Qualitative and Semi-Quantitative Assessment of Processing-Related Surface Contamination of One- and Two-Piece CAD/CAM Abutments before and after Ultrasonic Cleaning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Specimen Preparation
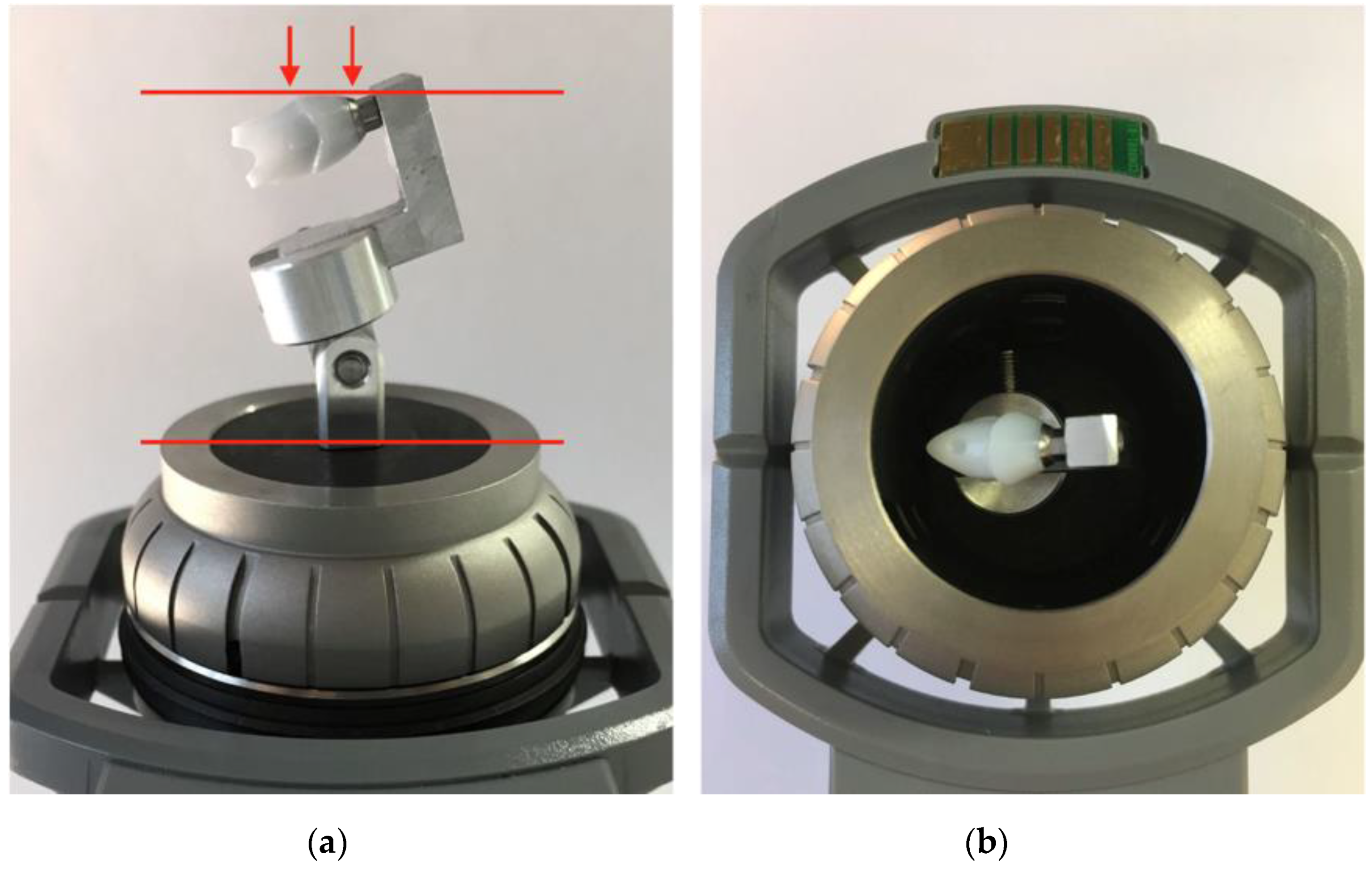
2.2. Cleaning of Abutment Samples
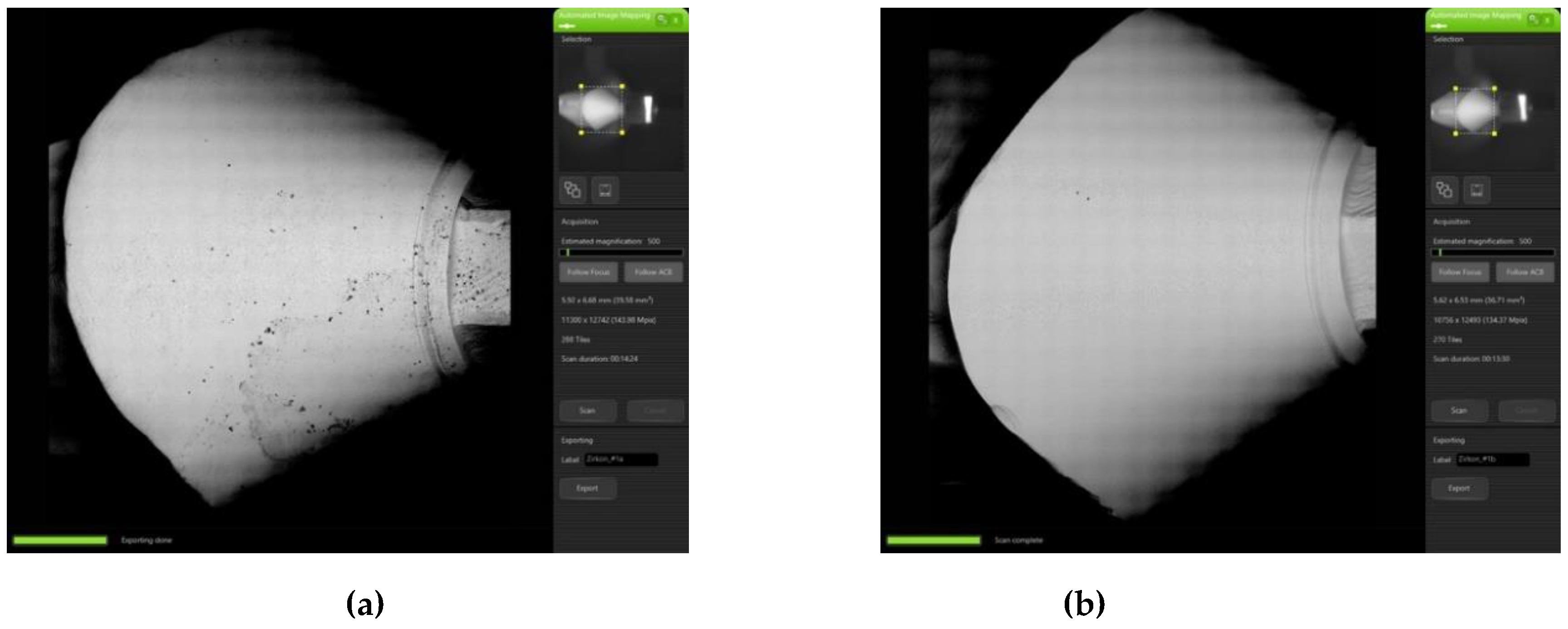
2.3. Qualitative Evaluation of Contamination
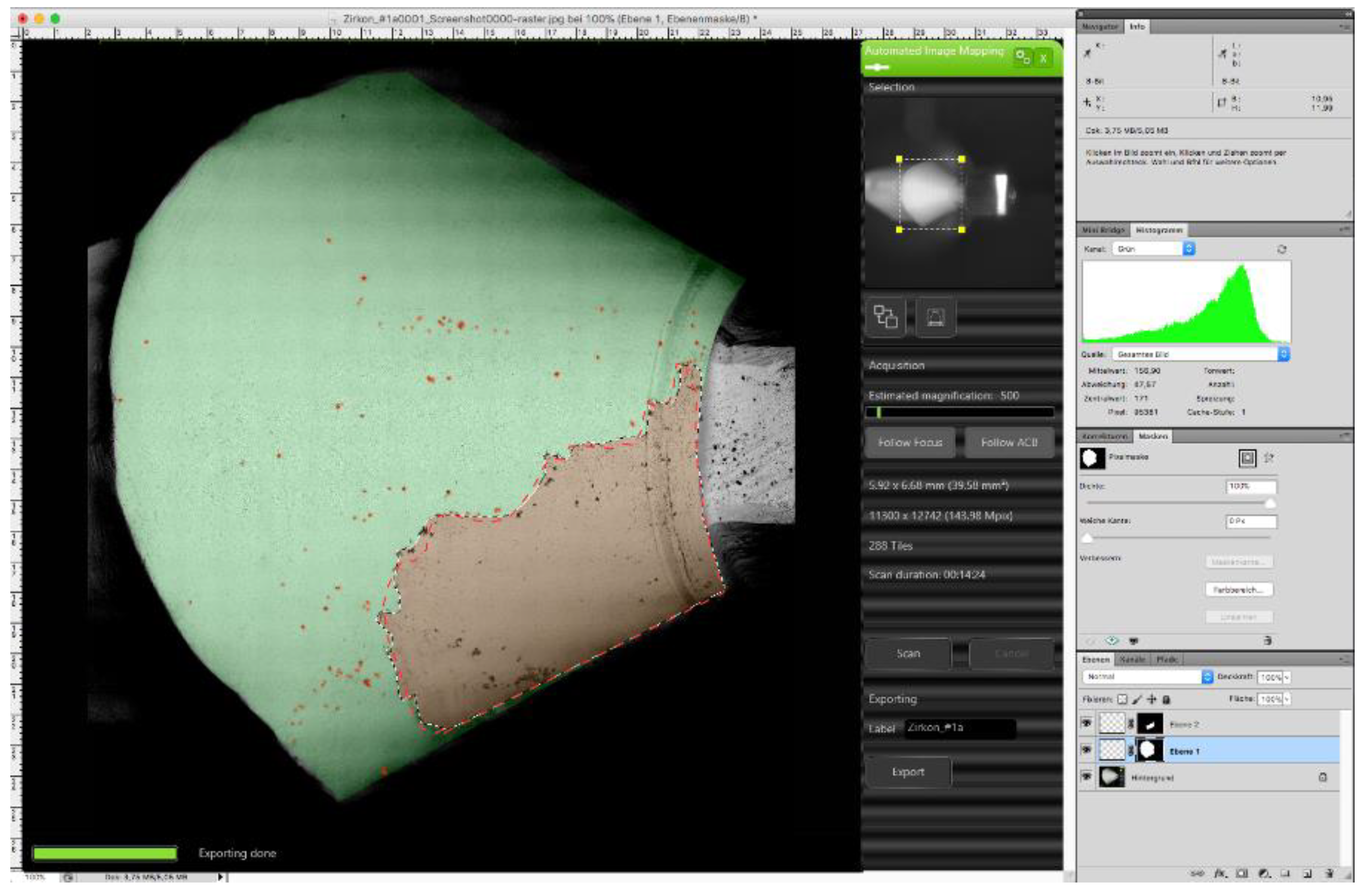
2.4. Semi-Quantitative Evaluation of Contamination
2.5. Statistical Analysis
3. Results
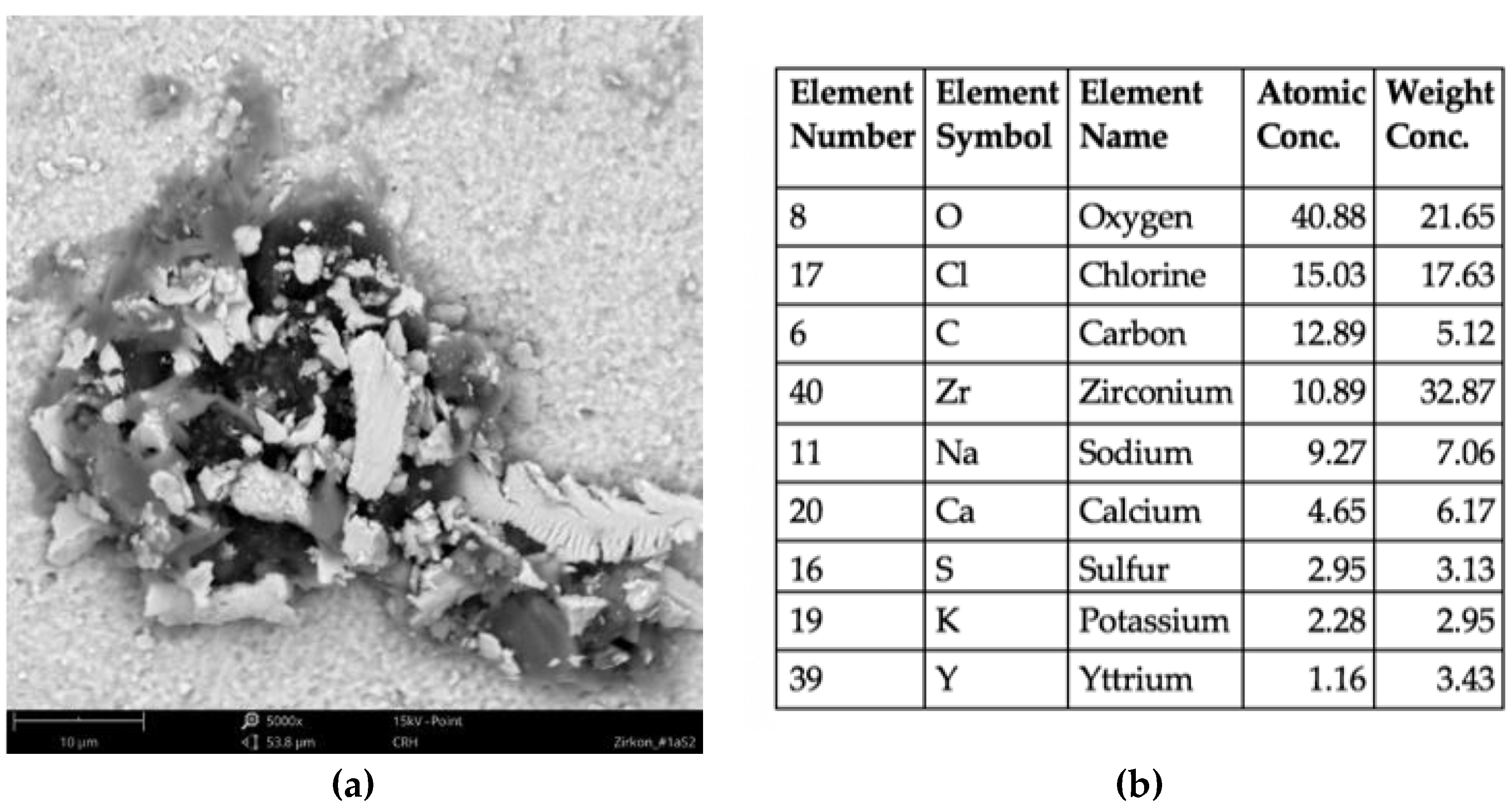
3.1. Qualitative Evaluation of Contamination
3.2. Semi-Quantitative Evaluation of Contamination
3.2.1. Comparison between Monotype and Hybrid Abutments CAD/CAM Abutments
3.2.2. Monotype CAD/CAM Abutments
3.2.3. Hybrid CAD/CAM Abutments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Valente, N.A.; Strasding, M.; Zwahlen, M.; Liu, S.; Sailer, I. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic single crowns. Clin. Oral Implants Res. 2018, 29, 199–214. [Google Scholar] [CrossRef] [Green Version]
- Vechiato-Filho, A.J.; Pesqueira, A.A.; De Souza, G.M.; dos Santos, D.M.; Pellizzer, E.P.; Goiato, M.C. Are zirconia implant abutments safe and predictable in posterior regions? A systematic review and meta-analysis. Int. J. Prosthodont. 2016, 29, 233–244. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Zarauz, C.; Strasding, M.; Sailer, I.; Zwahlen, M.; Zembic, A. A systematic review of the influence of the implant-abutment connection on the clinical outcomes of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin. Oral Implants Res. 2018, 29, 160–183. [Google Scholar] [CrossRef] [Green Version]
- Pitta, J.; Zarauz, C.; Pjetursson, B.; Sailer, I.; Liu, X.; Pradies, G. A systematic review and meta-analysis of the influence of abutment material on peri-implant soft tissue color measured using spectrophotometry. Int. J. Prosthodont. 2020, 33, 39–47. [Google Scholar] [CrossRef]
