The samples were processed via powder metallurgy technique. For nanomaterials, powder metallurgy processing is still the preferred technology when retaining the nanoscale characteristics of the parent powders is important for the final product. Compared to the classical metal processing by melting and casting, powder metallurgy processing consists of a series of steps, each of them having a critical influence on the final product.
Figure 1 shows the processing steps and variables that have been used for Cu-x Fe
3O
4 nanocomposites fabrication and characterization. Samples were obtained from individual Cu and Fe
3O
4 nanopowders with different Fe
3O
4 content, i.e., 5, 10, 15 and 20 wt.%, and under various processing conditions.
3.1.1. Nanopowder Blend
The SEM and Energy Dispersive Spectroscopy (EDS) investigations performed on nanopowder mixtures were used to evaluate the homogeneity of powder composition.
Figure 2 presents the SEM image of Cu-15%Fe
3O
4 blend in which Fe
3O
4 particle are uniformly distributed in the powder mixture. Elemental mapping showed a uniform distribution of Cu, O and Fe, which indicated a good structural homogeneity of the blend. Similar results were obtained for the other nanopowder compositions.
Powder blends are usually described in terms such as flowability, powder filling and density, which can be further used to calculate the consolidation parameters. The theoretical density,
ρm, was calculated with the following equation:
where the theoretical densities for Cu and Fe
3O
4 are 8.94 g/cm
3 and 5.2 g/cm
3, respectively [
2].
The flowability (flowability) of Cu-Fe3O4 nanopowder blends is virtually zero as they do not flow freely through the fluorimeter port. The apparent density of the powder mixture was determined under the specific conditions specified in the SR EN ISO 3923-1:2010, for non-flowing powders. The apparent density for Cu and Fe3O4 nanopowders were 1.29 ± 0.17 g/cm3 and 0.77 ± 0.07 g/cm3, respectively. In the calculations, average values of the apparent densities were taken for both copper and magnetite. The compactness of the mixtures varies between 13.88 and 13.87% and the apparent density between 11.38 and 12.4%.
Figure 3 presents the variation of apparent density and the compactness of the powder with the Fe
3O
4 content in the powder mixture. This graph shows that the apparent density of nanopowder mixtures decreased linearly with the Fe
3O
4 content from 12.4 to 11.38%, while the powder compactness was almost constant, as it shows only a slight increase with the Fe
3O
4 content in the mixture from 13.87 to 13.88%. Actually, the amount of Fe
3O
4 should not influence the compactness of the powders, because the size of both powders is in the nanometric range and the blend shows very good homogeneity.
3.1.2. Powder Consolidation
Because the Cu-Fe3O4 nanopowder mixture has magnetic properties due to the presence of magnetite in the mixture, steel molds, which are frequently used in powder presses, could not be used. When steel molds were used, the magnetite particles separated on the walls of the mold. Therefore, to press the magnetic nanopowders, a custom non-magnetic die-cast was made. By using this mold, the surface of the compacts was very smooth while preserving the homogeneity of the powder mixture. The consolidation process of Cu-xFe3O4 blends took place in a cold-rolled unidirectional press. Inevitably, powder agglomeration takes place when nanopowders are compressed in a die.
The electron microscopy images presented in
Figure 4 show nanoparticle agglomerations, which is inevitable in the consolidation of very fine, non-flowing powders. SEM investigations were performed on samples consolidation at different pressures (
Figure 4 and
Figure 5). To obtain the topography of the compacted samples, we used backscatter electron detector (BSED) to detect the elastically scattered electrons from atoms below the sample surface.
Figure 5 shows the SEM images of the Cu-15%Fe
3O
4 powder mixture pressed at 500 and 700 MPa. After pressing, a rearrangement of the nanoparticles takes place and a decrease in the porosity was observed in both samples. It is obvious that the powder mixture reorganized during pressing to increase compactness. A partial welding processing resulted in agglomerates in the range of 300–600 nm. At 700 MPa compaction pressures, the gliding of the formed agglomerates and the partial filling of the gaps is more noticeable than at 500 MPa (
Figure 5).
