Preclinical Study of a Multi-Layered Antimicrobial Patch Based on Thin Nanocomposite Amorphous Diamond Like Carbon Films with Embedded Silver Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Bandage Prototype
2.2. Microbiological Methods
2.3. Spread Plate Method
2.4. Tests with the Laboratory Animals
3. Results
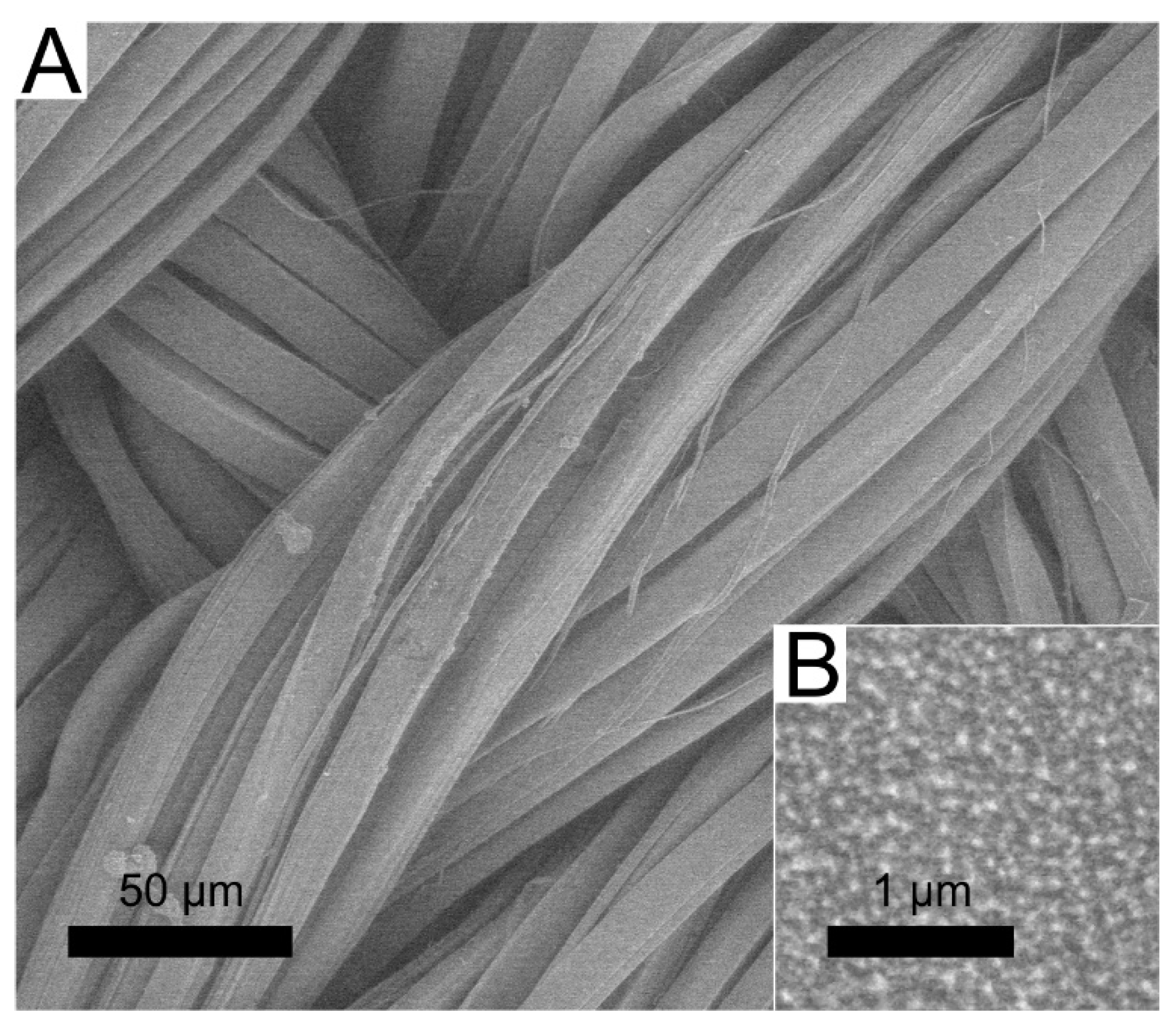
3.1. Deposition of DLC:Ag Thin Film on Silk
3.2. Antimicrobial Properties
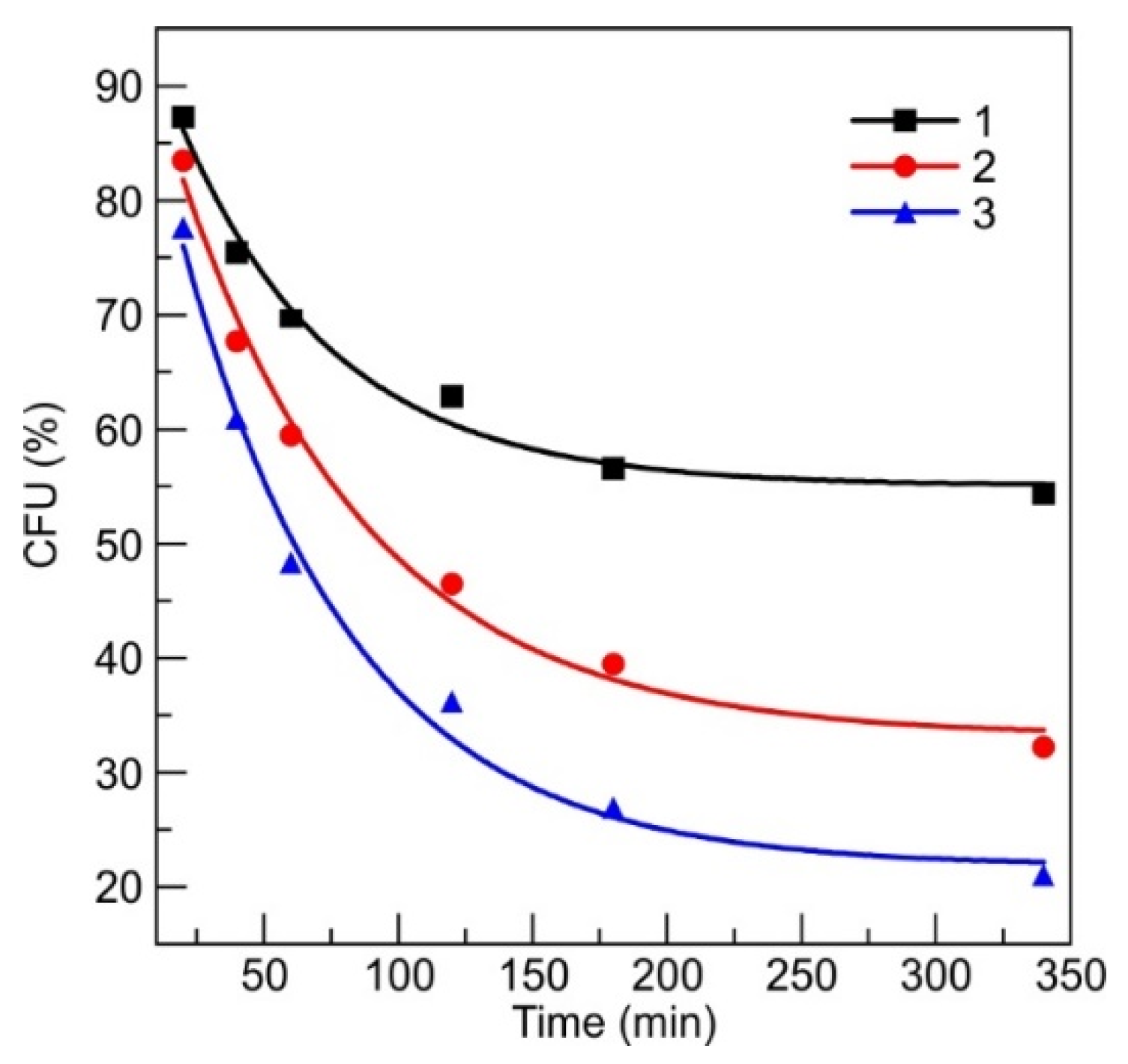
3.2.1. DLC:Ag Film
3.2.2. Antimicrobial Patch Prototype
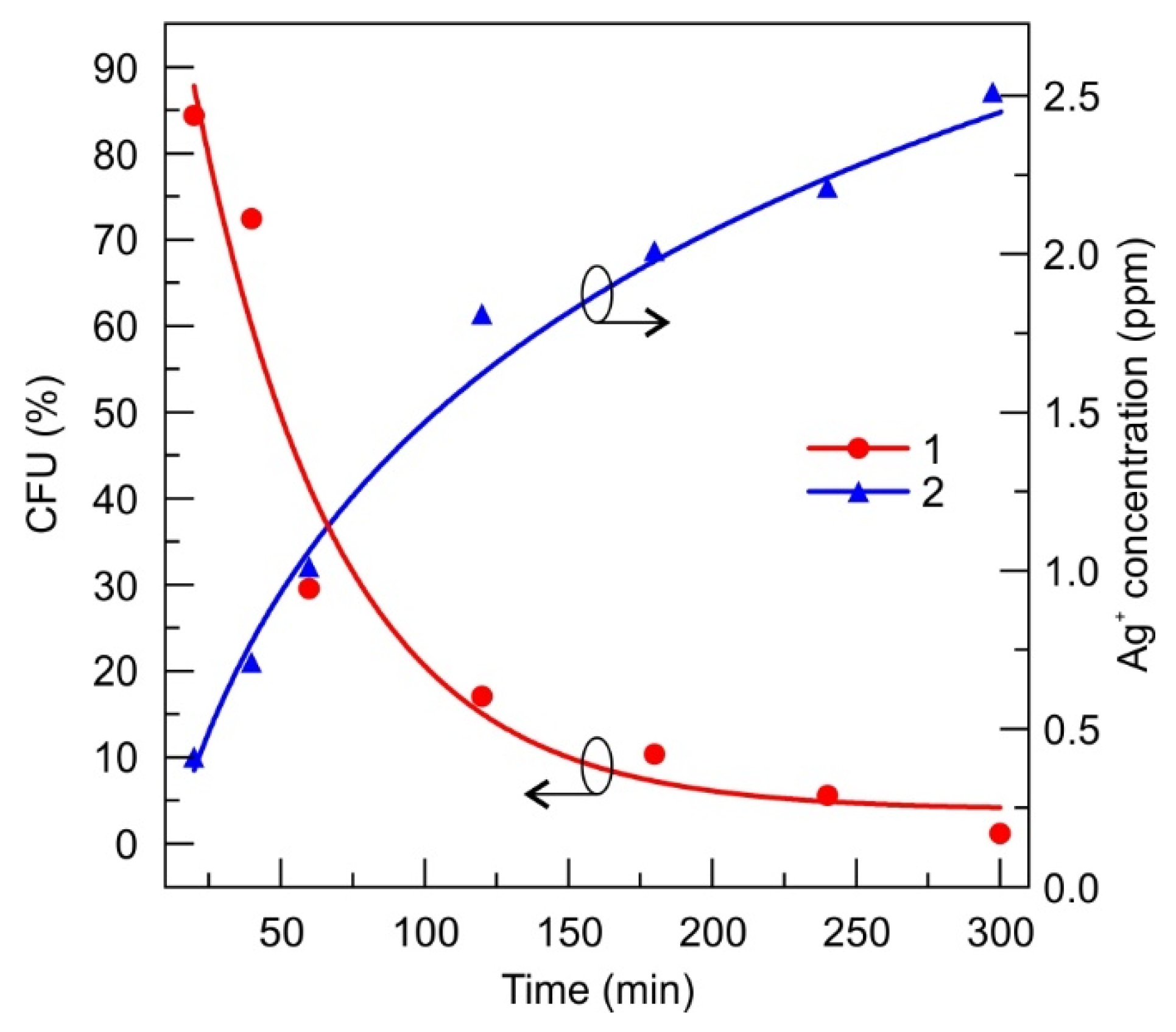
3.2.3. Silver-Ion-Saturated Water
3.3. Microscopic Analysis of Silver-Ion-Treated Bacteria
3.4. Short Preclinical Study with the Animal Model
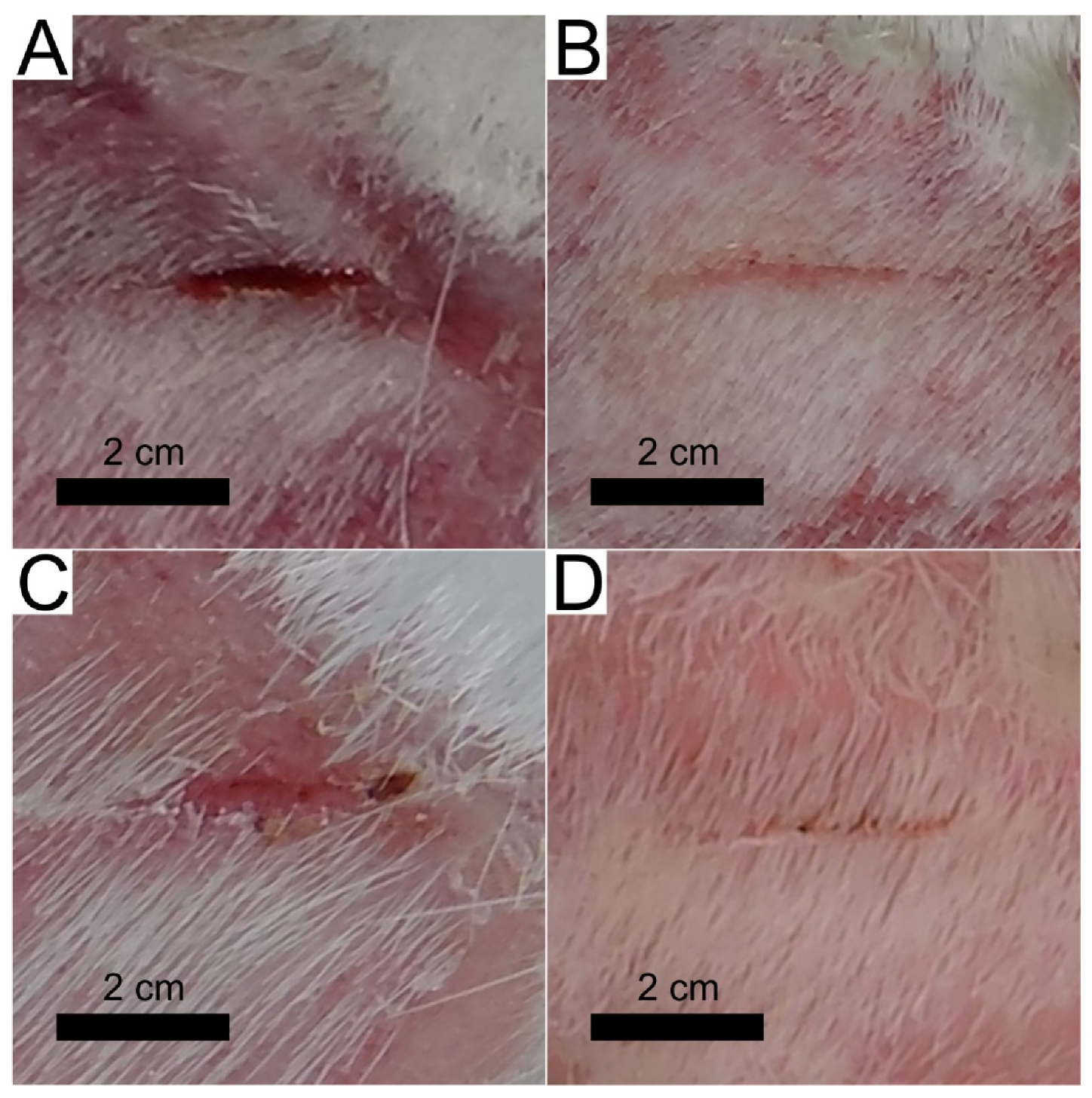
3.4.1. Wound Treatment with a Control Patch
3.4.2. Wound Treatment with a Patch Prototype
4. Discussion
5. Conclusions
- The DLC:Ag thin film coated on synthetic silk fabric, oxygen plasma etched and containing 3.4 at.% Ag, with a size of silver nanoparticles (NPs) from 3 to 63 nm and an average size of 23.7 nm, was able to release 4 ppm of silver ions after 24 h of soaking in water, and inactivated 83% of S. aureus (0.5 Mf) bacteria per 120 min, while silver-ion-saturated water of 4 ppm, inactivated only 64.1% of bacteria;
- After S. aureus bacteria treatment with silver ions (4 ppm concentration in bacteria suspension), 76% of bacteria were inactivated. Scanning electron microscopy analysis showed that silver NPs destroyed the bacteria cell and the bacteria affected by Ag ions had spots and perforated cell wall areas, where cytoplasm leakage out was obtained;
