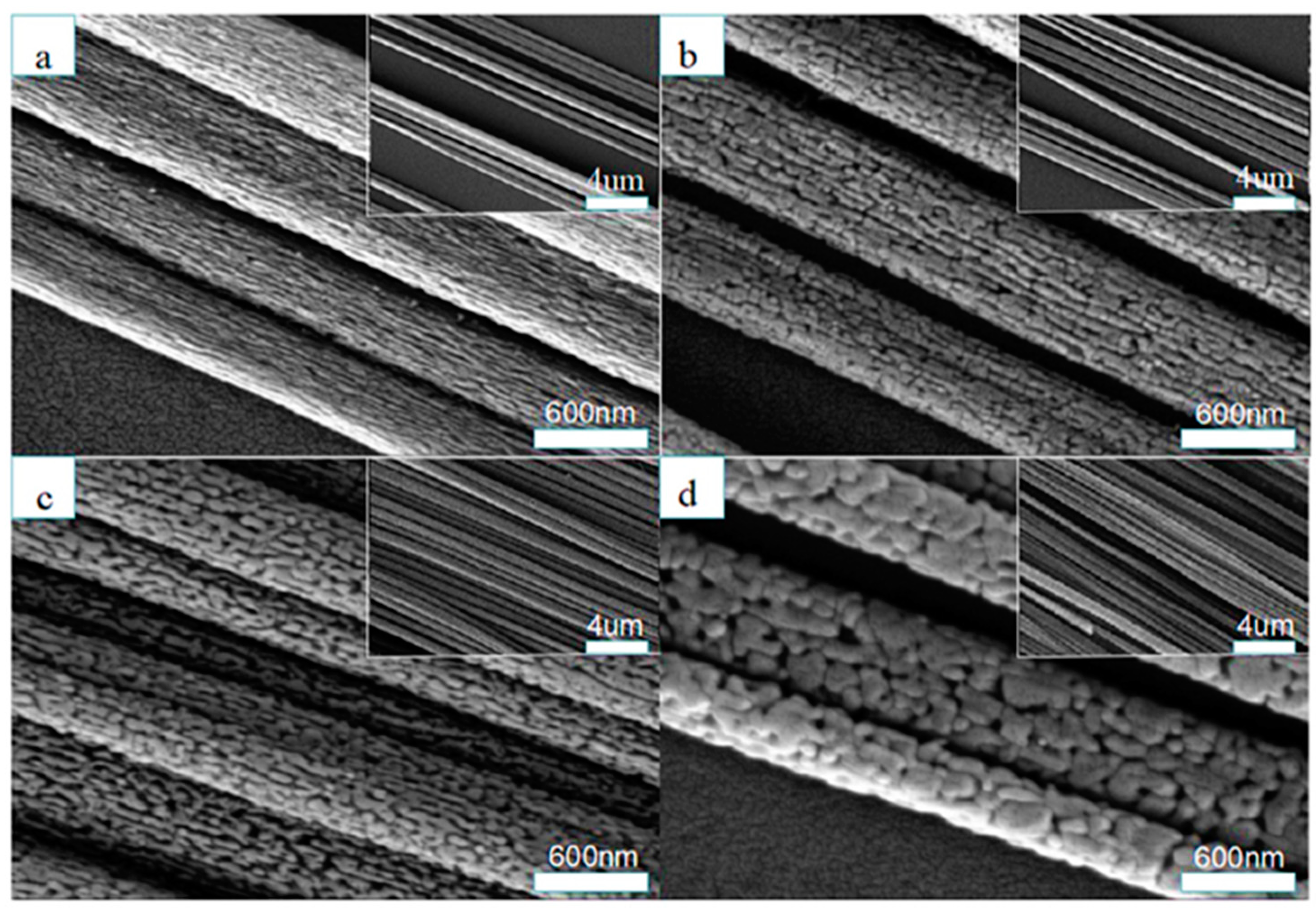

| Crystal Structure | System | Space Group | Lattice Constants (nm) | |||
|---|---|---|---|---|---|---|
| a | b | c | c/a | |||
| Rutile | Tetragonal | —P42/mmm | 0.4584 | - | 0.2953 | 0.644 |
| Anatase | Tetragonal | —I41/amd | 0.3733 | - | 0.937 | 2.51 |
| Brookite | Rhombohedral | —Pbca | 0.5436 | 0.9166 | - | 0.944 |
| Hydrothermal | |||||
|---|---|---|---|---|---|
| Metal Oxide | Materials/Parameters | Nanostructure Type | Sized | Crystalline Phases | Ref./Year |
| TiO2 | Titanium butoxide, hydrochloric acid and deionized water/ Temp. = 150 °C Time = 18 h | Nanorods | length = 5 μm diameter = 100 nm | rutile | [42]/2015 |
| TiO2 | Isopropyl titanate, hydrochloric acid Temp. = 150 °C Time = 3, 6, and 9 h | Nanorods | length = 4200 nm diameter = 120 nm | rutile | [44]/2020 |
| TiO2 | TiCl4, HCl and deionized water Temp. = 180 °C Time = 3 h | Nanorods | diameter = 21.20 nm length = NA | anatase/rutile mixed phases | [64]/2017 |
| TiO2–Ag2O | TiCl4, deionized water Temp. = 180 °C Time = 3 h | Composite Nanorods | diameter = 100 nm | rutile | [65]/2019 |
| Bi-TiO2 | [Bi(NO3)3·5H2O] and TiO2 nanoparticles with NaOH and water. Temp. = 140 °C Time = 24 h | Nanotubes | diameter = NA length = NA | anatase | [46]/2017 |
| TiO2 | Titanium (IV) isopropoxide, NaOH and ethanol. Temp. = 150 °C Time = 20 h | Nanowires | length = 1825 nm diameter = 30–50 nm | anatase | [66]/(2018) |
| Electrochemical Anodization | |||||
| TiO2 | Ti foil in ethylene glycol, acide phosphorique (H3PO4) and hydrofluoric acid HF. Anodization = 120 V Temp. = RT Time = 2 h | Nanotubes | length = 20 µm diameter = 80–120 nm | anatase/rutile mixed phases | [67]/2015 |
| 7TiO2 | Ti foil in ethylene glycol, deionized water and ammonium fluoride (NH4F) Anodization = 1 From 0 V to 60 V Time = 4 h Temp. = RT | Nanotubes | length = 50 μm diameter = 120 nm | anatase | [51]/2015 |
| TiO2 | Ti foil in NH4F and (NH4)2SO4 and deionized water Anodization = 20 V Time = 2 h Temp. = RT Thermal annealing at 750 °C for 4 h in air | Nanotubes | length = NA diameter = 50–80 nm | anatase | [68]/2020 |
