Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Scaffold
2.3. Physicochemical Characterization
2.4. In Vitro Study
2.5. Cell Seeding
2.6. Biocompatibility and Proliferation
2.7. Real-Time Quantitative PCR (RT-qPCR)
2.8. Histological Mineralization Analysis
2.9. Osteogenic and Chondrogenic Differentiation Analysis by Immunofluorescence
2.10. In Vivo Study
2.11. Subcutaneous Implantation in Mice to Evaluate Biocompatibility and Mineralization
3. Results
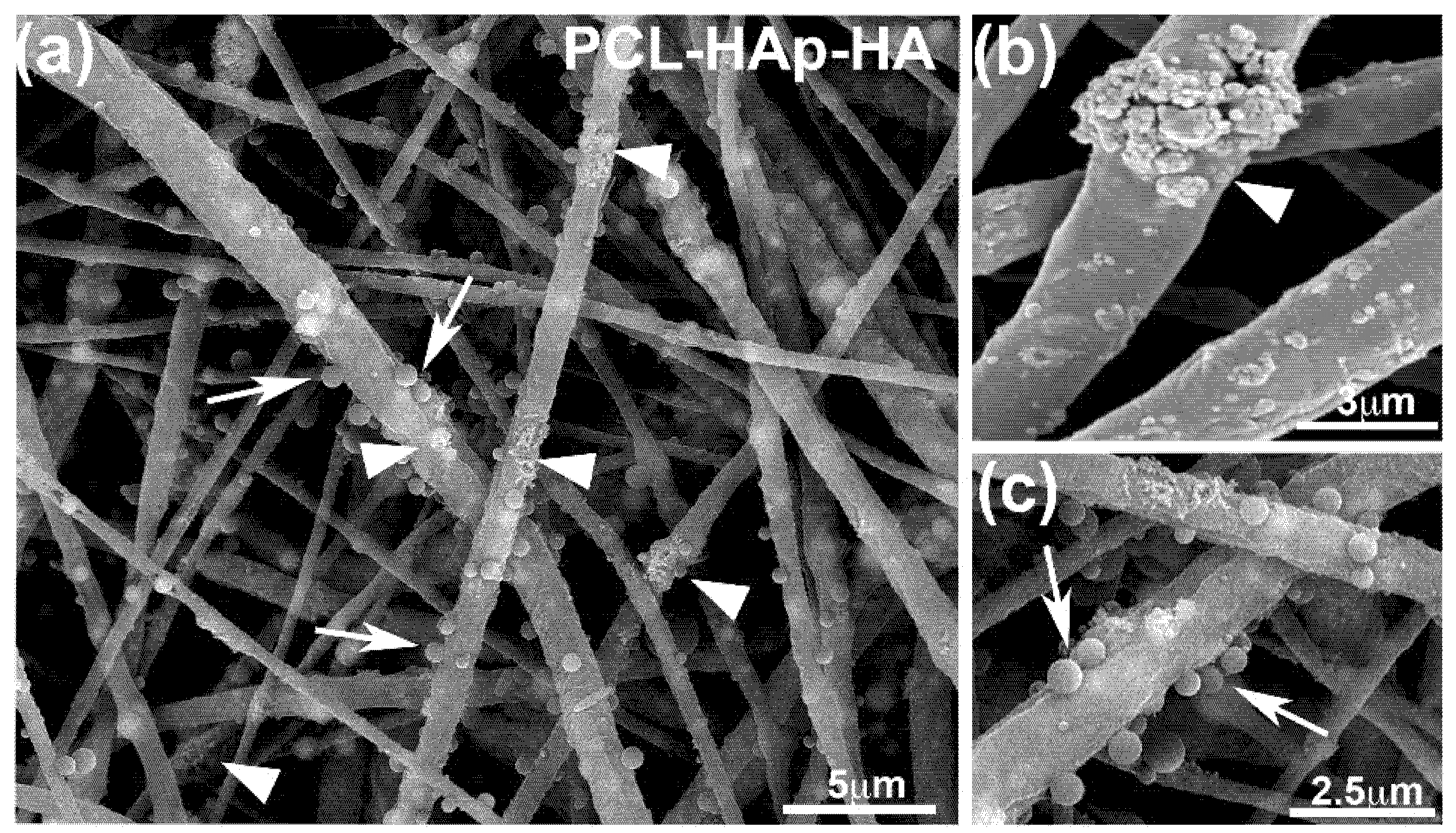
3.1. Structure of the Different Scaffolds
3.2. In Vitro Biocompatibility of the Scaffolds
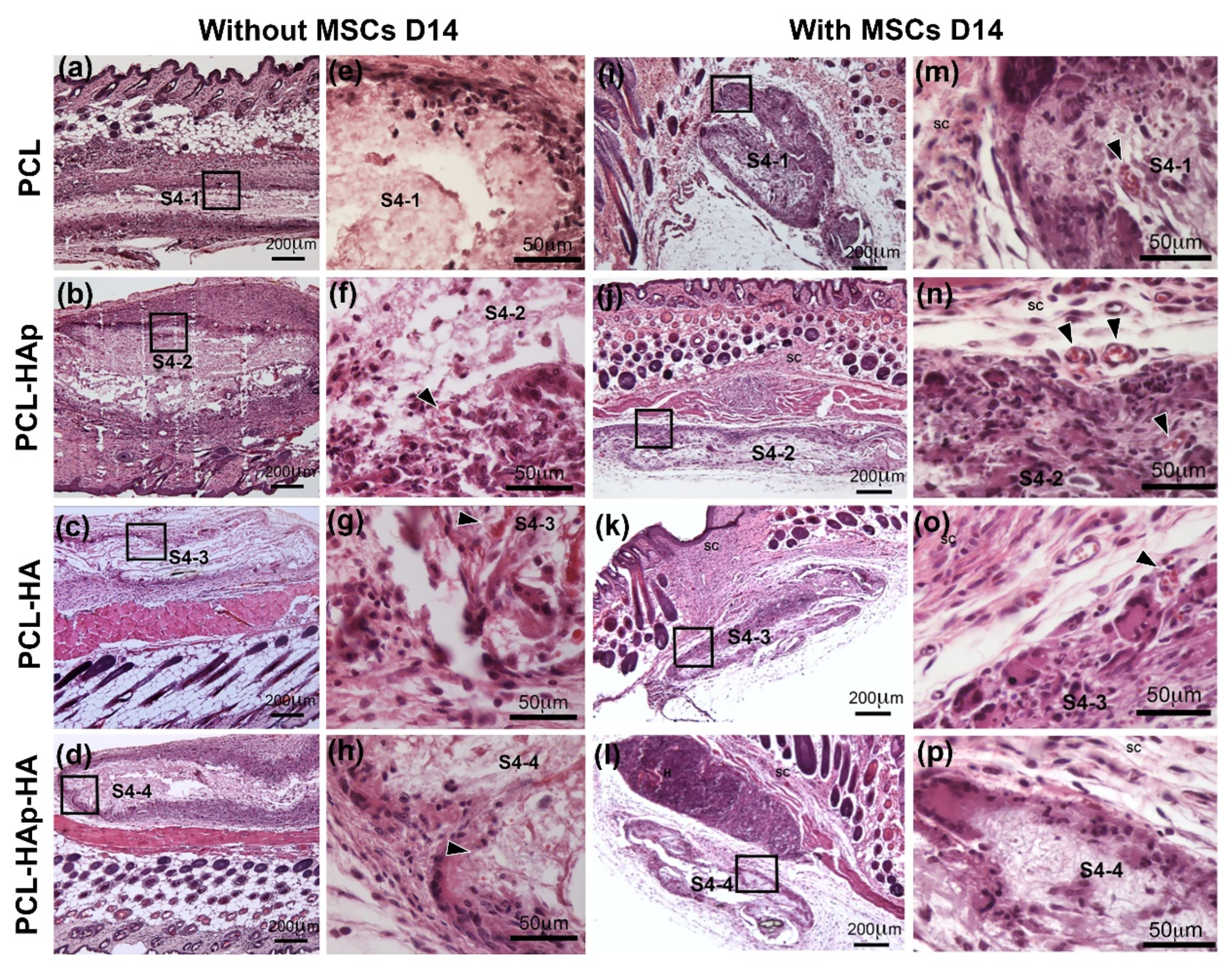
3.3. In Vivo Biocompatibility of the Scaffolds
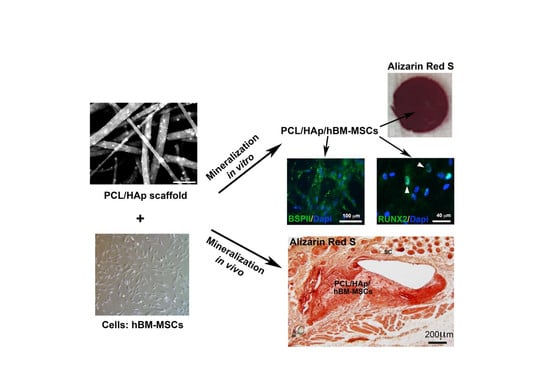
3.4. HAp and hBM-MSCs Can Accelerate in Vivo Mineralization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primer 2016, 2, 336–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldring, M.B.; Berenbaum, F. Emerging targets in osteoarthritis therapy. Curr. Opin. Pharm. 2015, 22, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Qian, Y.; Li, L.; Yao, G.; Yang, L.; Sun, Y. Polycaprolactone nanofiber scaffold enhances the osteogenic differentiation potency of various human tissue-derived mesenchymal stem cells. Stem Cell Res. Ther. 2017, 8, 148. [Google Scholar] [CrossRef]
- Murphy, M.B.; Moncivais, K.; Caplan, A.I. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Keller, L.; Pijnenburg, L.; Idoux-Gillet, Y.; Bornert, F.; Benameur, L.; Tabrizian, M.; Auvray, P.; Rosset, P.; María Gonzalo-Daganzo, R.; Gómez Barrena, E.; et al. Preclinical safety study of a combined therapeutic bone wound dressing for osteoarticular regeneration. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Eap, S.; Keller, L.; Schiavi, J.; Huck, O.; Jacomine, L.; Fioretti, F.; Gauthier, C.; Sebastian, V.; Schwinté, P.; Benkirane-Jessel, N. A living thick nanofibrous implant bifunctionalized with active growth factor and stem cells for bone regeneration. Int. J. Nano. Med. 2015, 10, 1061–1075. [Google Scholar] [CrossRef] [Green Version]
- Eap, S.; Ferrand, A.; Palomares, C.M.; Hébraud, A.; Stoltz, J.-F.; Mainard, D.; Schlatter, G.; Benkirane-Jessel, N. Electrospun nanofibrous 3D scaffold for bone tissue engineering. Biomed. Mater. Eng. 2012, 22, 137–141. [Google Scholar] [CrossRef]
- Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Eap, S.; Brasse, D.; Schwinté, P.; Arruebo, M.; Benkirane-Jessel, N. Nanoengineered implant as a new platform for regenerative nanomedicine using 3D well-organized human cell spheroids. Int. J. Nanomed. 2017, 12, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Eap, S.; Morand, D.; Clauss, F.; Huck, O.; Stoltz, J.-F.; Lutz, J.-C.; Gottenberg, J.-E.; Benkirane-Jessel, N.; Keller, L.; Fioretti, F. Nanostructured thick 3D nanofibrous scaffold can induce bone. Biomed. Mater. Eng. 2015, 25, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Eap, S.; Keller, L.; Ferrand, A.; Schiavi, J.; Lahiri, D.; Lemoine, S.; Facca, S.; Fioretti, F.; Mainard, D.; Agarwal, A.; et al. Nanomechanical Properties of Active Nanofibrous Implants After In Vivo Bone Regeneration. Nano LIFE 2013, 04, 1450001. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facca, S.; Ferrand, A.; Mendoza-Palomares, C.; Perrin-Schmitt, F.; Netter, P.; Mainard, D.; Liverneaux, P.; Benkirane-Jessel, N. Bone formation induced by growth factors embedded into the nanostructured particles. J. Biomed. Nanotechnol. 2011, 7, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Leach, J.K. Advancements in electrospinning of polymeric nanofibrous scaffolds for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 277–293. [Google Scholar] [CrossRef]
- Nisbet, D.R.; Forsythe, J.S.; Shen, W.; Finkelstein, D.I.; Horne, M.K. Review paper: A review of the cellular response on electrospun nanofibers for tissue engineering. J. Biomater. Appl. 2009, 24, 7–29. [Google Scholar] [CrossRef]
- Steipel, R.T.; Gallovic, M.D.; Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Electrospray for generation of drug delivery and vaccine particles applied in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110070. [Google Scholar] [CrossRef]
- Reddy, A.S.; Lakshmi, B.A.; Kim, S.; Kim, J. Synthesis and characterization of acetyl curcumin-loaded core/shell liposome nanoparticles via an electrospray process for drug delivery, and theranostic applications. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2019, 142, 518–530. [Google Scholar] [CrossRef]
