LDH-Co-Fe-Acetate: A New Efficient Sorbent for Azoic Dye Removal and Elaboration by Hydrolysis in Polyol, Characterization, Adsorption, and Anionic Exchange of Direct Red 2 as a Model Anionic Dye
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of LDH Samples
- (a)
- CoFe-Ac/pThe CoFe-Ac LDH where acetate anion is intercalated was synthesized with a molar ratio (Co/Fe) of the three following, previously described methods based on a forced hydrolysis reaction in a polyol medium [27]. Accordingly, a mixture of acetate salts dissolved in DEG with a total molar concentration of 0.1 mol/L that is heated at 130 °C under continuous stirring for 6 h. The corresponding LDH precipitated when the hydrolysis and alkalinity ratios h and b were fixed at 100 and 2, respectively, where h = nH2O/n (Co+Fe) and b = nNaOH/n (Co + Fe). The solid formed is separated by centrifugation. Then it is washed several times with ethanol, dried under air at 60 °C, and named CoFe-Ac/p. As it will be shown below by Mossbauer spectroscopy, the Fe2+ present in the precursor has been oxidized to Fe3+ in the polyol medium despite the reducing nature of this solvent. This oxidation is due to the presence of a large amount of water, which inhibits reduction, promotes oxidation, and the formation of hydroxides or oxides in addition to the easy oxidation of ferrous ions. The valence of Co2+ is preserved in these conditions [28,29].
- (b)
- CoFe-Ac/ExThe anion exchange properties of CoFe-Ac/p with carbonate anions were investigated by mixing 1 g of the synthesized CoFe-Ac/p LDH with 100 mL of a 2 M Na2CO3 solution. Despite the fact that the Co2 + in the cationic layers appears stable with respect to oxidation (see UV-Vis-NIR analysis), the exchange has been carried out as a precaution in an inert atmosphere. After equilibrating for 24 h at room temperature, the solid was separated by centrifugation, washed several times with ethanol, dried under air at 60 °C, and then named CoFe-Ac/Ex.
- (c)
- CoFe-CO3/AThe LDH intercalated with carbonate anions (CoFe-CO3) was prepared by coprecipitation in an aqueous medium [30]. An acid solution of CoCl2·4H2O and FeCl3, with a Co2+/Fe3+ molar ratio R = 3 and a total concentration of metallic cations of 0.75 mol/L, was added drop-by-drop to a vigorously stirred alkaline solution of NaOH (1 M) and Na2CO3 (2 M) in an inert atmosphere in order to avoid the oxidation of Co2+ into Co3+. The pH of the reaction mixture was adjusted to 10. The resulting slurry was aged at 70 °C for 24 h, separated by centrifugation, and washed extensively using distilled water until the supernatant was chloride-free, as indicated by the AgNO3 test. The product was dried at 60 °C under air and ground in an agate mortar. The obtained material is called CoFe-CO3/A.
2.2. Methods
2.2.1. Characterization
2.2.2. Adsorption Experiments
2.2.3. Theory and Modelling
Kinetic Study
Isotherm Study
- (a)
- Langmuir IsothermIn the Langmuir isotherm, it is assumed that the maximum adsorption is limited to a monolayer of molecules distributed homogeneously over the entire surface and without interactions between them [34]. It is given by the following linear equation.where is the equilibrium adsorption coefficient (L mg−1), Qmax is the maximum adsorption capacity (mg/g), Ce is the equilibrium concentration (mg L−1), and qe is the adsorbed amount at equilibrium (mg/g). and Qmax values were calculated from the slope and intercept of the plot of Ce/qe = f (Ce).
- (b)
- Freundlich IsothermThe Freundlich model is based on an empirical equation, which considers that the sorption occurred on a surface where the active sites have heterogeneous energetic distribution. Additionally, it supposes multilayer adsorption with interactions between the adsorbed molecules [34,35]. It is represented by the following linear equation.is the equilibrium concentration (mg·L−1), is the adsorbed amount at equilibrium (mg·g−1), and and 1/n are the Freundlich constants. The constant n is related to the energy and the intensity of adsorption and indicates the adsorption capacity (mg g−1). and 1/n values were inferred from the slope and intercept of the plot of = f ().