| p-Co3O4/n-TiO2 | Ti foil, NH4F ethylene glycol, cobalt (II) nitrate hexahydrate and water Anodization = 50 V Time = 1 h Temp. = 500 °C | Nanotubes | length = NA diameter = 80 nm | anatase | [48]/2018 |
| Pd decorated TiO2 | Ti foil in NH4F and ethylene glycol. Anodization = 60 V Time = 45 min Temp. = RT Thermal annealing at 6 h at 500 °C in ambient air | Nanotubes | length = NA diameter = 40 nm | anatase | [69]/2016 |
| PbS quantum dots/TiO2 | Ti foil in ethylene glycol solution containing NH4F and H2O Pb(NO3)2 dissolved in methanol and Na2S in methanol. Anodization = 50 V Time = 5 h Temp. = RT | Nanotubes | length = 18 μm diameter = 80 nm | anatase | [70]/2016 |
| Ni and Pd-modified TiO2 | Ti foil, NH4F, ethylene glycol and water. NiCl2 and PdCl2 Anodization = 20 V Temp. = 300 C Time = 3 h | Nanotubes | diameter = 50–60 nm length = 470 nm | anatase | [71]/2015 |
| TiO2 | Ti foil and NH4F with H2O in ethylene glycol Anodization = 50 V Time = 3 h Temp. = RT | Nanotubes | diameter = 100 nm length = NA | anatase | [72]/2016 |
| Co-doped TiO2 | Ti foil in glycol solution with ammonium fluoride and deionized water. Anodization = 30 V Time 2 h Temp. = RT | Nanotubes | diameter = 110 nm length = NA | anatase | [73]/2019 |
| Electrospinning | |||||
| TiO2 | Titanium tetraisopropoxid (C12H28O4Ti), ethanol, acetic acid Stirring time = 15 min Voltage = 18 kV Rate = 2 mL/min Calcination temp. = 300 °C, 500 °C, 700 °C, 900 °C | Nanofibers | lengths = NA diameters = 50 nm, 80 nm, 130 nm, 200 nm | anatase/rutile mixed phase | [40]/2015 |
| In2O3 beads @ TiO2-In2O3 composite | TBT (tetrabutyl titanate), indium nitrate hydrate, ethanol, DMF (dimethylformamide), PVP (polyvinylpyrrolidone) Stirring time = 6 h Voltage = 16.0 kV Rate = 0.25 mL·h−1 | Nanofibers | lengths = tens of µm diameter = 150–250 nm | polycrystalline TiO2-In2O3 composite | [60]/(2015) |