- Lops, D.; Bressan, E.; Parpaiola, A.; Sbricoli, L.; Cecchinato, D.; Romeo, E. Soft tissues stability of cad-cam and stock abutments in anterior regions: 2-year prospective multicentric cohort study. Clin. Oral Implants Res. 2015, 26, 1436–1442. [Google Scholar] [CrossRef]
- Gehrke, P.; Johannson, D.; Fischer, C.; Stawarczyk, B.; Beuer, F. In vitro fatigue and fracture resistance of one- and two-piece CAD/CAM zirconia implant abutments. Int. J. Oral Maxillofac. Implants 2015, 30, 546–554. [Google Scholar] [CrossRef]
- Parpaiola, A.; Toia, M.; Norton, M.; Cecchinato, D.; Bressan, E.; Lops, D. CAD/CAM Implant abutments: Peri-implant hard and soft tissue response with up to 4 years of follow-up- a retrospective cohort study evaluation. Int. J. Periodontics Restor. Dent. 2020, 40, 193–201. [Google Scholar] [CrossRef]
- Pietruski, J.K.; Skurska, A.; Bernaczyk, A.; Milewski, R.; Pietruska, M.J.; Gehrke, P.; Pietruska, M.D. Evaluation of concordance between CAD/CAM and clinical positions of abutment shoulder against mucosal margin: An observational study. BMC Oral Health 2018, 18, 73. [Google Scholar] [CrossRef] [Green Version]
- Elsayed, A.; Wille, S.; Al-Akhali, M.; Kern, M. Comparison of fracture strength and failure mode of different ceramic implant abutments. J. Prosthet. Dent. 2017, 117, 499–506. [Google Scholar] [CrossRef]
- Asgeirsson, A.G.; Sailer, I.; Gamper, F.; Jung, R.E.; Hammerle, C.H.F.; Thoma, D.S. Veneered zirconia abutments cemented on non-original titanium bases: 1-year results of a prospective case series. Clin. Oral Implants Res. 2019, 30, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Muhlemann, S.; Kraus, R.D.; Hammerle, C.H.F.; Thoma, D.S. Is the use of digital technologies for the fabrication of implant-supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clin. Oral Implants Res. 2018, 29, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.B.; Rheu, G.B.; Kim, Y.S.; Jeong, C.M.; Lee, J.Y.; Shin, S.W. Influence of Implant transmucosal design on early peri-implant tissue responses in beagle dogs. Clin. Oral Implants Res. 2014, 25, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Kondo, R.; Oshiro, W.; Matsuura, Y.; Furuhashi, A.; Tsukiyama, Y.; Koyano, K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016, 60, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Galindomoreno, P.; Leoncano, A.; Ortegaoller, I.; Monje, A.; Suarez, F.; Ovalle, F.; Spinato, S.; Catena, A. Prosthetic abutment height is a key factor in peri-implant marginal bone loss. J. Dent. Res. 2014, 93, 80S–85S. [Google Scholar] [CrossRef]
- Spinato, S.; Galindo-Moreno, P.; Bernardello, F.; Zaffe, D. Minimum abutment height to eliminate bone loss: Influence of implant neck design and platform switching. Int. J. Oral Maxillofac. Implants 2018, 33, 405–411. [Google Scholar] [CrossRef] [Green Version]
- Blanco, J.; Pico, A.; Caneiro, L.; Nóvoa, L.; Batalla, P.; Martín-Lancharro, P. Effect of abutment height on interproximal implant bone level in the early healing: A randomized clinical trial. Clin. Oral Implants Res. 2018, 29, 108–117. [Google Scholar] [CrossRef]
- Canullo, L.; Micarelli, C.; Iannello, G. Microscopical and chemical surface characterization of the gingival portion and connection of an internal hexagon abutment before and after different technical stages of preparation. Clin. Oral Implants Res. 2013, 24, 606–611. [Google Scholar] [CrossRef]
- Canullo, L.; Tallarico, M.; Peñarrocha-Oltra, D.; Monje, A.; Wang, H.L.; Peñarrocha-Diago, M. Implant abutment cleaning by plasma of argon: 5-year follow-up of a randomized controlled trial. J. Periodontol. 2016, 87, 434–442. [Google Scholar] [CrossRef]
- Canullo, L.; Micarelli, C.; Lembo-Fazio, L.; Iannello, G.; Clementini, M. Microscopical and microbiologic characterization of customized titanium abutments after different cleaning procedures. Clin. Oral Implants Res. 2014, 25, 328–336. [Google Scholar] [CrossRef]
- Canullo, L.; Penarrocha-Oltra, D.; Marchionni, S.; Bagan, L.; Penarrocha-Diago, M.A.; Micarelli, C. Soft tissue cell adhesion to titanium abutments after different cleaning procedures: Preliminary results of a randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e177–e183. [Google Scholar] [CrossRef]