- Bhattarai, D.P.; Aguilar, L.E.; Park, C.H.; Kim, C.S. A Review on Properties of Natural and Synthetic Based Electrospun Fibrous Materials for Bone Tissue Engineering. Membranes 2018, 8, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, A.J.; Gleeson, J.P.; Matsiko, A.; Thompson, E.M.; O’Brien, F.J. Effect of different hydroxyapatite incorporation methods on the structural and biological properties of porous collagen scaffolds for bone repair. J. Anat. 2015, 227, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Clegg, T.E.; Caborn, D.; Mauffrey, C. Viscosupplementation with hyaluronic acid in the treatment for cartilage lesions: A review of current evidence and future directions. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2013, 23, 119–124. [Google Scholar] [CrossRef]
- Vetrano, M.; Ranieri, D.; Nanni, M.; Pavan, A.; Malisan, F.; Vulpiani, M.C.; Visco, V. Hyaluronic Acid (HA), Platelet-Rich Plasm and Extracorporeal Shock Wave Therapy (ESWT) promote human chondrocyte regeneration in vitro and ESWT-mediated increase of CD44 expression enhances their susceptibility to HA treatment. PLoS ONE 2019, 14, e0218740. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Block, J.E. US-Approved Intra-Articular Hyaluronic Acid Injections are Safe and Effective in Patients with Knee Osteoarthritis: Systematic Review and Meta-Analysis of Randomized, Saline-Controlled Trials. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2013, 6, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Aragón, J.; Salerno, S.; De Bartolo, L.; Irusta, S.; Mendoza, G. Polymeric electrospun scaffolds for bone morphogenetic protein 2 delivery in bone tissue engineering. J. Colloid Interface Sci. 2018, 531, 126–137. [Google Scholar] [CrossRef]
- Abedalwafa, M.; Wang, F.; Wang, L.; Li, C. Biodegradable poly-epsilon-caprolactone (pcl) for tissue engineering applications: A review. Rev. Adv. Mater. Sci. 2013, 18, 123–140. [Google Scholar]
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Palomares, C.; Ferrand, A.; Facca, S.; Fioretti, F.; Ladam, G.; Kuchler-Bopp, S.; Regnier, T.; Mainard, D.; Benkirane-Jessel, N. Smart Hybrid Materials Equipped by Nanoreservoirs of Therapeutics. ACS Nano 2012, 6, 483–490. [Google Scholar] [CrossRef]
- Facca, S.; Lahiri, D.; Fioretti, F.; Messadeq, N.; Mainard, D.; Benkirane-Jessel, N.; Agarwal, A. In vivo osseointegration of nano-designed composite coatings on titanium implants. ACS Nano 2011, 5, 4790–4799. [Google Scholar] [CrossRef]