| Nb2O5-TiO2 | Titanium isopropoxide, polyvinylpyrrolidone, acetic acid, ethanol stirring time = 12 h Voltage = 18.0 kV, −4.0 kV Rate = 1.5 mL/h, Calcination temp. = 500 °C | Nanofibers | lengths = NA diameter = 121.3 nm | anatase/ rutile mixed phase | [58]/2019 |
| TiO2/V2O5 | Tetrabutyl titanate, poly-vinylpyrrolidone, ethanol, acetic acid stirred time = 20 min Ti/V molar ratio of 4:1 Annealing Temp = 450 °C | Nanofibers | length = µm range diameter = 60 nm | anatase/ rutile mixed phase | [74]/2016 |
| TiO2-SnO2 | Dimethylformamide, ethanol stirring time = 10 min SnCl2·2H2O stirring time = 8 h voltage = 18 kV | Nanofibers | N/A | rutile | [59]/2017 |
| Hydrothermal | Electrochemical Anodization | Electrospinning | |||
|---|---|---|---|---|---|
| Advantages | Disadvantages | Advantages | Disadvantages | Advantages | Disadvantages |
| Simple, easy and low-cost synthesis method | The reaction takes long time | Production of High quality of 1D nanostructures specially nanotubes | Mainly used for nanotubes growth | Ease to fabricated composites | Limited control of structure porosity |
| Production of High quality of 1D nanostructures specially nanorods | Utilization of highly concentrated NaOH solution | Ordered and aligned structure | The mass-produced is limited | High efficiency | Use of toxic solvents |
| The morphology is controlled by synthesis parameters | Difficult in achieving uniform size | high aspect ratio (length/diameter ratio) | Utilization of toxic electrolyte: Hydrofluoric acid HF | Process simplicity | - |
| Easy addition of additives for doping | length/diameter ratio is smaller than the ratio produced by electrochemical anodization | Growth at room temperature | high production cost | Mass production | - |
| - | - | Aspect ratio controlled by synthesis parameters | Difficulties in separation of film from substrate | - | - |
| Material | Synthesis Method | Target Gas/ Concentration | Response (S) | Temperature/ Humidity | Response/ Recovery Time | Ref./Year |
|---|---|---|---|---|---|---|
| TiO2 NTs | Electrochemical anodization | H2/100 ppm | S = (Ig/Ia) 3.5 | RT/Dry air | 0.7 s/0.9 s | [67]/2015 |