Thermodynamic Parameters
3. Results
3.1. Characterization of Adsorbents
3.1.1. X-ray Diffraction
3.1.2. Morphology
3.1.3. Spectroscopy Studies
3.1.4. Thermal Analysis
3.1.5. Chemical Analysis
3.1.6. Surface Area Measurements
3.2. Adsorption Study
3.2.1. Effect of Contact Time
3.2.2. Kinetic Modelling
3.2.3. Effect of pH
3.2.4. Effect of Temperature and Thermodynamic Study
3.2.5. Adsorption Isotherms
3.3. X-ray and IR Characterizations of the LDHs after Adsorption
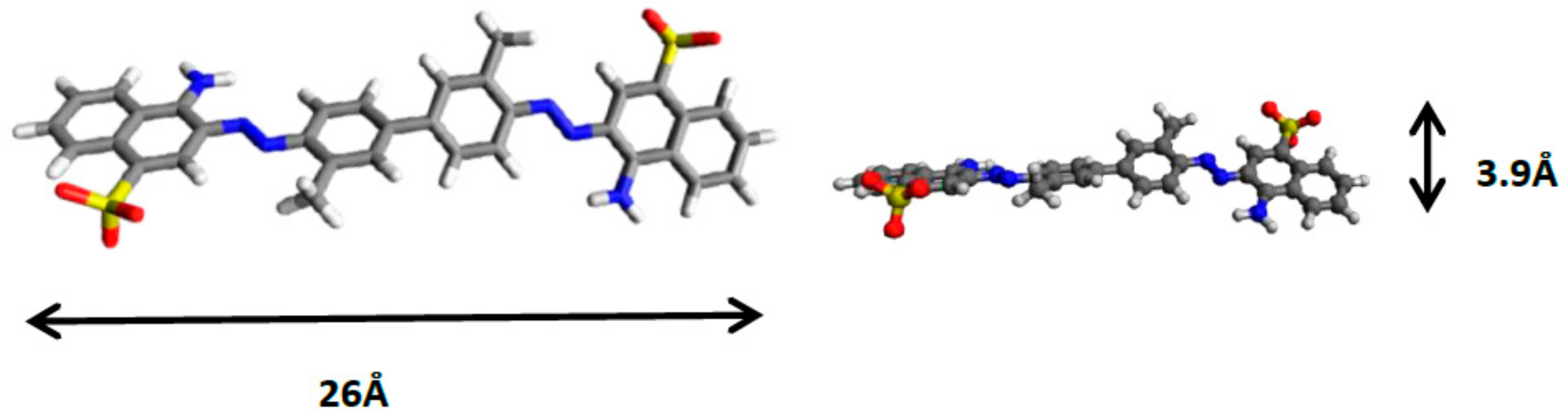
4. Discussion: Mechanism of Direct Red 2 Removal and Comparison with Previous Works
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Rives, V.; del Arco, M.; Martín, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Richetta, M. Characteristics, Preparation Routes and Metallurgical Applications of LDHs: An Overview. J. Mater. Sci. Eng. 2017, 6, 397. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.; Dash, B.; Pandey, S. Layered double hydroxides: A brief review from fundamentals to application as evolving biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Duan, X.; Evans, D.G. Layered Double Hydroxydes. Struct. Bond. 2006, 119, 1–234. [Google Scholar]
- Del Arco, M.; Trujillano, R.; Rives, V. Cobalt–iron hydroxycarbonates and their evolution to mixed oxides with spinel structure. J. Mater. Chem. 1998, 8, 761–767. [Google Scholar] [CrossRef]
- Faour, A.; Prévot, V.; Taviot-Gueho, C. Microstructural study of different LDH morphologies obtained via different synthesis routes. J. Phys.Chem. Solids 2010, 71, 487–490. [Google Scholar] [CrossRef]
- Qiao, C.; Zhang, Y.; Zhu, Y.; Cao, C.; Bao, X.; Xu, J. One-step synthesis of zinc–cobalt layered double hydroxide (Zn-Co-LDH) nanosheets for high-efficiency oxygen evolution reaction. J. Mater. Chem. A 2015, 3, 6878–6883. [Google Scholar] [CrossRef]
- Zaghouane-Boudiaf, H.; Boutahala, M.; Arab, L. Removal of methyl orange from aqueous solution by uncalcined and calcined MgNiAl layered double hydroxides (LDHs). J. Chem. Eng. 2012, 187, 142–149. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K.M. Zn-Cr layered double hydroxide: Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants. Separ. Purif. Tech. 2012, 91, 73–80. [Google Scholar] [CrossRef]
- Reichle, W.-T. Synthesis of anionic clay minerals (Mixed Metal Hydroxides, Hydrotalcite). Solid State Ionics 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Ulibarri, M.A.; Pavlovic, I.; Barriga, C.; Hermosín, M.C.; Cornejo, J. Adsorption of anionic species on hydrotalcite-like compounds: Effect of interlayer anion and crystallinity. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]
- Chibwe, K.; Jones, W. Intercalation of organic and inorganic anions into layered double hydroxides. J. Chem. Soc. Chem. Commun. 1989, 14, 926–927. [Google Scholar] [CrossRef]
