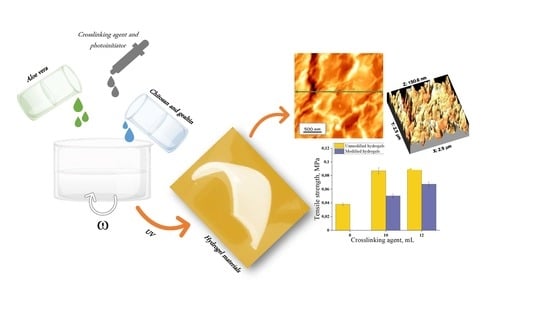
Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hydrogels
2.3. Methodology of Measurements
2.3.1. Analysis of the Chemical Structure of Hydrogels
2.3.2. Sorption Properties of Hydrogels
2.3.3. Mechanical Properties of Hydrogels
2.3.4. Morphological Properties of Hydrogels
2.3.5. Cytotoxicity of Hydrogels
3. Results and Discussion
3.1. Results of Syntheses Performed with Crosslinking Agent with Different Amounts and Molecular Weights
3.2. Chemical Analysis of Hydrogels
3.3. Morphological Properties of Hydrogels
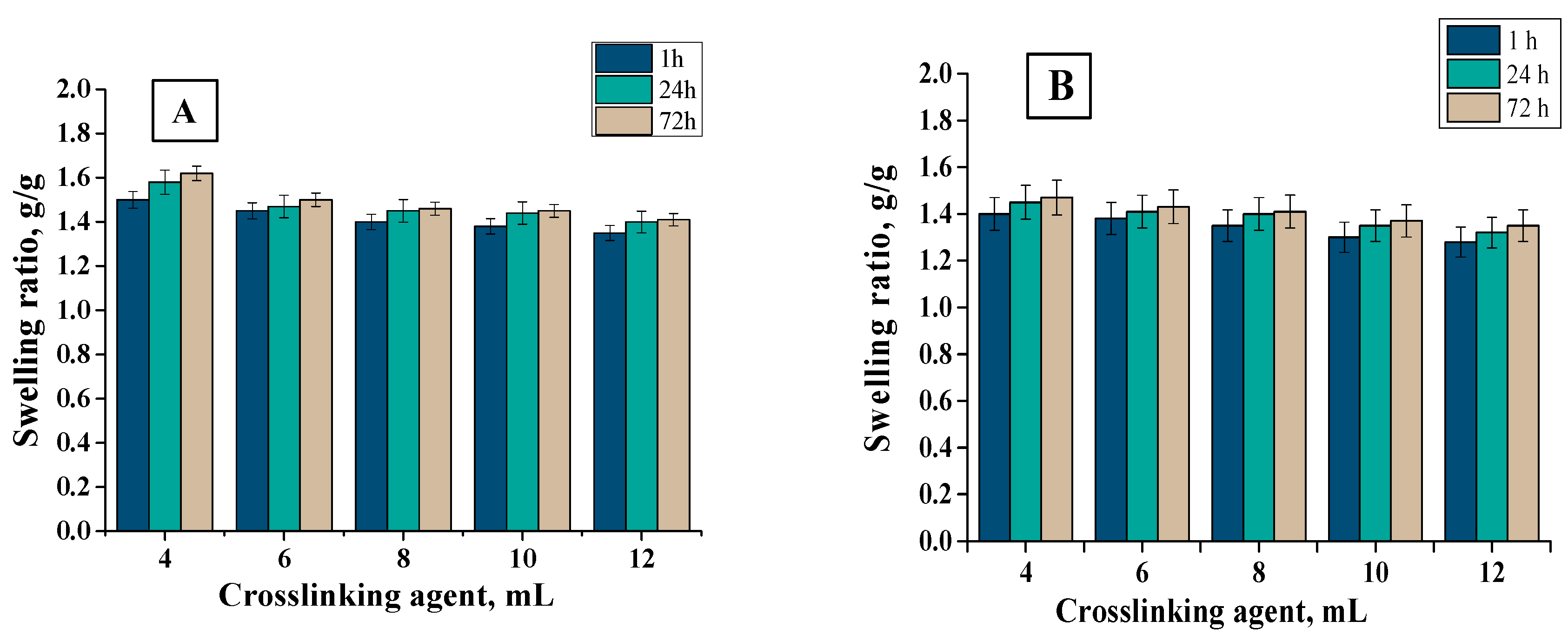
3.4. Sorption Properties of Hydrogels
3.5. Mechanical Properties of Hydrogels
3.6. Cytotoxicity of the Hydrogels
4. Conclusions
- FTIR analysis confirmed the presence of characteristic absorption bands from functional groups found in chitosan. For hydrogel samples modified with Aloe vera, two new peaks were observed in the wave number: 1642 cm−1 and 3435 cm−1, which confirmed the presence of this additive in the hydrogel material.