| TiO2 NFs | Electrospinning | H2/50 ppm | S = (Ra/Rg) 30 | 190 °C with UV irradiation/Dry air | 2s/6.9 s | [40]/2015 |
| TiO2 NRs | Hydrothermal | H2/2000 ppm | S = (∆I/I × 100) 215% | 200 °C/Dry air | NA | [42]/2015 |
| p-Co3O4/n-TiO2 NTs | Electrochemical anodization | H2/1000 ppm | S = (∆I/I) 6 | 200 °C/50% | 10 min/5 min | [48]/2018 |
| Pd decorated TiO2 NTs | Electrochemical anodization | H2/10ppm | S = (∆R/R) 1.25 | 180 °C/in dry synthetic air | 20 s/40 s | [69]/2016 |
| PdAu decorated TiO2 NWs | Hydrothermal | H2/5 ppm | S = (∆I/I×100) 350% | RT/- | 42 s/NA | [45]/2016 |
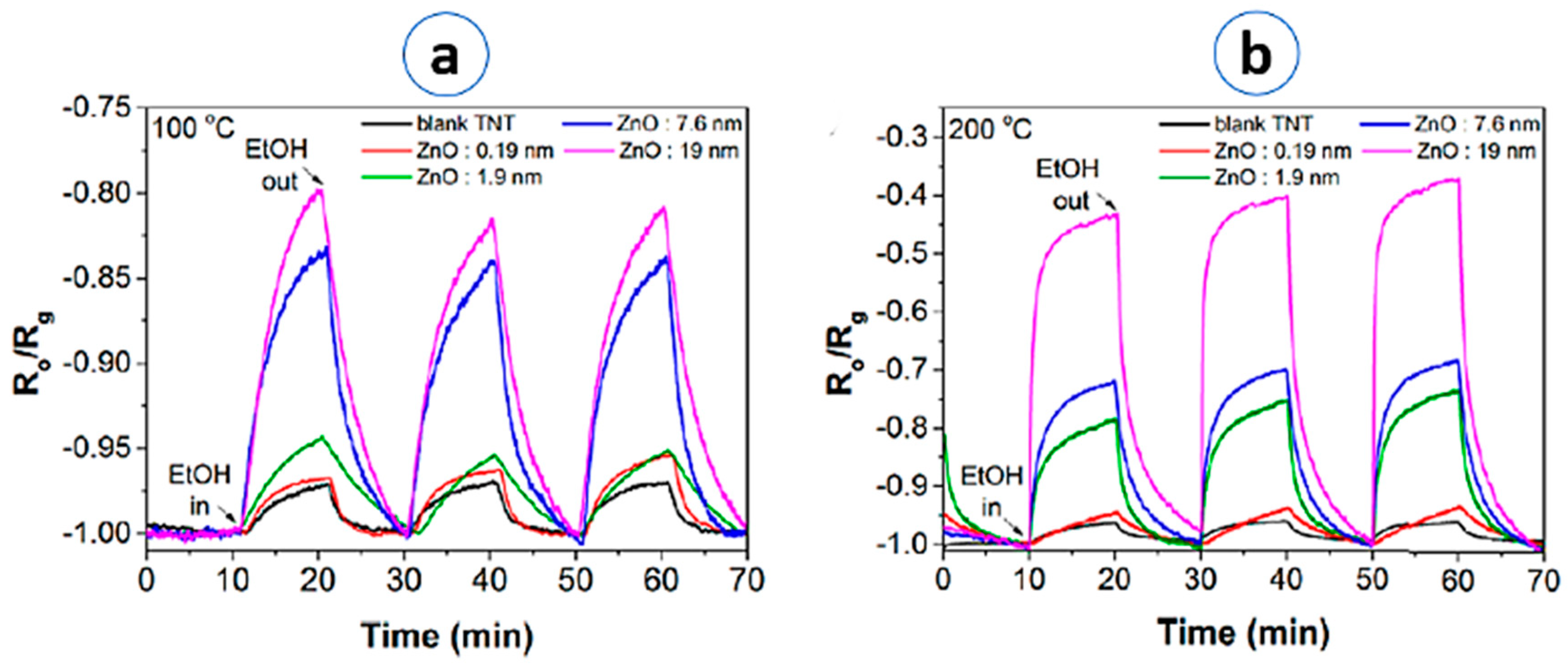
| TiO2/ZnO core-shell NTs | Electrochemical anodization | Ethanol/1930 ppm | S = (Ra/Rg) 0.8 | 100 °C/- | NA | [47]/2018 |
| Al2O2/TiO2 | Thermal oxidation | Ethanol/1000 ppm | S = (Ra/Rg) 1108.9 | 650 °C/- | 4 min/20 min | [61]/2017 |
| Nb2O5-TiO2 NFs | Electrospinning | Ethanol/500 ppm | S = (Ra/Rg) 21.64 | 250 °C0/45% | NA | [58]/2019 |
| TiO2/V2O5 NFs | Electrospinning | Ethanol/100 ppm | S = (Ra/Rg) 24.6 | 350 °C/30% | 6 s/7s | [74]/2016 |
| Ni-TiO2 NTs | Electrochemical anodization | Acetone/1000 ppm | S = (∆R/R×100) 82% | 100 °C/- | NA | [71]/2015 |
| TiO2-SnO2 core-shell NFs | Electrospinning | Acetone/100 ppm | S = (Ra/Rg) 13.5 | 280 °C/- | 2 s/60 s | [59]/2017 |
| TiO2 NTs | Electrochemical anodization | Acetone/50 vol% | S = (∆R/R×100) 115% | Light irradiation at RT/- | NA | [68]/2020 |
| TiO2 NWs | Vapor-Phase growth | CO/1 ppm | S = (NA) 11% | 400 °C/- | NA | [63]/2016 |
| TiO2 NRs | Hydrothermal | Ammonia/20 ppm | S = (∆R/R × 100) 14.1% | RT/50% | 61 s/9 s | [44]/ 2020 |
| Pbs QDs/TiO2 NTs | Electrochemical anodization | Ammonia/100 ppm | S = (Ra/Rg) 17.49 | RT/- | NA | [70]/2016 |
| TiO2 NTs | Electrochemical anodization | H2S/6 to38 ppm | S = (∆R/R × 100) 12 to 144% | 70 °C/10% | NA | [72]/2016 |
| Co-doped TiO2 NTs | Electrochemical anodization | H2S/50 ppm | S = (Ra/Rg) 199.16% | 300 °C/50% | 15 s/4 s | [73]/2019 |
| Material | Synthesis Method | Target Gas/ Concentration | Response (S) | Temperature/ Humidity | Response/ Recovery Time | Ref./Year |