- Nakajima, K.; Odatsu, T.; Shinohara, A.; Baba, K.; Shibata, Y.; Sawase, T. Effects of cleaning methods for custom abutment surfaces on gene expression of human gingival fibroblasts. J. Oral Sci. 2017, 59, 533–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piattelli, A.; Pontes, A.E.; Degidi, M.; Iezzi, G. Histologic studies on osseointegration: Soft tissues response to implant surfaces and components. A review. Dent. Mater. 2011, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Wu, W.; Rozo, C.; Hallab, N.J.; Benevenia, J.; Gause, W.C. Micrometer-sized titanium particles can induce potent Th2-type responses through TLR4-independent pathways. J. Immunol. 2011, 187, 6491–6498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ADA Council on Scientific Affairs and ADA Council on Dental Practice. Infection control recommendations for the dental office and the dental laboratory. J. Am. Dent. Assoc. 1996, 127, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Lee, S.H.; Her, S.B.; Chang, W.G.; Lim, B.S. Effects of surface treatments on the susceptibilities of low temperature degradation by autoclaving in zirconia. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Basilio Mde, A.; Cardoso, K.V.; Antonio, S.G.; Rizkalla, A.S.; Santos Junior, G.C.; Arioli Filho, J.N. Effects of artificial aging conditions on yttria-stabilized zirconia implant abutments. J. Prosthet. Dent. 2016, 116, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehrke, P.; Tabellion, A.; Fischer, C. Microscopical and chemical surface characterization of CAD/CAM zircona abutments after different cleaning procedures. A qualitative analysis. J. Adv. Prosthodont. 2015, 7, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Li, M.; Komasa, S.; Agariguchi, A.; Yang, Y.; Zeng, Y.; Takao, S.; Zhang, H.; Tashiro, Y.; Kusumoto, T.; et al. Decontamination of titanium surface using different methods: An in vitro study. Materials 2020, 13, 2287. [Google Scholar] [CrossRef]