- Marangoni, R.; Bouhent, M.; Taviot-Guého, C.; Wypych, F.; Leroux, F. Zn2Al layered double hydroxides intercalated and adsorbed with anionic blue dyes: A physico-chemical characterization. J. Colloid Interf. Sci. 2009, 333, 120–127. [Google Scholar] [CrossRef]
- Abdolmohammad-Zadeh, H.; Nejati, K.; Ghorbani, E. Synthesis, Characterization, and Application of Zn–Al Layered Double Hydroxide as a Nano-Sorbent for the Removal of Direct Red 16 from Industrial Wastewater Effluents. Chem. Eng. Commun. 2015, 202, 1349–1359. [Google Scholar] [CrossRef]
- Alexandrica, M.C.; Silion, M.; Hritcu, D.; Popa, M.I. Layered Double Hyrdoxides as adsorbents for anionic dye removal from aqueous solutions. J. Environ. Eng. Manag. 2015, 14, 381–388. [Google Scholar]
- Costantino, U.; Coletti, N.; Nocchetti, M.; Aloisi, G.G.; Elisei, F.; Latterini, L. Surface Uptake and Intercalation of Fluorescein Anions into Zn−Al−Hydrotalcite. Photophysical Characterization of Materials Obtained. Langmuir 2000, 16, 10351–10358. [Google Scholar] [CrossRef]
- Wu, P.; Wu, T.; He, W.; Sun, L.; Li, Y.; Sun, D. Adsorption properties of dodecylsulfate-intercalated layered double hydroxide for various dyes in water. Colloids Surf. A Physicochem. Eng. Aspects 2013, 436, 726–731. [Google Scholar] [CrossRef]
- El Hassani, K.; Jabkhiro, H.; Kalnina, D.; Beakou, B.H.; Anouar, A. Effect of drying step on layered double hydroxides properties: Application in reactive dye intercalation. Appl. Clay Sci. 2019, 182, 105246. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthick, K.; Kundu, S. Evolution of layered double hydroxides (LDH) as high performance water oxidation electrocatalysts: A review with insights on structure, activity and mechanism. Mater. Today Energy 2017, 6, 1–26. [Google Scholar] [CrossRef]
- Wang, R.; Wu, X.; Zou, C.; Li, X.; Du, Y. NOx Removal by Selective Catalytic Reduction with Ammonia over a Hydrotalcite-Derived NiFe Mixed Oxide. Catalysts 2018, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Carja, G.; Chiriac, H.; Lupu, N. New magnetic organic–inorganic composites based on hydrotalcite-like anionic clays for drug delivery. J. Magn. Magn. Mater. 2007, 311, 26–30. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Zhou, Y.; Song, Y.; He, J.; Evans, D.G. Synthesis of CoFe alloy nanoparticles embedded in a MgO crystal matrix using a single-source inorganic precursor. R. Soc. Chem. Chem. Commun. 2010, 46, 3911–3913. [Google Scholar] [CrossRef] [PubMed]
- Bouberka, Z.; Khenifi, A.; Sekrane, F.; Bettahar, N.; Derriche, Z. Adsorption of Direct Red 2 on bentonite modified by cetyltrimethylammonium bromide. J. Chem. Engr. 2008, 136, 295–305. [Google Scholar]