In the manufacturing of the parts by powder metallurgy processes, an important technological property of any new powder blend is the compressibility of the powder mixture, as a measure of the volume reduction under uniaxial pressure in a closed die. Knowing the compressibility of the Cu-xFe3O4 powder, the appropriate compact density at a given pressure can be determined, as well as the decrease in the powder volume due to the applied pressure. Further in processing, a high compact density can reduce the dimensional changes produced during sintering.
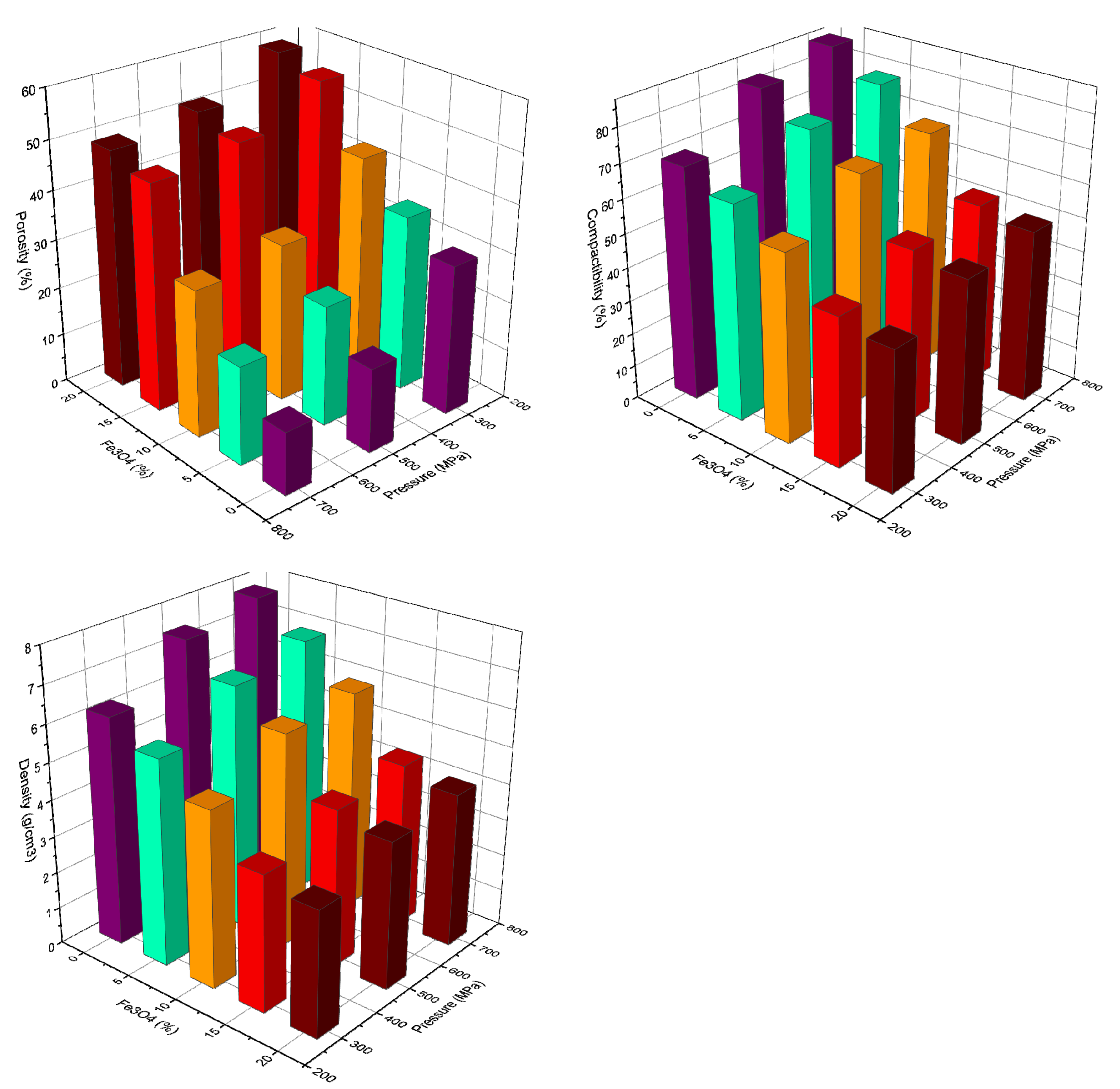
The compressibility of Cu-xFe
3O
4 powders was determined experimentally as compact density function of the applied pressure. The 3-D charts of the powder porosity, compressibility and density as a function of the Fe
3O
4 amount in the mixture and compacting pressure is presented in
Figure 6, along with copper nanopowder, which is the matrix of the composites. The compressibility data start from the point of apparent density of Cu, i.e., 1.24 g/cm
3 and increase asymptotically to the theoretical density.
