First-Principles Study on the Cu/Fe Interface Properties of Ternary Cu-Fe-X Alloys
Abstract
1. Introduction
2. Models and Computation Method
3. Results and Discussion
3.1. Bulk and Surface Calculation
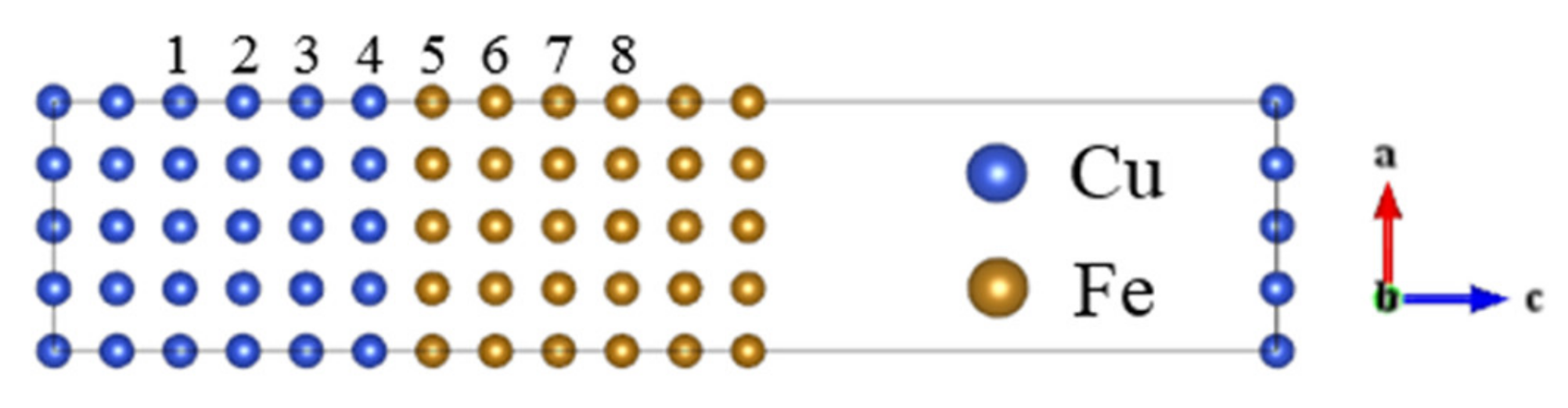
3.2. Model Geometry of Interface
3.3. Work of Adhesion and Interfacial Energy
3.4. Electronic Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hong, S.I.; Song, J.S. Strength and conductivity of Cu-9Fe-1.2X (X = Ag or Cr) filamentary microcomposite wires. Metall. Mater. Trans. A 2001, 32, 985–991. [Google Scholar] [CrossRef]
- Hong, S.I. Copper-Iron Filamentary Microcomposites. Adv. Eng. Mater. 2001, 3, 475–479. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Sun, B. Effect of Ag on the thermal stability of deformation processed Cu-Fe in situ composites. J. Alloys Compd. 2009, 469, 580–586. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Da, S.; Sun, B. Microstructure and strength of Cu-Fe-Ag in situ composites. Mater. Sci. Eng. A 2007, 452, 367–373. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Da, S.; Sun, B. Microstructure and properties of Cu-11Fe-6Ag in situ composite after thermo-mechanical treatments. J. Alloy. Compd. 2007, 438, 268–273. [Google Scholar] [CrossRef]
- Fu, C.L.; Freeman, A.J. Electronic and magnetic properties of the fcc Fe(001) thin films: Fe/Cu(001) and Cu/Fe/Cu(001). Phys. Rev. B 1987, 35, 925–932. [Google Scholar] [CrossRef]
- Polzonetti, G.; Di Castro, V.; Furlani, C. The Growth Mode of Copper and Iron for the Cu/Fe and Fe/Cu Interfaces. Surf. Interface Anal. 1994, 22, 211–213. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, W.; Seidman, D.N.; Wolverton, C. First-principles study of the nucleation and stability of ordered precipitates in ternary Al-Sc-Li alloys. Acta Mater. 2011, 59, 3012–3023. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Zhou, N.G. First-principles calculations on Mg/Al2CO interfaces. Appl. Surf. Sci. 2013, 285, 879–884. [Google Scholar] [CrossRef]
- Wang, J.; Enomoto, M.; Shang, C. First-principles study on the interfacial segregation at coherent Cu precipitate/Fe matrix interface. Scripta Mater. 2020, 185, 425–446. [Google Scholar] [CrossRef]
- Zhao, Y.; Wen, Z.; Hua, H.; Wei, G.; Han, P. Density functional theory study of the interfacial properties of Ni/Ni 3 Si eutectic alloy. Appl. Surface Sci. 2014, 303, 205–209. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Han, Y.; Dai, Y.; Wang, J.; Sun, B. Role of the third element in accelerating Fe diffusivities in Cu from first principles. J. Alloys Compd. 2015, 639, 642–647. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Han, Y.; Dai, Y.; Wang, J.; Sun, B. First-principles study on the solubility of iron in dilute Cu-Fe-X alloys. J. Alloys Compd. 2016, 691, 992–996. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Xiaozhi, W.U.; Gan, L.; Wei, Q. Temperature effects on the generalized planar fault energies and twinnabilities of Al, Ni and Cu: First principles calculations. Comput. Mater. Sci. 2014, 88, 124–130. [Google Scholar] [CrossRef]
- Abhilash, R.; Gian, B.; Guido, F. Core Level Spectra of Organic Molecules Adsorbed on Graphene. Materials 2018, 11, 518–532. [Google Scholar]