- Gehrke, P.; Smeets, R.; Gosau, M.; Friedrich, R.E.; Madani, E.; Duddeck, D.; Fischer, C.; Tebbel, F.; Sader, R.; Hartjen, P. The influence of an ultrasonic cleaning protocol for CAD/CAM abutment surfaces on cell viability and inflammatory response in vitro. In Vivo 2019, 33, 689–698. [Google Scholar] [CrossRef]
- Aleksejuniene, J.; Scheie, A.A.; Holst, D. Inter-individual variation in the plaque formation rate of young individuals. Int. J. Dent. Hyg. 2006, 4, 35–40. [Google Scholar] [CrossRef]
- Linkevicius, T.; Vindasiute, E.; Puisys, A.; Linkeviciene, L.; Maslova, N.; Puriene, A. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin. Oral Implants Res. 2013, 24, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Vindasiute, E.; Puisys, A.; Peciuliene, V. The influence of margin location on the amount of undetected cement excess after delivery of cement-retained implant restorations. Clin. Oral Implants Res. 2011, 22, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, P.; Bleuel, K.; Fischer, C.; Sader, R. Influence of margin location and luting material on the amount of undetected cement excess on CAD/CAM implant abutments and cement-retained zirconia crowns: An in-vitro study. BMC Oral Health 2019, 19, 111. [Google Scholar] [CrossRef] [Green Version]
- Olefjord, I.; Hansson, S. Surface analysis of four dental implant systems. Int. J. Oral Maxillofac. Implants 1993, 8, 32–40. [Google Scholar] [PubMed]
- Canullo, L.; Penarrocha, M.; Monje, A.; Catena, A.; Wang, H.L.; Penarrocha, D. Association between clinical and microbiologic cluster profiles and peri-implantitis. Int. J. Oral Maxillofac. Implants 2017, 32, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Tallarico, M.; Chu, S.; Penarrocha, D.; Ozcan, M.; Pesce, P. Cleaning, disinfection, and sterilization protocols employed for customized implant abutments: An international survey of 100 universities worldwide. Int. J. Oral Maxillofac. Implants 2017, 32, 774–778. [Google Scholar] [CrossRef]