In the case of Cu powder, the compact density does not exceed 8.0 g/cm3 at 700 MPa. Although pure copper has a good plasticity, the compressibility is reduced compared to powders of irregular shapes. The reduced compressibility is due to the large particle surface area (~ 50 m2/g), the anosized dimension and spherical shape of the particles, which results in a low-pressure distribution on a reduced number of surfaces in contact between nanoparticles.
Regarding the porosity of the compacts,
Figure 6 shows that at constant pressure, the porosity increases with increasing Fe
3O
4 content, a maximum value of 58.49% being obtained for a composite containing 20%Fe
3O
4 pressed at 300 Mpa. At a constant percentage of Fe
3O
4, the porosity decreases with increasing compaction pressure, with a minimum value of 12.75% being obtained for Cu powder pressed at 700 MPa. This value increased rapidly to 20.02% when 5%Fe
3O
4 was added to copper nanopowder.
Experiments showed that an increase in Fe3O4 content in the nanopowder mixture contributes to a decrease in the density of presses and their compaction, together with increased porosity. The maximum compaction value of nanopowders was obtained at a pressure of 700 MPa. Because the die did not hold up under 700 MPa pressure requiring repeated adjustments, the subsequent compact samples were obtained at 500 MPa, when the compaction value of the nanopowders varies between 48.83 and 75.41%, depending on the Fe3O4 content in the blend. It was found that samples containing 5–10% Fe3O4, have porosities closer to the copper nanometric powders.
During powder compression, various porosities were observed for compacts containing different amounts of Fe
3O
4. SEM microscopy images show that the size of the pressed pores increases in proportion to the increase in Fe
3O
4 content in the powder mixture. The pore size of presses containing 5–20% Fe
3O
4 is between 1.17 and 4.5 μm, as shown in
Figure 6.
3.1.3. Sintering of Cu-xFe3O4 Compacts
Sintering of the Cu-x Fe
3O
4 compacts was carried out according to the sintering temperature–time schedule illustrated in
Figure 7. The densification of the Cu-xFe
3O
4 composites during the sintering process was investigated taking into account the sintering temperature, Fe
3O
4 content and sintering time. The remnant porosity and density of the sintered nanocomposites were used as indicators of system sinterability. Characterization of Fe
3O
4 nanocomposites obtained by pressing-sintering consists in determining the structure and physical-mechanical properties such as thermal conductivity, porosity, heating behavior, magnetic properties, cold deformability and Vickers micro-hardness. The structure of Cu-xFe
3O
4 nanocomposites was observed by scanning and transmission electron microscopy (SEM and high resolution—HRTEM), and X-ray diffraction.
The X-ray diffraction measurements of the sintered Cu-xFe
3O
4 nanocomposites show only peaks for the Cu matrix and secondary quasi-crystalline peaks for the reinforced Fe
3O
4 particles.
Figure 8 shows the X-ray diffraction of Cu-5% Fe
3O
4 nanocomposite sintered at 800 °C. Similar peaks for the Cu and Fe
3O
4 phases were obtained for all the samples. However, an additional peak was observed for composites containing more than 10% Fe
3O
4, which was associated with the CuFeO
2 phase, also called delaphosite, formed during sintering at 800 °C [
2].