- Herrig, F.; Music, D.; Völker, B.; Hans, M. Ab Initio Guided Low Temperature Synthesis Strategy for Smooth Face-Centred Cubic FeMn Thin Films. Materials 2018, 8, 384. [Google Scholar] [CrossRef]
- Pang, H.; Li, M.; Gao, C.; Huang, H. Phase Transition of Single-Layer Molybdenum Disulfide Nanosheets under Mechanical Loading Based on Molecular Dynamics Simulations. Materials 2018, 11, 502. [Google Scholar] [CrossRef] [PubMed]
- Benali, A.; Lacaze-Dufaure, C.; Morillo, J. Density functional study of copper segregation in aluminum. Surf. Sci. 2011, 605, 341–350. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeir, T.C. Smithells Metals Reference Book, 8th ed.; Elsevier: Oxford, UK, 2004. [Google Scholar]
- Yu, J.; Xin, L.; Wang, J.; Jing, C.; Huang, W. First-principles study of the relaxation and energy of bcc-Fe, fcc-Fe and AISI-304 stainless steel surfaces. Appl. Surf. Sci. 2009, 255, 9032–9039. [Google Scholar] [CrossRef]
- Acet, M.; Auml, H.; Wassermann, H.F.; Pepperhoff, W. High-temperature moment-volume instability and anti-Invar of gamma-Fe. Phys. Rev. B 1994, 49, 6012. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Adams, J.B.; Van Schilfgaarde, M. Density-functional calculation of CeO2 surfaces and prediction of effects of oxygen partial pressure and temperature on stabilities. J. Chem. Phys. 2005, 123, 064701. [Google Scholar] [CrossRef]
- Han, H.; Yin, G.; Wang, H.; Wang, C.; Shao, K.; Zhang, W.; Dai, J.; Ping, H. First-principles investigation on the geometries, stabilities and defective properties of fluoride surfaces. Comput. Mater. Sci. 2017, 133, 159–166. [Google Scholar] [CrossRef]
- Vitos, L.; Ruban, A.; Skriver, H.; Kollar, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202. [Google Scholar] [CrossRef]
- Finnis, M.W. The theory of metal-ceramic interfaces. J. Phys. Condens. Matter 1996, 8, 5811. [Google Scholar] [CrossRef]
- Liu, R.; Yin, X.; Feng, K.; Xu, R. First-principles calculations on Mg/TiB2 interfaces. Comput. Mater. Sci. 2018, 149, 373–378. [Google Scholar] [CrossRef]
- Hashibon, A.; Elsässer, C.; Mishin, Y.; Gumbsch, P. First-principles study of thermodynamical and mechanical stabilities of thin copper film on tantalum. Phys. Rev. B 2007, 76, 245434. [Google Scholar] [CrossRef]
- Chunmei, L.I.; Chen, Z.Q.; Zeng, S.M.; Cheng, N.P.; Chen, T.X. Intermetallic phase formation and evolution during homogenization and solution in Al-Zn-Mg-Cu alloys. Sci. China 2013, 56, 2827–2838. [Google Scholar]





| Parameter | Cu | γ–Fe | ||||||
|---|---|---|---|---|---|---|---|---|
| GGA (this work) | GGA [18] | GGA [22] | Exp. [23] | GGA (this work) | GGA-PBE [24] | GGA-PW91 [24] | Exp. [25] | |
| /Å | 3.63 | 3.63 | 3.64 | 3.62 | 3.45 | 3.47 | 3.47 | 3.65 |
| V/Å3·cell−1 | 48.00 | 47.99 | 48.23 | 47.24 | 40.95 | 41.93 | 41.85 | 48.79 |
| Elements (X) | ||
|---|---|---|
| Cu107X | Fe107X | |
| B | 4.802 | 7.103 |
| Si | 5.659 | 7.235 |
| P | 5.258 | 6.595 |
| Al | 4.447 | 4.691 |
| Ge | 4.514 | 5.605 |
| S | 3.529 | 4.261 |
| Mg | 1.73 | 0.911 |
| Ag | 2.294 | 1.296 |
| Cd | 0.32 | 0.44 |
| Sn | 3.56 | 3.416 |
| In | 2.286 | 1.637 |
| Sb | 3.618 | 3.742 |
| Zr | 8.143 | 8.071 |
| Bi | 2.459 | 1.877 |
| Layer Number/n | σ/J·m−2 | |