- Setti, N.D.; Jouini, N.; Derriche, Z. Sorption study of an anionic dye—Benzopurpurine 4B—On calcined and uncalcined Mg-Al layered double hydroxides. J. Phys. Chem. Solids 2010, 71, 556–559. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Drici Setti, N.; Derriche, Z.; Jouini, N. New acetate-intercalated CoNiFe, ZnNiFe and CoZnFe layered double hydroxides: Synthesis by forced hydrolysis in polyol medium and characterization. J. Adv. Chem. 2014, 10, 93097–93107. [Google Scholar]
- Poul, L.; Ammar, S.; Jouini, N.; Fievet, F.; Villain, F. Synthesis of Inorganic Compounds (Metal, Oxide and Hydroxide) in Polyol Medium: A Versatile Route Related to the Sol-Gel Process. J. Sol-Gel Sci. Technol. 2003, 26, 261–265. [Google Scholar] [CrossRef]
- Beji, Z.; Ben Chaabane, A.; Smiri, L.S.; Ammar, S.; Fiévet, F.; Jouini, N.; Greneche, J.M. Synthesis of nickel-zinc ferrite nanoparticles in polyol: Morphological, structural and magnetic properties. Phys. Stat. Solid A 2005, 203, 504–512. [Google Scholar] [CrossRef]
- Aramendia, M.A.; Borau, V.; Jimé, N.C.; Maria, M.J.; José, R.F.; Rafael, R.J. Synthesis, Characterization, and 1H and 71Ga MAS NMR Spectroscopy of a Novel Mg/Ga Double Layered Hydroxide. J. Solid State Chem. 1997, 131, 78–83. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelosterstoffe. Kungliga Svenska Vetenskapsakademiem. Handlingar 1898, 24, 1–39. [Google Scholar]
- Namasivayam, C.; Sumithra, C. Adsorptive removal of catechol on waste Fe (III)/Cr (III) hydroxide: Equilibrium and kinetics study. Ind. Eng. Chem. Res. 2004, 43, 7581–7587. [Google Scholar] [CrossRef]
- Weber, J.R.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Wooa, S.H. Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour. Technol. 2009, 100, 2803–2809. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Deliyanni, E.A.; Peleka, E.N.; Lazaridis, N.K. Comparative study of phosphates removal from aqueous solutions by nanocrystalline akaganéite and hybrid surfactant-akaganéite. Sep. Purif. Tech. 2007, 52, 478–486. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Peleka, E.N.; Komvokis, V.G.; Mavros, P.P. Iron-modified hydrotalcite-like materials as highly efficient phosphate sorbents. J. Colloid Interf. Sci. 2010, 342, 427–436. [Google Scholar] [CrossRef]
- Trujillano, R.; Holgado, M.J.; Pigazo, F.; Rives, V. Preparation, physicochemical characterisation and magnetic properties of Cu-Al layered double hydroxides with CO32− and anionic surfactants with different alkyl chains in the interlayer. Phys. B Condenser. Mater. 2006, 373, 267–273. [Google Scholar] [CrossRef]
- Taibi, M.; Ammar, S.; Schoenstein, F.; Jouini, N.; Fiévet, F.; Chauveau, T.; Greneche, J.-M. Powder and film of nickel and iron-layered double hydroxide: Elaboration in polyol medium and characterization. J. Phys. Chem. Solids. 2008, 69, 1052–1055. [Google Scholar] [CrossRef] [Green Version]
- Oriakhi, O.; Farr, I.V.; Lerner, M.M. Incorporation of poly (acrylic acid), poly (vinylsulfonate) and poly (styrenesulfonate) within layered double hydroxides. J. Mater. Chem. 1996, 6, 103. [Google Scholar] [CrossRef]