SEM images obtained on Cu-xFe
3O
4 nanocomposites confirm a good distribution of the particle reinforcement in the copper matrix.
Figure 9 shows that the reinforcement particles have rounded and rectangular shapes and they are distributed almost evenly over the surface of the composite material. SEM analysis of these samples also allows for the EDS analysis to be performed on the surface of the particle agglomerates. It has been found that agglomerates contain both magnetite and copper.
A detailed structural analysis of the nanocomposite samples was performed using high-resolution transmission microscopy. Samples for TEM observation were obtained by sampling small portions of the nanocomposite, which were ionically thinned and then collected on a carbon-coated copper grid.
Figure 10 shows the bright field transmission electron microscopy (TEMBF) image of the nanostructure of Cu-15%Fe
3O
4 composite. At high resolution, the nanostructure of the composite clearly shows the grains of the cupper matrix and the agglomerates of magnetite nanoparticles incorporated in the copper matrix (
Figure 10b). The grain size was also measured, showing that both the grains of the copper matrix and the Fe
3O
4 particles that reinforced the grains have nanometric dimensions.
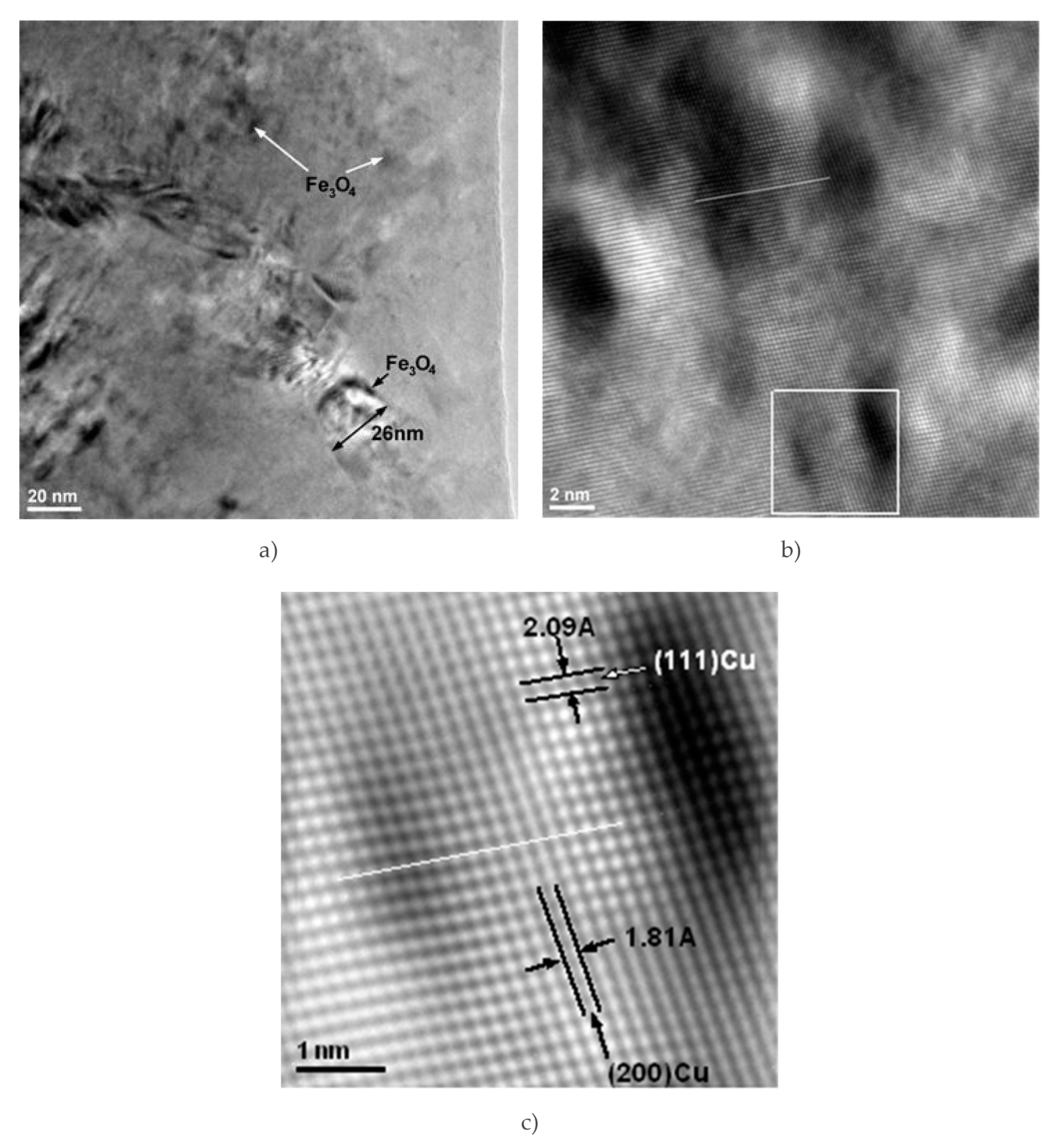
The interface between Cu matrix and reinforcing particles was also observed using scanning electron microscopy. In
Figure 11, Fe
3O
4 particles can be observed at the boundaries of large Cu grains (e.g., the grain of 26 nm in size,
Figure 11a), as well inside the Cu grains (indicated by arrows in