- The patch prototype was successfully prepared by applying the DLC:Ag thin-film-coated synthetic silk fabric, which contained the 3.4 at.% Ag. The special cellulose membrane and silver ion accumulation layer were sewed layer by layer on thin-film-coated synthetic silk. The patch demonstrated stable properties for 6 months when stored at room temperature. The prototype increased the healing speed of infected wounds, killed the MRSA inside the wound with more than 50% efficiency per day (completely killed after 3 days), sustained the anti-stick to wound properties and reduced the scar marks.
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, S.Y.; Tatsumura, Y. Alexander Fleming (1881–1955): Discoverer of Penicillin. Singapore Med. J. 2015, 56, 366–367. [Google Scholar] [CrossRef]
- Strohsahl, C.M.; Miller, B.L.; Krauss, T.D. Detection of Methicillin-Resistant Staphylococcus Aureus (MRSA) Using the NanoLantern Biosensor. Proc. SPIE Front. Pathog. Detect. Nanosensors Syst. 2009, 7167, 71670S. [Google Scholar] [CrossRef]
- Morrison, M.L.; Buchanan, R.A.; Liaw, P.K.; Berry, C.J.; Brigmon, R.L.; Riester, L.; Abernathy, H.; Jin, C.; Narayan, R.J. Electrochemical and Antimicrobial Properties of Diamondlike Carbon-Metal Composite Films. Diam. Relat. Mater. 2006, 15, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Cloutier, M.; Tolouei, R.; Lesage, O.; Lévesque, L.; Turgeon, S.; Tatoulian, M.; Mantovani, D. On the Long Term Antibacterial Features of Silver-Doped Diamondlike Carbon Coatings Deposited via a Hybrid Plasma Process. Biointerphases 2014, 9, 029013. [Google Scholar] [CrossRef] [PubMed]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-Doped Nanocomposite Carbon Coatings (Ag-DLC) for Biomedical Applications—Physiochemical and Biological Evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Belkaid, Y.; Segre, J.A. Dialogue between Skin Microbiota and Immunity. Science 2014, 346, 954–959. [Google Scholar] [CrossRef]