- Bruna, F.; Celis, R.; Pavlovic, I.; Barriga, C.; Cornejo, J.; Ulibarri, M.A. Layered double hydroxides as adsorbents and carriers of the herbicide (4-chloro-2-methylphenoxy)acetic acid (MCPA): Systems Mg-Al, Mg-Fe and Mg-Al-Fe. J. Hazard Mater. 2009, 168, 1476–1481. [Google Scholar] [CrossRef]
- Choy, J.-H.; Kwon, Y.-M.; Han, K.-S.; Song, S.-W.; Chang, S.H. Intra- and inter-layer structures of layered hydroxy double salts, Ni1−xZn2x(OH)2(CH3CO2)2x·nH2O. Mater. Lett. 1998, 34, 356–363. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Verberckmoes, A.A.; Weckhuysen, M.; Schoonheydt, R.A. Spectroscopy and coordination chemistry of cobalt in molecular sieves. Microporous Mesoporous Mater. 1998, 22, 16. [Google Scholar] [CrossRef] [Green Version]
- Noronha, F.B.; Perez, C.A.; Schmal, M.; Férty, R. Determination of cobalt species in niobia supported catalysts. Phys. Chem. Chem. Phys. 1999, 1, 2861. [Google Scholar] [CrossRef]
- Sharma, P.-K.; Dutta, R.-K.; Pandey, A.-C.; Layek, S.; Verma, H.C. Effect of iron doping concentration on magnetic properties of ZnO nanoparticles. J. Magn. Magn. Mater. 2009, 321, 2587–2591. [Google Scholar] [CrossRef]
- Morpurgo, S.; Lo Jacono, M.; Porta, P. Copper-zinc-cobalt-chromium hydroxycarbonates and oxides. J. Solid State Chem. 1995, 119, 246–253. [Google Scholar] [CrossRef]
- Taibi, M.; Ammar, S.; Jouini, N.; Fievet, F. Layered nickel-cobalt hydroxyacetates and hydroxycarbonates: Chimie douce synthesis and structural features. J. Phys. Chem. Solids. 2006, 67, 932–937. [Google Scholar] [CrossRef]
- Carja, G.; Nakamura, R.; Aida, T.; Niiyama, H. Textural properties of layered double hydroxides: Effect of magnesium substitution by copper or iron. Microporous Mesoporous Mater. 2001, 47, 275–284. [Google Scholar] [CrossRef]
- Aramendıa, M.A.; Borau, V.; Jimenez, C.; Marinas, J.M.; Ruiz, J.R.; Urbano, F.J. Comparative Study of Mg/M(III) (M = Al, Ga, In) Layered Double Hydroxides Obtained by Coprecipitation and the Sol–Gel Method. J. Solid State Chem. 2002, 168, 156–161. [Google Scholar] [CrossRef]
- Dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; Costa, L.M.D.; Silva, L.H.M.D.; Tronto, J.; Pinto, F.G. Removal of Acid Green 68:1 from aqueous solutions by calcined and uncalcined layered double hydroxides. Appl. Clay Sci. 2013, 80–81, 189–195. [Google Scholar] [CrossRef]
- Li, Y.; Gao, B.; Wu, T.; Chen, W.; Li, X.; Wang, B. Adsorption kinetics for removal of thiocyanate from aqueous solution by calcined hydrotalcite. Colloids Surf. A Physicochem. Eng. Aspects 2009, 325, 38–43. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Adsorption characteristics of brilliant green dye on kaolin. J. Hazard Mater. 2009, 161, 387–395. [Google Scholar] [CrossRef]