- Canullo, L.; Penarrocha, D.; Micarelli, C.; Massidda, O.; Bazzoli, M. Hard tissue response to argon plasma cleaning/sterilisation of customised titanium abutments versus 5-second steam cleaning: Results of a 2-year post-loading follow-up from an explanatory randomised controlled trial in periodontally healthy patients. Eur. J. Oral Implantol. 2013, 6, 251–260. [Google Scholar]
- Esposito, M. The Editor’s point of view: On the study on argon-plasma cleaning of customised abutments. Eur. J. Oral Implantol. 2015, 8, 213. [Google Scholar]
- Kern, M. On the scientific evidence that the sterilization of customized implant abutments is required. Eur. J. Oral Implantol. 2015, 8, 219. [Google Scholar]
- Aronsson, B.O. On Argon-plasma cleaning—Some comments from regulatory and scientific perspectives. Eur. J. Oral Implantol. 2015, 8, 211–212. [Google Scholar]
- Mehl, C.; Kern, M.; Zimmermann, A.; Harder, S.; Huth, S.; Selhuber-Unkel, C. Impact of cleaning procedures on adhesion of living cells to three abutment materials. Int. J. Oral Maxillofac. Implants 2017, 32, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Wiedenmann, F.; Liebermann, A.; Spintzyk, S.; Eichberger, M.; Stawarczyk, B. Influence of different cleaning procedures on tensile bond strength between zirconia abutment and titanium base. Int. J. Oral Maxillofac. Implants 2019, 34, 1318–1327. [Google Scholar] [CrossRef]
- Pils, D.; Baeppler, R.J.; Junker, R.; Kielbassa, A.M.; Nothdurft, F.P. Application of a standard autoclaving protocol does not harm structural integrity of two-piece zirconia abutments under detachment force testing. Clin. Oral Investig. 2019, 23, 3133–3137. [Google Scholar] [CrossRef]
- Fadanelli, M.A.; Amaral, F.L.; Basting, R.T.; Turssi, C.P.; Sotto-Maior, B.S.; Franca, F.M. Effect of steam autoclaving on the tensile strength of resin cements used for bonding two-piece zirconia abutments. J. Oral Implantol. 2017, 43, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Schneider-Feyrer, S.; Huber, C.; Krifka, S.; Rosentritt, M. The effect of sterilization and ultrasonic cleaning on resin cement interface of customized dental implant abutments. J. Mech. Behav. Biomed. Mater. 2020, 104, 103660. [Google Scholar] [CrossRef] [PubMed]