Figure 11a). The presence of reinforcement particles inside the Cu crystallites and at the grain boundary is due to intense diffusion processes occurring during sintering. Diffusion at the interface between the matrix and the nanoparticles could also explain the formation of an intermediate diffusion layer containing CuFeO
2.
The presence of Fe
3O
4 nanometric precipitates embedded in the Cu crystallites distorts the crystal lattice. In
Figure 11b, it is obvious that there is a distortion of the crystalline lattice. The distortion is shown along the white straight line marked on the image, where the local curvature of the crystalline planes is distorted compared to the straight line. The inverse Fourier transform of the image marked with a white square in
Figure 11b clearly shows the distortion of the lattice. The distortion of the crystalline lattice is observed by the curvature of the lattice, indicating the existence of nanocrystalline particles (either magnetite or delaphosite).
Influence of Temperature and Fe3O4 Content on Sinterability
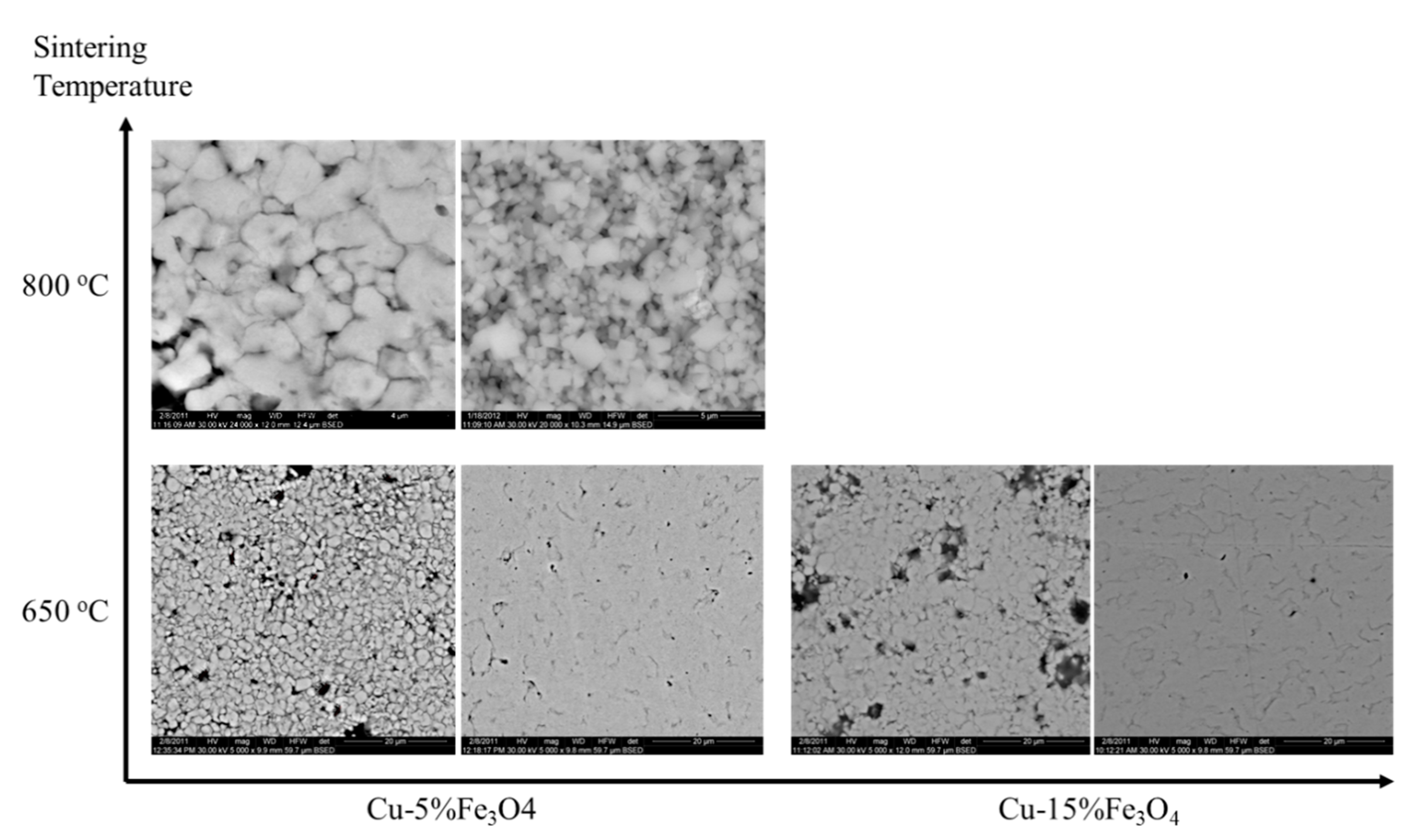
During sintering, microstructural changes occur due to the diffusion on the surface, at the interface and in volume. Under heat and pressure conditions, the kinetic energy and the mobility of atoms increase, which cause them to migrate to low energy sites such as cracks and non-even surface to reach equilibrium positions. Surface diffusion results in smoothing the surface of particles and pores. The shape, size and distribution of the pores are dictated by the complex developments that take place during consolidation-sintering process. In the SEM microscopy images of
Figure 12, the changes in the morphology of Cu-5%Fe
3O
4 and Cu-15%Fe
3O