- Samples modified with Aloe vera were characterized by higher sorption properties than unmodified ones. In the case of unmodified hydrogels, higher swelling ratios were calculated for samples obtained using PEGDA 700 than PEGDA 575. This dependence resulted from the different structure of hydrogels obtained. A decrease in swelling ratios was observed for samples obtained with a larger amount of crosslinking agent due to the formation of hydrogels with higher crosslinking density.
- Hydrogels modified with Aloe vera compared to these unmodified ones obtained with the same amount of crosslinking agent were characterized by greater flexibility. Aloe vera application caused increase in percentage elongation of hydrogels’ samples to 23%. Hydrogels containing 10 mL of Aloe vera and 10 and 12 mL PEGDA 700 showed the best mechanical properties of modified samples.
- Tested hydrogels did not exhibit cytotoxicity toward L929 murine fibroblasts. Viability of the cells incubated for 24 h with these materials was above 70%.
- Unmodified hydrogels had a well-developed specific surface with a relatively high roughness. The application of Aloe vera as a modifying agent caused smoothing of the hydrogel surface.
- The selection of the appropriate conditions for the synthesis of hydrogels allowed us to obtain materials with good strength parameters at the same time with appropriate flexibility. Moreover, these materials did not show cytotoxic properties in relation to the analyzed cells. Due to the above mentioned properties, these hydrogels are an interesting material for biomedical applications, among others, as dressing materials to wounds with difficulty healing.
Author Contributions
Funding
Conflicts of Interest
References
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Tyliszczak, B.; Rudnicka, K.; Urbaniak, M.; Michlewska, U.; Królczyk, J.B.; Gajda, P.; Pielichowski, K. Measurement methodology toward determination of structure-property relationships in acrylic hydrogels with starch and nanogold designed for biomedical applications. Measurement 2020, 156, 107608–107619. [Google Scholar] [CrossRef]
- Boso, D.; Maghin, E.; Carrario, E.; Giagante, M.; Pavan, P.; Piccoli, M. Extracellular matrix-derived hydrogels as biomaterial for different skeletal muscle tissue replacements. Materials 2020, 13, 2483. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.W.; Chan, W.P.; Tai, P.W.; Lo, H.Y.; Wu, T.Y. Roles of silk fibroin on characteristics of hyaluronic acid/silk fibroin hydrogels for tissue engineering of nucleus polposus. Materials 2020, 13, 2750. [Google Scholar] [CrossRef]
- Ilgina, P.; Ozayb, H.; Ozayb, O. A new dual stimuli responsive hydrogel: Modeling approaches for the prediction of drug loading and release profile. Europ. Polym. J. 2019, 113, 244–253. [Google Scholar] [CrossRef]
- Timaeva, O.I.; Arkharova, N.A.; Orekhov, A.S.; Klechkovskaya, V.V.; Mulakov, S.P.; Kuz’micheva, G.M.; Pashkin, I.I. New hydrogels in the poly-N-vinylpyrrolidone–RE(NO3)3xH2O (RE = La, Gd, Yb) system: Fabrication, structure, bactericidal properties. Polymer 2020, 186, 122079. [Google Scholar] [CrossRef]
- García-Fernández, L.; Olmeda-Lozano, M.; Benito-Garzón, L.; Perez-Caballer, A.; Roman, J.S.; Vázques-Lasa, B. Injectable hydrogel-based drug delivery system for cartilage regeneration. Mat. Sci. Eng. C. 2020, 110, 110702–110721. [Google Scholar] [CrossRef]
- Chouhan, G.; Moakes, R.J.A.; Esmaeili, M.; Hill, L.J.; deCogan, F.; Hardwicke, J.; Rauz, S.; Logan, A.; Grover, L.M. A self-healing hydrogel eye drop for the sustained delivery of decorin to prevent corneal scarring. Biomaterials 2019, 210, 41–50. [Google Scholar] [CrossRef]
- Tamahkar, E.; Ozkahraman, B.; Süloğlu, A.K.; İdil, N.; Perçin, I. A novel multilayer hydrogel wound dressing for antibiotic release. J. Drug Deliv. Sci. Tech. 2020, 58, 101536–101551. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803–118821. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saud. Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dai, G.; Hong, Y. Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater. 2019, 95, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Varshney, L.; Francis, S. Synthesis and characterization of tragacanth gum based hydrogels by radiation method for use in wound dressing application. Rad. Phys. Chem. 2017, 135, 94–105. [Google Scholar] [CrossRef]
- Parente, M.E.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jimenez-Kairuz, A. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosm. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef]
- Hasija, V.; Sharma, K.; Kumar, V.; Sharma, S.; Sharma, V. Green synthesis of agar/Gum Arabic based superabsorbent as an alternative for irrigation in agriculture. Vacuum 2018, 157, 458–464. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 2018, 217, 123–143. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Bengoechea, C.; Romero, A.; Guerrero, A. A natural-based polymeric hydrogel based on functionalized soy protein. Eur. Polym. J. 2016, 85, 164–174. [Google Scholar] [CrossRef]