- Bankoglu Gungor, M.; Karakoca Nemli, S. The effect of resin cement type and thermomechanical aging on the retentive strength of custom zirconia abutments bonded to titanium inserts. Int. J. Oral Maxillofac. Implants 2018, 33, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Tallarico, M.; Penarrocha, M.; Corrente, G.; Fiorellini, J.; Penarrocha, D. Plasma of argon cleaning treatment on implant abutments in periodontally healthy patients: Six years postloading results of a randomized controlled trial. Int. J. Periodontics Restor. Dent. 2017, 37, 683–690. [Google Scholar] [CrossRef]
- Farronato, D.; Fumagalli, D.; Asa’ad, F.; Rasperini, G. Decontamination of customized laser-microtextured titanium abutments: A comparative in vitro study of different cleaning procedures. The Int. J. Periodontics Restor. Dent. 2018, 38, e87–e95. [Google Scholar] [CrossRef] [Green Version]
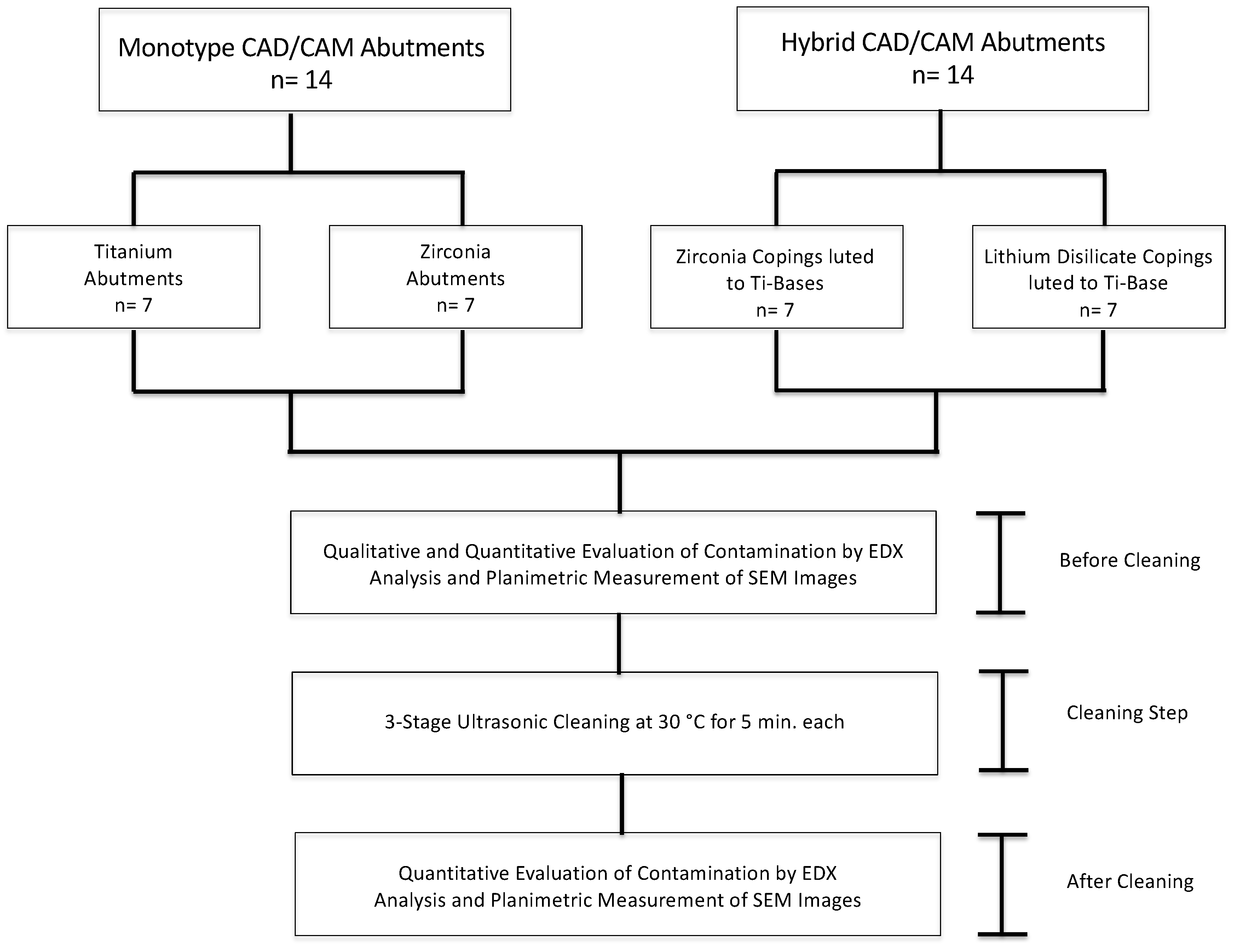

| Group | Abutment Type | Material | Product & Manufacturer | No. of Samples |
|---|---|---|---|---|
| A | Monotype Abutment | Titanium Grade 5 | CADAbut F, CAD Titanium, Semados SCX, BEGO Implant Systems | 7 |
| B | Monotype Abutment | Y-TZP Zirconia | CADAbut F, BeCe CAD Zirconia XH, Semados SCX, BEGO Implant Systems | 7 |
| C | Hybrid Abutment | Y-TZP Zirconia Coping/Ti-Base | CADAbut D, Zirconia LT CAD on Ti-Base, Semados SCX, BEGO Implant Systems | 7 |
| D | Hybrid Abutment | Lithium Disilicate Coping/Ti-Base | CADAbut D, IPS e.max CAD LT/ Ivoclar Vivadent AG on Ti-Base, Semados SCX, BEGO Implant Systems | 7 |
| Element Number | Element Symbol | Element Name | Atomic Conc. (%) Max./Min. |
|---|---|---|---|
| 8 | O | Oxygen | 74.63–60.21 |
| 14 | Si | Silicium | 28.71–0.92 |
| 40 | Zr | Zirconium | 19.66–1.01 |
| 19 | K | Potassium | 9.19–0.58 |
| 56 | Ba | Barium | 5. 97–0.79 |
| 13 | Al | Aluminum | 4.25–0.58 |
| 20 | Ca | Calcium | 3.54–0.00 |
| 11 | Na | Sodium | 2.84–1.64 |
| 39 | Y | Yttrium | 2.24–0.00 |
| 16 | S | Sulfur | 1.53–0.00 |
| 12 | Mg | Magnesium | 1.52–0.00 |
| 30 | Zn | Zinc | 0.91–0.00 |