- Facca, S.; Cortez, C.; Mendoza-Palomares, C.; Messadeq, N.; Dierich, A.; Johnston, A.P.R.; Mainard, D.; Voegel, J.-C.; Caruso, F.; Benkirane-Jessel, N. Active multilayered capsules for in vivo bone formation. Proc. Natl. Acad. Sci. USA 2010, 107, 3406–3411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessel, N.; Oulad-Abdelghani, M.; Meyer, F.; Lavalle, P.; Haîkel, Y.; Schaaf, P.; Voegel, J.-C. Multiple and time-scheduled in situ DNA delivery mediated by beta-cyclodextrin embedded in a polyelectrolyte multilayer. Proc. Natl. Acad. Sci. USA 2006, 103, 8618–8621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eap, S.; Ferrand, A.; Schiavi, J.; Keller, L.; Kokten, T.; Fioretti, F.; Mainard, D.; Ladam, G.; Benkirane-Jessel, N. Collagen implants equipped with ’fish scale’-like nanoreservoirs of growth factors for bone regeneration. Nano. Med. 2014, 9, 1253–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessel, N.; Atalar, F.; Lavalle, P.; Mutterer, J.; Decher, G.; Schaaf, P.; Voegel, J.C.; Ogier, J. Bioactive Coatings Based on a Polyelectrolyte Multilayer Architecture Functionalized by Embedded Proteins. Adv. Mater. 2003, 15, 692–695. [Google Scholar] [CrossRef]
- Jessel, N.B.; Schwinté, P.; Donohue, R.; Lavalle, P.; Boulmedais, F.; Darcy, R.; Szalontai, B.; Voegel, J.C.; Ogier, J. Pyridylamino-β-cyclodextrin as a Molecular Chaperone for Lipopolysaccharide Embedded in a Multilayered Polyelectrolyte Architecture. Adv. Funct. Mater. 2004, 14, 963–969. [Google Scholar] [CrossRef]
- Aragon, J.; Navascues, N.; Mendoza, G.; Irusta, S. Laser-treated electrospun fibers loaded with nano-hydroxyapatite for bone tissue engineering. Int. J. Pharm. 2017, 525, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Biazar, E.; Heidari, M.; Asefnejad, A.; Asefnezhad, A.; Montazeri, N. The relationship between cellular adhesion and surface roughness in polystyrene modified by microwave plasma radiation. Int. J. Nanomed. 2011, 6, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venugopal, J.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Electrospun-modified nanofibrous scaffolds for the mineralization of osteoblast cells. J. Biomed. Mater. Res. A 2008, 85, 408–417. [Google Scholar] [CrossRef]
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Wang, B.; Wang, Y.; Lou, D. Polymer-controlled core–shell nanoparticles: A novel strategy for sequential drug release. RSC Adv. 2014, 4, 30430–30439. [Google Scholar] [CrossRef]