- Kannan, K.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—A comparative study. Dyes Pigments 2001, 51, 25. [Google Scholar] [CrossRef]
- El Gaini, L.; Lakraimi, M.; Sebbar, E.; Meghea, A.; Bakasse, M. Removal of indigo carmine dye from water to Mg-Al-CO3-calcined layered double hydroxides: Review. J. Hazard Mater. 2009, 61, 627–632. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Lv, L.; He, J.; Evans, D.G.; Duan, X. Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides. J. Hazard Mater. B 2006, 133, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Venkat, S.M.; Indra, D.M.; Vimal, C.S. Use of bagasse fly ash as an adsorbent for the removal of brilliant green dye from aqueous solution. Dyes. Pigments 2007, 73, 269–278. [Google Scholar]
- Ni, Z.-M.; Xia, S.-J.; Wang, L.-G.; Xing, F.-F.; Pan, G.-X. Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: Adsorption property and kinetic studies. J. Colloid Interf. Sci. 2007, 316, 284–291. [Google Scholar] [CrossRef]
- Lian, L.; Guo, L.; Guo, C. Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J. Hazard Mater. 2009, 161, 126–131. [Google Scholar] [CrossRef]
- Zaki, A.B.; El-Sheikh, M.Y.; Evans, J.; El-Safty, S.A. Kinetics and Mechanism of the Sorption of Some Aromatic Amines onto Amberlite IRA-904 Anion-Exchange Resin. J. Colloid Interf. Sci. 2000, 221, 58–63. [Google Scholar] [CrossRef]
- Gupta, V.K. Equilibrium Uptake, Sorption Dynamics, Process Development, and Column Operations for the Removal of Copper and Nickel from Aqueous Solution and Wastewater Using Activated Slag, a Low-Cost Adsorbent. Ind. Eng. Chem. Res. 1998, 37, 192–202. [Google Scholar] [CrossRef]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3, 3973–3993. [Google Scholar] [CrossRef]
- Toth, J. Gas-(Dampf-)Adsorption auf festen Oberflecken inhomogener Aktivität. Acta Chim. Acad. Sci. Hung. 1962, 32, 39. [Google Scholar]
- Auxilio, A.R.; Andrews, P.C.; Junk, P.C.; Spiccia, L.; Neumann, D.; Raverty, W.; Vanderhoek, N. Adsorption and intercalation of Acid Blue 9 on Mg–Al layered double hydroxides of variable metal composition. Polyhedron 2007, 26, 3479–3490. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, Z.; Qiu, Y.; Zhao, J. Enhanced adsorption of acid brown 14 dye on calcined Mg/Fe layered Enhanced adsorption of acid brown 14 dye on calcined Mg/Fe layered double hydroxide with memory effect. J. Chem. Eng. 2013, 219, 69–77. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zhou, X.M.; Liu, Z.; Li, X. Different dye removal mechanisms between monodispersed and Different dye removal mechanisms between monodispersed and uniform hexagonal thin plate-like MgAleCO32− LDH and its calcined product in efficient removal of Congo red from water. J. Alloy. Compd. 2016, 673, 265–271. [Google Scholar] [CrossRef]