4 compacted at 500 MPa and sintered at 650 °C is observed, along with the temperature changes observed for Cu-5%Fe
3O
4.
During sintering, the diffusion of atoms begins at the surface of particles and pores and takes place at the surface of crystalline grains, especially those with crystal defects induced during compaction. At higher temperatures, diffusion in volume predominates and the nucleation of new crystallization centers occurs, especially in strongly deformed areas. Recrystallization begins followed by the gradual growth of newly formed crystalline grains.
After sintering, the samples contracted by approximately 6.67–10%, depending on the Fe
3O
4 content. Samples containing 15–20% Fe
3O
4 contracted less than those containing 5% reinforcement nanoparticles. The sintering shrinkage was determined according to ISO 4497-2008. When considering the porosity of a sample compaction with the applied pressure (see
Figure 6), it is observed that the pores of the sintered composites have shrunk in size or disappeared completely.
X-ray diffraction analysis also provides additional information to understand the phase composition and crystallinity of sintered composites with different Fe3O4 content. For example, samples with a Fe3O4 content of less than 10% that were sintered at 650 °C showed no change in phase composition compared to the initial powders. For samples containing more than 15% Fe3O4 and sintered at 800 °C, a new phase was observed. The new phase delafossite was detected in a percentage of 1–7%, depending on the concentration of Fe3O4. The composition of this phase suggests that it occurs at the interface between Cu and Fe3O4 nanoparticles during sintering at 800 °C.