|---|---|---|---|---|---|---|
| TiO2 NWs | Hydrothermal | NO2/100 ppm | S = (Ra/Rg) 3.1 | RT/50%RH | 10 s/19 s | [66]/(2018) |
| TiO2 NRs | Hydrothermal and annealing | NO2/40 ppm | S = (Rg/Ra) 1300 | RT/dry air | NA | [64]/(2017) |
| TiO2-Al2O3 Core-shell NWs | Thermal oxidation | NO2/1000 ppm | S = (Rg/Ra) 1.9 | 650 °C/dry air | 180 s/180 s | [61]/(2017) |
| MoS2-Decorated TiO2 NTs | Anodization and Hydrothermal | NO2/100 ppm | S = (Rg/Ra) 1.1 | 150 °C/45%RH | NA | [50]/(2016) |
| TiO2-In2O3 Composite NFs | Electrospinning | NO2/97 ppm | S = (∆R/Ra) 95 | RT/26%RH | 6.7 s/NA | [60]/(2015) |
| TiO2 NTs | Anodization | NO2/50 ppm | S = (∆I/Ig) 17 | 200 °C/50%RH | NA | [48]/(2018) |
| TiO2 Networked NWs | Vapor-phase growth | NO2/50 ppm | S = (∆R/Ra×100) 8% | 400 °C/dry air | NA | [63]/(2016) |
| TiO2-Ag2O Composite NRs | Hydrothermal and sputtering | NO2/0.5 ppm | S = (Rg/Ra) 3.1 | 250 °C/dry air | 87 s/112 s | [65]/(2019) |
| TiO2@Au Heterojunction NRs | Hydrothermal and chemical approach | NO2/5 ppm | S = (∆R/Ra) 135.5 | 250 °C/dry air | 40 s/43 s | [94]/(2017) |
| TiO2 NRs Array | Acid vapor oxidation | O2/16% vol. | S = (Rg/Ra) 2.1 | RT/dry air | 55 s/51 s | [95]/(2016) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, N.; Singh, M.; Moumen, A.; Duina, G.; Comini, E. 1D Titanium Dioxide: Achievements in Chemical Sensing. Materials 2020, 13, 2974. https://doi.org/10.3390/ma13132974
Kaur N, Singh M, Moumen A, Duina G, Comini E. 1D Titanium Dioxide: Achievements in Chemical Sensing. Materials. 2020; 13(13):2974. https://doi.org/10.3390/ma13132974
Chicago/Turabian StyleKaur, Navpreet, Mandeep Singh, Abderrahim Moumen, Giorgio Duina, and Elisabetta Comini. 2020. "1D Titanium Dioxide: Achievements in Chemical Sensing" Materials 13, no. 13: 2974. https://doi.org/10.3390/ma13132974
APA StyleKaur, N., Singh, M., Moumen, A., Duina, G., & Comini, E. (2020). 1D Titanium Dioxide: Achievements in Chemical Sensing. Materials, 13(13), 2974. https://doi.org/10.3390/ma13132974