- Demitri, C.; De Benedictis, V.M.; Madaghiele, M.; Corcione, C.E.; Maffezzoli, A. Nanostructured active chitosan-based films for food packaging applications: Effect of graphene stacks on mechanical properties. Measurement 2016, 90, 418–423. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Rudnicka, K.; Gatkowska, J.; Sobczak-Kupiec, A.; Jampilek, J. In vitro biosafety of pro-ecological chitosan-based hydrogels modified with natural substances. J. Biomed. Mater. Res. Part A 2019, 107, 2501–2511. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterization and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Mohandas, A.; Bhatanagar, I.; Se-Kwon, K. Antimocrobial activity of chitosan-carbon nanotube hydrogels. Materials 2014, 7, 3946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziminska, M.; Wilson, J.J.; McErlean, E.; Dunne, N.; McCarthy, H.O. Synthesis and Evaluation of Thermoresponsive Degradable Chitosan-Grafted PNIPAAM Hydrogel as a “Smart” Gene Delivery System. Materials 2020, 13, 2530. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Kulshrestha, N.; Gupta, P.N. Nano composite solid polymer electrolytes based on biodegradable polymers starch and poly (vinyl alcohol). Measurement 2016, 82, 490–499. [Google Scholar] [CrossRef]
- Van Nieuwenhove, I.; Salamon, A.; Peters, K.; Graulus, G.J.; Martins, J.C.; Frankel, D.; Kersemans, K.; De Vos, F.; Van Vlierberghe, S.; Dubruel, P. Gelatin- and starch-based hydrogels. Part A: Hydrogel development, characterization and coating. Carbohydr. Polym. 2016, 15, 129–139. [Google Scholar] [CrossRef]
- Fan, M.; Ma, Y.; Zhang, Z.; Mao, J.; Tan, H.; Hu, X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering. Mater. Sci. Eng. C 2015, 56, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Bociaga, D.; Bartniak, M.; Grabarczyk, J.; Przybyszewska, K. Sodium alginate hydrogels for direct bioprinting—The effect of composition selection and applied solvents on the bioink properties. Materials 2019, 12, 2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Y.; Lu, J.; Cheng, Y.; Li, Q.; Wang, H. Lignin-based hydrogels: A review of preparation, properties, and application. Int. J. Biol. Macromol. 2019, 135, 1006–1019. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Ino, K.; Robert, J.; Shiku, H. Electrochemical printing of calcium alginate/gelatin hydrogel. Electrochim. Acta 2018, 281, 429–436. [Google Scholar] [CrossRef]
- Gilarska, A.; Lewandowska-Łańcucka, J.; Horak, W.; Nowakowska, W. Collagen/chitosan/hyaluronic acid-based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bialik-Wąs, K.; Kijkowska, R.; Sobczak-Kupiec, A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surf. B Biointerfaces 2017, 160, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Bordoloi, M.; Deka Baruah, H.P. Aloe vera: A multipurpose industrial crop. Ind. Crops Prod. 2016, 94, 951–963. [Google Scholar] [CrossRef]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of Aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Majumder, R.; Das, C.K.; Mandal, M. Lead Bioactive Compounds of Aloe vera as Potential Anticancer Agent. Pharm. Res. 2019, 148, 104416–104427. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of biological properties and clinical effectiveness of Aloe vera: A systematic review. J. Trad. Comp. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Oliveira, M.B.; Mano, J.F.; Reis, R.L. Bio-inspired Aloe vera sponges for biomedical applications. Carb. Polym. 2014, 112, 264–270. [Google Scholar] [CrossRef]
- Bhaarathy, V.; Venugopal, J.; Gandhimathi, C.; Ponpandian, N.; Mangalaraj, D.; Ramakrishna, S. Biologically improved nanofibrous scaffolds for cardiac tissue engineering. Mater. Sci. Eng. C. 2014, 44, 268–277. [Google Scholar] [CrossRef]
- Jamnongkan, T.; Kaewpirom, S. Potassium release kinetics and water retention of controlled-release fertilizers based on chitosan hydrogels. J. Polym. Environ. 2010, 18, 413–421. [Google Scholar] [CrossRef]
- Nagpal, M.; Singh, S.K.; Mishra, D. Superporous hybrid hydrogels based on polyacrylamide and chitosan: Characterization and in vitro drug release. Int. J. Pharm. Investig. 2013, 3, 88–94. [Google Scholar] [PubMed] [Green Version]
- Kumirska, J.; Czerwicka, M.; Kaczynski, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Nejatzadeh-Barandozi, F.; Enferadi, S. FT-IR study of the polysaccharides isolated from the skin juice, gel juice, and flower of Aloe vera tissues affected by fertilizer treatment. Org. Med. Chem. Lett. 2012, 2, 33–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Z.X.; Cheong, K.Y. Effects of drying temperature and ethanol concentration on bipolar switching characteristics of natural Aloe vera-based memory devices. Phys. Chem. Chem. Phys. 2015, 17, 26833–26853. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe vera Hydrogel Films for Biomedical Applications. Proc. CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- ISO-10993-5-2009. In Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity; Technical Committee: ISO/TC 194 Biological and Clinical Evaluation of Medical Devices: Geneva, Switzerland, 2009.
- Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.T.; Kaviyarasan, V. Fungal chitosan based nanocomposites sponges—An alternative medicine for wound dressing. Int. J. Biol. Macromol. 2017, 104, 1905–1915. [Google Scholar] [CrossRef] [PubMed]













| 1% HD Chitosan in 0.05% Acetic Acid Solution [mL] | Photoinitiator * [mL] | PEGDA 575 [mL] | Name of the Sample |
|---|---|---|---|
| 50 | 0.5 | 2 | 2–575 |
| 4 | 4–575 | ||
| 6 | 6–575 | ||
| 8 | 8–575 | ||
| 10 | 10–575 | ||
| 12 | 12–575 |
| 1% HD Chitosan in 0.05% Acetic Acid [mL] | Photoinitiator * [mL] | PEGDA 700 [mL] | Name of the Sample |
|---|---|---|---|
| 50 | 0.5 | 2 | 2–700 |
| 4 | 4–700 | ||
| 6 | 6–700 | ||
| 8 | 8–700 | ||
| 10 | 10–700 | ||
| 12 | 12–700 |
| 1% HD Chitosan in 0.05% Acetic Acid Solution [mL] | Photoinitiator * [mL] | PEGDA 700 [mL] | Name of the Sample |
|---|---|---|---|
| 50 | 0.5 | 8 | 8–700-a |
| 10 | 10–700-a | ||
| 12 | 12–700-a |
| Crosslinking Agent [mL] | Observations for PEGDA 575 | Observations for PEGDA 700 |
|---|---|---|
| 2 | No hydrogel crosslinking | No hydrogel crosslinking |
| 4 | ||
| 6 | Crosslinking/very brittle material → not adequate for mechanical tests  | Crosslinking/very brittle material → not adequate for mechanical tests  |
| 8 | ||
| 10 | The material can be used to prepare samples for mechanical tests  | |
| 12 |
| Crosslinking Agent [mL] | Observations for PEGDA 700 with Aloe vera |
|---|---|
| 8 | Crosslinking/very brittle material → not adequate for mechanical tests |
| 10 | The material can be used for mechanical tests |
| 12 |
| Parameter | Value [µm] |
|---|---|
| 10 mL PEGDA 700 | |
| Rz | 34.40 |
| Rv | 14.50 |
| 12 mL PEGDA 700 | |
| Rz | 44.40 |
| Rv | 27.00 |
| 10 mL PEGDA 700 with Aloe vera | |
| Rz | 91.74 |
| Rv | 52.00 |
| 12 mL PEGDA 700 with Aloe vera | |
| Rz | 27.70 |
| Rv | 17.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głąb, M.; Kędzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. https://doi.org/10.3390/ma13143073
Drabczyk A, Kudłacik-Kramarczyk S, Głąb M, Kędzierska M, Jaromin A, Mierzwiński D, Tyliszczak B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials. 2020; 13(14):3073. https://doi.org/10.3390/ma13143073
Chicago/Turabian StyleDrabczyk, Anna, Sonia Kudłacik-Kramarczyk, Magdalena Głąb, Magdalena Kędzierska, Anna Jaromin, Dariusz Mierzwiński, and Bożena Tyliszczak. 2020. "Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use" Materials 13, no. 14: 3073. https://doi.org/10.3390/ma13143073
APA StyleDrabczyk, A., Kudłacik-Kramarczyk, S., Głąb, M., Kędzierska, M., Jaromin, A., Mierzwiński, D., & Tyliszczak, B. (2020). Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials, 13(14), 3073. https://doi.org/10.3390/ma13143073