- Thomson, C.H. Biofilms: Do They Affect Wound Healing? Int. Wound J. 2011, 8, 63–67. [Google Scholar] [CrossRef]
- Thet, N.T.; Alves, D.R.; Bean, J.E.; Booth, S.; Nzakizwanayo, J.; Young, A.E.R.; Jones, B.V.; Jenkins, A.T.A. Prototype Development of the Intelligent Hydrogel Wound Dressing and Its Efficacy in the Detection of Model Pathogenic Wound Biofilms. ACS Appl. Mater. Interfaces 2016, 8, 14909–14919. [Google Scholar] [CrossRef]
- Osińska-Jaroszuk, M.; Ginalska, G.; Belcarz, A.; Uryniak, A. Vascular Prostheses with Covalently Bound Gentamicin and Amikacin Reveal Superior Antibacterial Properties than Silver-Impregnated Ones—An In Vitro Study. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Barillo, D.J.; Pozza, M.; Margaret-Brandt, M. A Literature Review of the Military Uses of Silver-Nylon Dressings with Emphasis on Wartime Operations. Burns 2014, 40, S24–S29. [Google Scholar] [CrossRef]
- N Oktar, F.; Yetmez, M.; Ficai, D.; Ficai, A.; Dumitru, F.; Pica, A. Molecular Mechanism and Targets of the Antimicrobial Activity of Metal Nanoparticles. Curr. Top. Med. Chem. 2015, 15, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Mooney, E.K.; Lippitt, C.; Friedman, J. Silver Dressings. Plast. Reconstr. Surg. 2006, 117, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial Cellulose as a Material for Wound Treatment: Properties and Modifications. A Review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J. Silver Dressings: Their Role in Wound Management. Int. Wound J. 2006, 3, 282–294. [Google Scholar] [CrossRef]