|---|---|---|
| Cu(100) | γ–Fe(100) | |
| 4 | 1.45 | 3.30 |
| 6 | 1.48 | 3.36 |
| 8 | 1.48 | 3.37 |
| 10 | 1.47 | 3.36 |
| 12 | 1.46 | 3.38 |
| Ref | 1.485, 1.532 [22] 1.79 J/m2 [28] | 2.956, 2.973 [14] |
| Elements | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| B | 1.431 | 1.439 | 1.751 | 0.188 | 0.276 | 0.663 | 0.616 | 0.666 |
| Si | −0.240 | −0.284 | −0.312 | −1.165 | −1.076 | −0.928 | −1.099 | −1.052 |
| P | 0.107 | 0.033 | 0.010 | −1.065 | −0.954 | −0.784 | −0.953 | −0.915 |
| Al | −0.763 | −0.777 | −0.792 | −1.235 | −0.817 | −0.414 | −0.621 | −0.585 |
| Ge | 0.084 | 0.039 | 0.012 | −0.673 | −0.384 | −0.038 | −0.251 | −0.235 |
| S | 0.635 | 0.535 | 0.769 | −0.544 | 0.048 | 0.696 | 0.497 | 0.516 |
| Mg | −0.121 | −0.119 | −0.111 | 0.000 | 0.685 | 1.438 | 1.171 | 1.177 |
| Ag | 0.595 | 0.602 | 0.604 | 0.744 | 1.564 | 2.450 | 2.293 | 2.258 |
| Cd | 0.649 | 0.648 | 0.642 | 0.643 | 1.536 | 2.503 | 2.212 | 2.176 |
| Sn | 0.518 | 0.476 | 0.459 | −0.050 | 0.573 | 1.225 | 0.854 | 0.862 |
| In | 0.503 | 0.484 | 0.484 | 0.255 | 1.042 | 1.877 | 1.538 | 1.514 |
| Sb | 0.878 | 0.800 | 0.766 | 0.063 | 0.564 | 1.087 | 0.740 | 0.727 |
| Zr | 0.536 | 0.453 | 0.495 | −0.336 | 0.328 | 0.966 | 0.598 | 0.620 |
| Bi | 1.916 | 1.844 | 1.818 | 1.349 | 2.055 | 2.863 | 2.409 | 2.395 |
| Elements | Interfacial Distance (Å) | ||
|---|---|---|---|
| Cu | 3.822 | 2.441 | 1.827 |
| B | 4.185 | 2.409 | 1.827 |
| Si | 4.045 | 2.418 | 1.825 |
| P | 3.856 | 2.477 | 1.827 |
| Al | 3.988 | 2.452 | 1.831 |
| Ge | 3.793 | 2.367 | 1.832 |
| S | 3.604 | 2.406 | 1.838 |
| Mg | 3.786 | 2.479 | 1.827 |
| Ag | 3.775 | 2.502 | 1.827 |
| Cd | 3.786 | 2.451 | 1.837 |
| Sn | 3.488 | 2.507 | 1.847 |
| In | 3.481 | 2.510 | 1.849 |
| Sb | 3.394 | 2.494 | 1.847 |
| Zr | 3.972 | 2.416 | 1.844 |
| Bi | 3.027 | 2.528 | 1.855 |
| Element | Cu | Ag | Al | B | Bi | Cd | Ge | In |
|---|---|---|---|---|---|---|---|---|
| /J·m−2 | 0.819 | 0.852 | 0.659 | 0.288 | 0.750 | 0.869 | 0.582 | 0.641 |
| Element | Mg | P | S | Sb | Si | Sn | Zr | |
| /J·m−2 | 0.845 | 0.443 | 0.474 | 0.584 | 0.503 | 0.684 | 0.579 |
| Element | Cu | Ag | Al | B | Bi | Cd | Ge | In |
|---|---|---|---|---|---|---|---|---|
| 10.79 | 10.00 | 9.71 | 10.92 | 12.84 | 10.69 | 13.27 | 11.94 | |
| Element | Mg | P | S | Sb | Si | Sn | Zr | |
| 9.74 | 12.40 | 12.17 | 13.12 | 12.10 | 12.62 | 8.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, M.; Gao, H.; Wang, J.; Sun, B. First-Principles Study on the Cu/Fe Interface Properties of Ternary Cu-Fe-X Alloys. Materials 2020, 13, 3112. https://doi.org/10.3390/ma13143112
Wang Y, Li M, Gao H, Wang J, Sun B. First-Principles Study on the Cu/Fe Interface Properties of Ternary Cu-Fe-X Alloys. Materials. 2020; 13(14):3112. https://doi.org/10.3390/ma13143112
Chicago/Turabian StyleWang, Yufei, Min Li, Haiyan Gao, Jun Wang, and Baode Sun. 2020. "First-Principles Study on the Cu/Fe Interface Properties of Ternary Cu-Fe-X Alloys" Materials 13, no. 14: 3112. https://doi.org/10.3390/ma13143112
APA StyleWang, Y., Li, M., Gao, H., Wang, J., & Sun, B. (2020). First-Principles Study on the Cu/Fe Interface Properties of Ternary Cu-Fe-X Alloys. Materials, 13(14), 3112. https://doi.org/10.3390/ma13143112