- Zhu, M.; Li, H.; Xie, M.; Xin, H. Sorption of anionic dye by uncalcined and calcined Layered Double Hydroxides. J. Hazard Mater. 2005, 120, 163–171. [Google Scholar] [CrossRef]
- Huang, G.; Sun, Y.; Zhao, C.; Zhao, Y.; Song, Z.; Chen, S.; Ma, J.; Du, J.; Yin, Z. Water—n-BuOH solvothermal synthesis of ZnAl-LDHs with different morphologies and its calcined product in efficient dyes removal. J. Colloid Interf. Sci. 2017, 494, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Poul, L.; Jouini, N.; Fievet, F. Layered hydroxide metal acetates (metal = zinc, cobalt, and nickel): Elaboration via hydrolysis in polyol medium and comparative study. Chem. Mater. 2000, 12, 3123–3132. [Google Scholar] [CrossRef]
- Prevot, V.; Forano, C.; Besse, J.P. Hydrolysis in polyol: New route for hybrid-layered double hydroxides preparation. Chem. Mater. 2005, 17, 6695–6701. [Google Scholar] [CrossRef]
- Mandal, S.; Lerner, D.A.; Marcotte, N.; Tichit, D. Structural characterization of azoic dye hosted layered double hydroxides. Z. Krist. 2009, 224, 282–286. [Google Scholar] [CrossRef]











| Compound | d003 (Å) | a Parameter (Å) |
|---|---|---|
| CoFe-Ac/p | 12.70 | 3.11 |
| CoFe-Ac/Ex | 7.67 | 3.12 |
| CoFe-CO3/A (reference) | 7.57 | 3.12 |
| Compound | Mass Fraction (%) | Molar Ratio CoII/FeIII | X = FeIII/CoII + FeIII | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | Fe | C | H | Solution | Solid | Solution | Solid | |||
| CoFe-Ac/p | 28.68 | 8.96 | 6.80 | 4.34 | 3.0 | 3.0 | 0.25 | 0.25 | ||
| Chemical formula | DEG | H2O% | Total weight loss % | |||||||
| Exp. | Exp. | Cal. | ||||||||
| Co0.75Fe0.25·(OH)2·Ac0.25,1.50H2O, 0.094DEG | 0.094 | 16.0 | 56.0 | 54.4 | ||||||
| Method/Adsorbent | ||||
|---|---|---|---|---|
| Pseudo-First-Order | qexp (mg/g) | k1 (min−1) | qe (mg/g) | R2 |
| CoFe-Ac/p | 250 | 0.494 | 234.54 | 0.9599 |
| CoFe-Ac/Ex | 153 | 0.026 | 101.24 | 0.9652 |
| CoFe-CO3/A | 128 | 0.016 | 70.24 | 0.9128 |
| Pseudo-Second-Order | qe (mg/g) | k2·103 (g/mg·min) | R2 | - |
| CoFe-Ac/P | 250 | 8.42 | 0.9996 | - |
| CoFe-Ac/Ex | 161.3 | 0.529 | 0.9999 | - |
| CoFe-CO3/A | 136.98 | 0.426 | 0.9978 | - |
| Intraparticule Diffusion | kp(mg/g·mn1/2) | C (mg/g) | R2 | - |
| CoFe-Ac/p | 5.4882 | 227.25 | 0.8108 | - |
| CoFe-Ac/Ex | 3.2649 | 107.65 | 0.9271 | - |
| CoFe-CO3/A | 1.7811 | 98.875 | 0.9768 | - |
| Compound | ΔH° (kJ/mol) | ΔS° (J/mol·K) | ΔG° (kJ/mol) | ||
|---|---|---|---|---|---|
| 283K | 293K | 323K | |||
| CoFe-Ac/P | 41.31 | 202.54 | −16.00 | −19.04 | −24.11 |
| CoFe-Ac/Ex | 8.75 | 78.89 | −13.57 | −14.76 | −16.73 |
| CoFe-CO3/A | 11.48 | 84.88 | −12.54 | −13.81 | −15.93 |
| Method/Adsorbent | ||||
|---|---|---|---|---|
| Langmuir Isothem | Qmax (mg/g) | KL (l/mg) | R2 | Qmax (exp) (mg/g) |
| CoFe-Ac/p | 588.23 | 0.404 | 0.9999 | 588 |
| CoFe-Ac/Ex | 175.44 | 0.034 | 0.9998 | 170 |
| CoFe-CO3/A | 128.205 | 0.426 | 0.9998 | 127 |
| Freundlich Isotherm | Kf (mg/g) | n | R2 | - |