- Morones-Ramirez, J.R.; Winkler, J.A.; Spina, C.S.; Collins, J.J. Silver Enhances Antibiotic Activity Against Gram-Negative Bacteria. Sci. Transl. Med. 2013, 5, 190ra81. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Sampson, B.; Rowe, A. Sequential Changes in Trace Metal, Metallothionein and Calmodulin Concentrations in Healing Skin Wounds. J. Anat. 1999, 195, 375–386. [Google Scholar] [CrossRef]
- Tang, C.; Hu, D.; Cao, Q.; Yan, W.; Xing, B. Silver Nanoparticles-Loaded Activated Carbon Fibers Using Chitosan as Binding Agent: Preparation, Mechanism, and Their Antibacterial Activity. Appl. Surf. Sci. 2017, 394, 457–465. [Google Scholar] [CrossRef]
- Paul, R. Diamond-Like-Carbon Coatings for Advanced Biomedical Applications. Glob. J. Nanomed. 2017, 2, 555598. [Google Scholar] [CrossRef]
- Wang, X.; Herting, G.; Odnevall Wallinder, I.; Blomberg, E. Adsorption of Bovine Serum Albumin on Silver Surfaces Enhances the Release of Silver at PH Neutral Conditions. Phys. Chem. Chem. Phys. 2015, 17, 18524–18534. [Google Scholar] [CrossRef]
- Rieger, K.A.; Cho, H.J.; Yeung, H.F.; Fan, W.; Schiffman, J.D. Antimicrobial Activity of Silver Ions Released from Zeolites Immobilized on Cellulose Nanofiber Mats. ACS Appl. Mater. Interfaces 2016, 8, 3032–3040. [Google Scholar] [CrossRef]
- Catanzano, O.; D’Esposito, V.; Pulcrano, G.; Maiolino, S.; Ambrosio, M.R.; Esposito, M.; Miro, A.; Ungaro, F.; Formisano, P.; Catania, M.R.; et al. Ultrasmall Silver Nanoparticles Loaded in Alginate–Hyaluronic Acid Hybrid Hydrogels for Treating Infected Wounds. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 626–634. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk Surfaces Coated with Triangular Silver Nanoplates: Antibacterial Action Based on Silver Release and Photo-Thermal Effect. Nanomaterials 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Bui, V.; Park, D.; Lee, Y.-C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers (Basel) 2017, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondarenko, O.M.; Sihtmäe, M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Bacterial Plasma Membrane Is the Main Cellular Target of Silver Nanoparticles in Escherichia Coli and Pseudomonas Aeruginosa. Prepr. Serv. Biol. 2018, 1–38. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Shidlovskiy, I.P.; Nikolaeva, E.D.; Sukovatiy, A.G.; Vasiliev, A.D.; Shishatskaya, E.I. Antibacterial Properties of Films of Cellulose Composites with Silver Nanoparticles and Antibiotics. Polym. Test. 2018, 65, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Gravante, G.; Caruso, R.; Sorge, R.; Nicoli, F.; Gentile, P.; Cervelli, V. Nanocrystalline Silver: A Systematic Review of Randomized Trials Conducted on Burned Patients and an Evidence-Based Assessment of Potential Advantages Over Older Silver Formulations. Ann. Plast. Surg. 2009, 63, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Marx, D.E.; Barillo, D.J. Silver in Medicine: The Basic Science. Burns 2014, 40, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, E.E.; Puglia, D.; Fortunati, E.; Bertoglio, F.; Bruni, G.; Visai, L.; Kenny, J.M. Cellulose Nanocrystals as Templates for Cetyltrimethylammonium Bromide Mediated Synthesis of Ag Nanoparticles and Their Novel Use in PLA Films. Carbohydr. Polym. 2017, 157, 1557–1567. [Google Scholar] [CrossRef]
- Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliauskas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial Properties of Diamond-like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials (Basel) 2016, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface Chemical and Biological Characterization of Flax Fabrics Modified with Silver Nanoparticles for Biomedical Applications. Mater. Sci. Eng. C 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia Coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankauskaitė, V.; Vitkauskienė, A.; Lazauskas, A.; Baltrusaitis, J.; Prosyčevas, I.; Andrulevičius, M. Bactericidal Effect of Graphene Oxide/Cu/Ag Nanoderivatives against Escherichia Coli, Pseudomonas Aeruginosa, Klebsiella Pneumoniae, Staphylococcus Aureus and Methicillin-Resistant Staphylococcus Aureus. Int. J. Pharm. 2016, 511, 90–97. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Wettability and Antibacterial Activity of Modified Diamond-like Carbon Films. Appl. Surf. Sci. 2009, 255, 8377–8382. [Google Scholar] [CrossRef]
- Biao, L.; Tan, S.; Zhang, X.; Gao, J.; Liu, Z.; Fu, Y. Synthesis and Characterization of Proanthocyanidins-Functionalized Ag Nanoparticles. Colloids Surf. B Biointerfaces 2018, 169, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Abalkhil, T.A.; Alharbi, S.A.; Salmen, S.H.; Wainwright, M. Bactericidal Activity of Biosynthesized Silver Nanoparticles against Human Pathogenic Bacteria. Biotechnol. Biotechnol. Equip. 2017, 31, 411–417. [Google Scholar] [CrossRef] [Green Version]
- López-Carballo, G.; Higueras, L.; Gavara, R.; Hernández-Muñoz, P. Silver Ions Release from Antibacterial Chitosan Films Containing in Situ Generated Silver Nanoparticles. J. Agric. Food Chem. 2013, 61, 260–267. [Google Scholar] [CrossRef]