Influence of Powder Properties and Pressures on Sinterability
The particle size and shape of the mixture influence the density of the compact and implicitly the sintering density. The finer the granulation of the powders, the greater their sinterability is.
Table 1 presents data on the technological points of reference copper powders with medium size in the micron (< 40 μm) and nanometric (< 35 nm) range, pressed at 500 MPa. It is found that the sinterability of nanoparticles is higher than micron-size particles. The explanation for this can be found in the mechanism of the sintering process. The high specific surface area of nanopowders (50 m
2/g) results in multiple paths for the transport of atoms by surface diffusion. At the same time, the small size of powder nanoparticles can lead to a reduced size of crystalline grains, which promotes the transport of material through diffusion to the boundary of crystalline grains during sintering. The spherical shape of nanoparticles of nanopowder mixtures has a negative effect on sinterability. Thus, the nanoscale size and spherical shape of the particulate granules results in a lower compact density than in the case of medium-sized powders. A lower compact density means a wider inner surface that favors the sintering process. Densification on sintering increases as the density of compacts is lower, as confirmed by experimental data.
Density and Porosity of the Composite
Figure 13 shows the variation of density and porosity of composites with Fe
3O
4 content on samples sintered at 650 °C and 800 °C. The compaction pressure was 500 MPa. It was observed that the density of nanocomposites obtained by sintering at 650 °C, decreases with increasing magnetite content, while increasing porosity. At 5–10% Fe
3O
4, the composite porosity does not exceed 25%. When the sintering temperature is 800 °C, the density decreases for nanocomposites containing 5–10% Fe
3O
4, then increases with increasing Fe
3O
4 concentration. This increase in the density of nanocomposites sintered at 800 °C can be explained by the formation of the new phase (CuFeO
2), previously observed by X-ray diffraction. Nanocomposites have densities close to the theoretical ones and the remaining porosity is much reduced. Densification indexes for sintering are approximately 0.2, and were calculated based on the following formula:
where
is the density of the sintered composite (g/cm
3),
is the density of the compact (g/cm
3), and
is the true density of the powder mixture (g/cm
3).
The Influence of Sintering Time on Sinterability
It was found that the variation in the sintering time from 60 to 75 min did not significantly affect the porosity of the nanocomposites. As can be seen from
Figure 14a,b, the BSED electron microscopy images obtained for samples pressed at 500 MPa and sintered at 650 °C for 60 min, do not essentially differ from those sintered at 75 min. Therefore, a prolonged sintering time is not necessary because the structural transformations that take place during sintering are completed in one hour.
The structural transformations of copper matrix and nanocomposites with 5% and 15% Fe
3O
4 content are presented in
Figure 15a–c. A non-homogeneity of sintered copper is observed (
Figure 15a), while the nanocomposite with 5% Fe
3O
4 has a homogeneous structure, the average size of nanoparticles being 44–82 nm. Nanocomposite with 15% Fe
3O
4 has a higher porosity and a structural homogeneity lower than 5% Fe
3O
4.
The microscopy images showed that the reinforcement particles are uniformly distributed in the nanocomposite. However, research results show that it is difficult to obtain high density of materials, i.e., small porosities, and strong bond between the particles. Agglomeration of the consolidated nanopowders is still a challenging issue. Nanoparticles are strongly influenced by the Van der Waals attraction forces. These forces determine a temporary distribution of loads on each individual nanoparticle, which can lead to rapid agglomeration of nanoparticles. These agglomerates are very difficult to destroy during pressing and sintering, thus contributing to the formation of intergranular voids and the increase of the residual porosity in nanocomposites. When the sintering temperature is 800 °C, advanced compaction occurs in the nanocomposite and the porosity decreases to 3.5%.