- Beane, O.S.; Darling, E.M. Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann. Biomed. Eng. 2012, 40, 2079–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, L.; Wagner, Q.; Schwinté, P.; Benkirane-Jessel, N. Double compartmented and hybrid implant outfitted with well-organized 3D stem cells for osteochondral regenerative nanomedicine. Nanomedicine 2015, 10, 2833–2845. [Google Scholar] [CrossRef] [PubMed]





| Scaffold | Composition (wt.%) | |||
|---|---|---|---|---|
| PCL | PVP | HAp | HA | |
| PCL (S4-1) | 90.79 | 9.21 | - | - |
| PCL-HAp (S4-2) | 56.47 | 5.88 | 37.65 | |
| PCL-HA (S4-3) | 90.58 | 9.19 | - | 0.24 |
| PCL-HAp-HA (S4-4) | 56.39 | 5.87 | 37.59 | 0.15 |
| Gene Product | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| ALP | CCACGTCTTCACATTTGGTG | GCAGTGAAGGGCTTCTTGTC |
| BSPII | GAGTGAGAGGGCAGAGGAAA | CGTGGCCTGTACTTAAAGACC |
| RUNX2 | CCAACCCACGAATGCACTATC | TAGTGAGTGGTGGCGGACATAC |
| COLII | CGTCCAGATGACCTTCCTACG | TGAGCAGGGCCTTCTTGAG |
| Beta-actin | GATGAGATTGGCATGGCTTT | CACCTTCACCGTTCCAGTTT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaida, R.; Pijnenburg, L.; Irusta, S.; Himawan, E.; Mendoza, G.; Harmouch, E.; Idoux-Gillet, Y.; Kuchler-Bopp, S.; Benkirane-Jessel, N.; Hua, G. Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration. Materials 2020, 13, 3087. https://doi.org/10.3390/ma13143087
Smaida R, Pijnenburg L, Irusta S, Himawan E, Mendoza G, Harmouch E, Idoux-Gillet Y, Kuchler-Bopp S, Benkirane-Jessel N, Hua G. Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration. Materials. 2020; 13(14):3087. https://doi.org/10.3390/ma13143087
Chicago/Turabian StyleSmaida, Rana, Luc Pijnenburg, Silvia Irusta, Erico Himawan, Gracia Mendoza, Ezeddine Harmouch, Ysia Idoux-Gillet, Sabine Kuchler-Bopp, Nadia Benkirane-Jessel, and Guoqiang Hua. 2020. "Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration" Materials 13, no. 14: 3087. https://doi.org/10.3390/ma13143087
APA StyleSmaida, R., Pijnenburg, L., Irusta, S., Himawan, E., Mendoza, G., Harmouch, E., Idoux-Gillet, Y., Kuchler-Bopp, S., Benkirane-Jessel, N., & Hua, G. (2020). Potential Implantable Nanofibrous Biomaterials Combined with Stem Cells for Subchondral Bone Regeneration. Materials, 13(14), 3087. https://doi.org/10.3390/ma13143087