- Tamulevičius, S.; Meškinis, Š.; Tamulevičius, T.; Rubahn, H.G. Diamond like Carbon Nanocomposites with Embedded Metallic Nanoparticles. Reports Prog. Phys. 2018, 81, 024501. [Google Scholar] [CrossRef]
- Zakarienė, G.; Novoslavskij, A.; Meškinis, Š.; Vasiliauskas, A.; Tamulevičienė, A.; Tamulevičius, S.; Alter, T.; Malakauskas, M. Diamond like Carbon Ag Nanocomposites as a Control Measure against Campylobacter Jejuni and Listeria Monocytogenes on Food Preparation Surfaces. Diam. Relat. Mater. 2018, 81, 118–126. [Google Scholar] [CrossRef]
- Hartman, D. Perfecting Your Spread Plate Technique. J. Microbiol. Biol. Educ. 2011, 12, 204–205. [Google Scholar] [CrossRef] [PubMed]
- Dorsett-Martin, W.A. Rat Models of Skin Wound Healing: A Review. Wound Repair Regen. 2004, 12, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Innes, M.E.; Umraw, N.; Fish, J.S.; Gomez, M.; Cartotto, R.C. The Use of Silver Coated Dressings on Donor Site Wounds: A Prospective, Controlled Matched Pair Study. Burns 2001, 27, 621–627. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the Antibacterial Property of Carbon Films. Diam. Relat. Mater. 2008, 17, 1416–1419. [Google Scholar] [CrossRef]
- Juknius, T. Smart Patch. WO/2018/002817, 1 April 2018. [Google Scholar]
- McFarland, J. The Nephelometer: An Instrument for Estimating the Number of Bacteria in Suspensions Used for Calculating the Opsonic Index and for Vaccines. JAMA J. Am. Med. Assoc. 1907, 49, 1176–1178. [Google Scholar] [CrossRef]
- Wickerham, L.J. Taxonomy of Yeasts. Tech. Bull. U. S. Dep. Agric. 1951, 1029, 1–55. [Google Scholar]