| CoFe-Ac/P | 433.93 | 21.64 | 0.9914 | - |
| CoFe-Ac/Ex | 1.82 | 5.12 | 0.9848 | - |
| CoFe-CO3/A | 81.95 | 12.93 | 0.9252 | - |
| LDH | Ratio M2+/M3+ | Dye | Initial d003 (Å) | Final d003 (Å) | Qmax (mg/g) | Reference |
|---|---|---|---|---|---|---|
| MgFe-CO3 | 3/1 | Acid Brown 14 | ≈7.8 | ≈7.8 | 41.7 | [66] |
| C(MgFe-CO3) | - | ≈7.8 | 370.0 | |||
| MgAl-CO3 | 2/1 | Congo Red | 7.58 | 7.88 | 129.9 | [67] |
| C(MgAl-CO3) | - | 7.92 | 143.27 | |||
| MgAl-CO3 | 2/1 | Brilliant Blue R | 7.43 | 7.55 | 54.59 | [68] |
| C(MgAl-CO3) | - | 7.76 | 613.6 | |||
| MgAl-CO3 | 2/1 | Direct Red 2 | 7.57 | 7,77 | 153.88 | [25] |
| C(MgAl-CO3) | - | 23.77 | 417.3 | |||
| MgAl-CO3 | 3/1 | Acid Green 68:1 | 7.6 | 7.6 | 99.1 | [51] |
| C(MgAl-CO3) | - | 7.3 | 154.8 | |||
| ZnAl-CO3 | 2/1 | Congo Red | 7.6 | Not given | Not given | [69] |
| C(ZnAl-CO3) | - | 30.0 | 1540 | |||
| ZnAl-CO3 | RR (X-3B) | 7.6 | Not given | Not given | ||
| C(ZnAl-CO3) | - | 7.6–8.0 | 390 | |||
| MgAl-SO4 | 3/1 | Remazol Brilliant Red 3FB | 8.12 | 7.9 | 85 | [19] |
| ZnAl-NO3 | 2/1 | Direct Red 16 | 8.84 | 11.78 | 69.85 | [15] |
| MgAl-NO3 | 2/1 | Reactive blue 19 | 8.79 | 8.41 | 281 | [16] |
| MgAl-SDS | 2/1 | Direct Blue G-RB | 25.5 | Not given | 707.76 | [18] |
| ZnAl-Cl | 2/1 | Evan Blue (EB) | 7.73 | 20.6 | 0.512 mmol/g | [14] |
| CoFe-Ac/p | 3/1 | Direct Red 2 | 12.70 | 23.77/8.28 | 588 (0.812 mmol/g) | This work |
| CoFe-Ac/Ex | 7.67 | 7.67 | 170 (0.235 mmol/g) | |||
| CoFe-CO3/A | 7.57 | 7.57 | 127 (0.175 mmol/g) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drici-Setti, N.; Lelli, P.; Jouini, N. LDH-Co-Fe-Acetate: A New Efficient Sorbent for Azoic Dye Removal and Elaboration by Hydrolysis in Polyol, Characterization, Adsorption, and Anionic Exchange of Direct Red 2 as a Model Anionic Dye. Materials 2020, 13, 3183. https://doi.org/10.3390/ma13143183
Drici-Setti N, Lelli P, Jouini N. LDH-Co-Fe-Acetate: A New Efficient Sorbent for Azoic Dye Removal and Elaboration by Hydrolysis in Polyol, Characterization, Adsorption, and Anionic Exchange of Direct Red 2 as a Model Anionic Dye. Materials. 2020; 13(14):3183. https://doi.org/10.3390/ma13143183
Chicago/Turabian StyleDrici-Setti, Nawal, Paolo Lelli, and Noureddine Jouini. 2020. "LDH-Co-Fe-Acetate: A New Efficient Sorbent for Azoic Dye Removal and Elaboration by Hydrolysis in Polyol, Characterization, Adsorption, and Anionic Exchange of Direct Red 2 as a Model Anionic Dye" Materials 13, no. 14: 3183. https://doi.org/10.3390/ma13143183
APA StyleDrici-Setti, N., Lelli, P., & Jouini, N. (2020). LDH-Co-Fe-Acetate: A New Efficient Sorbent for Azoic Dye Removal and Elaboration by Hydrolysis in Polyol, Characterization, Adsorption, and Anionic Exchange of Direct Red 2 as a Model Anionic Dye. Materials, 13(14), 3183. https://doi.org/10.3390/ma13143183