| Patch No. | Wound Bed Length (mm) | Capillary Net Growth | Swelling | Redness | Bacteria CFU | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Patch 1 | 31 ± 3 | 30 ± 2 | 25 ± 1 | n | y | n | y | n | n | y | y | n | - | - | - |
| Patch 2 | 30 ± 3 | 28 ± 1 | 26 ± 1 | n | y | n | y | n | n | y | y | n | 184 ± 10 | 128 ± 8 | 76 ± 11 |
| Patch 3 | 26 ± 2 | 22 ± 1 | 19 ± 2 | y | y | n | n | n | n | n | n | n | - | - | - |
| Patch 4 | 32 ± 2 | 30 ± 2 | 28 ± 1 | y | y | n | n | n | n | n | n | n | 91 ± 5 | 54 ± 6 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juknius, T.; Juknienė, I.; Tamulevičius, T.; Ružauskas, M.; Pamparienė, I.; Oberauskas, V.; Jurkevičiūtė, A.; Vasiliauskas, A.; Tamulevičius, S. Preclinical Study of a Multi-Layered Antimicrobial Patch Based on Thin Nanocomposite Amorphous Diamond Like Carbon Films with Embedded Silver Nanoparticles. Materials 2020, 13, 3180. https://doi.org/10.3390/ma13143180
Juknius T, Juknienė I, Tamulevičius T, Ružauskas M, Pamparienė I, Oberauskas V, Jurkevičiūtė A, Vasiliauskas A, Tamulevičius S. Preclinical Study of a Multi-Layered Antimicrobial Patch Based on Thin Nanocomposite Amorphous Diamond Like Carbon Films with Embedded Silver Nanoparticles. Materials. 2020; 13(14):3180. https://doi.org/10.3390/ma13143180
Chicago/Turabian StyleJuknius, Tadas, Indrė Juknienė, Tomas Tamulevičius, Modestas Ružauskas, Ina Pamparienė, Vaidas Oberauskas, Aušrinė Jurkevičiūtė, Andrius Vasiliauskas, and Sigitas Tamulevičius. 2020. "Preclinical Study of a Multi-Layered Antimicrobial Patch Based on Thin Nanocomposite Amorphous Diamond Like Carbon Films with Embedded Silver Nanoparticles" Materials 13, no. 14: 3180. https://doi.org/10.3390/ma13143180