| 15 | P | Phosphorus | 0.72–0.00 |
| Element Number | Element Symbol | Element Name | Atomic Conc. % Max./Min. |
|---|---|---|---|
| 6 | C | Carbon | 80.54–44.52 |
| 8 | O | Oxygen | 30.84–9.24 |
| 7 | N | Nitrogen | 20.84–0.00 |
| 40 | Zr | Zirconium | 12.59–3.81 |
| 39 | Y | Yttrium | 9.18–1.05 |
| 22 | Ti | Titanium | 4.50–0.00 |
| 14 | Si | Silicium | 1.60–0.00 |
| 13 | Al | Aluminum | 1.11–0.00 |
| Element Number | Element Symbol | Element Name | Atomic Conc. (%) Max./Min. |
|---|---|---|---|
| 22 | Ti | Titanium | 80.70–28.36 |
| 6 | C | Carbon | 65.06–31.94 |
| 40 | Zr | Zirconium | 31.95–0.00 |
| 13 | Al | Aluminum | 14.61–3.42 |
| 11 | Na | Sodium | 5.04–0.00 |
| 39 | Y | Yttrium | 3.55–0.00 |
| 23 | V | Vanadium | 2.60–1.15 |
| 20 | Ca | Calcium | 2.40–2.01 |
| 16 | S | Sulfur | 1.14–0.00 |
| Element Number | Element Symbol | Element Name | Atomic Conc. (%) Max./Min. |
|---|---|---|---|
| 6 | C | Carbon | 97.29–0.00 |
| 8 | O | Oxygen | 64.25–43.37 |
| 40 | Zr | Zirconium | 50.84–32.26 |
| 39 | Y | Yttrium | 5.79–3.49 |
| Descriptive Analysis | ||||||
|---|---|---|---|---|---|---|
| N | Mean | Median | Min. | Max. | St. Dev. | |
| Pixel total before cleaning | 28 | 459,105.0 | 468,155.0 | 327,680.0 | 515,278.0 | 42,943.82 |
| Pixel of contaminants before cleaning | 28 | 11,631.4 | 2347.0 | 28.0 | 116,728.0 | 23,133.89 |
| % Contamination before cleaning | 28 | 2.4 | 0.5 | 0.0 | 24.6 | 4.89 |
| Pixel total after cleaning | 28 | 437,911.2 | 436,259.0 | 388,941.0 | 502,181.0 | 32,825.33 |
| Pixel of contaminants after cleaning | 28 | 537.1 | 360.5 | 26.0 | 1875.0 | 568.20 |
| % Contamination after cleaning | 28 | 0.1 | 0.1 | 0.0 | 0.4 | 0.12 |
| % Difference before/after | 28 | 2.3 | 0.3 | -0.1 | 24.6 | 4.88 |
| box plot of projected surface contamination of all abutment samples before and after cleaning in pixels and percent | 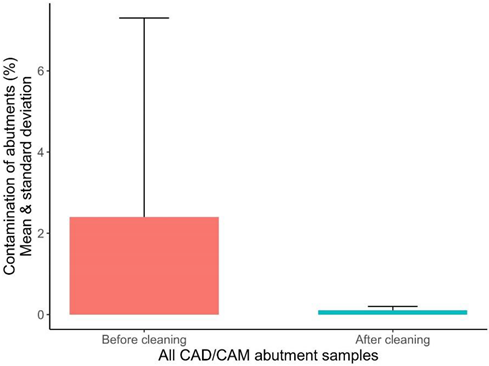 | |||||
| Monotype Abutments: Titanium/Zirconia | ||||||
| N | Mean | Median | Min. | Max. | St. Dev. | |
| % Contamination before cleaning | 14 | 4.861380 | 2.899032 | 0.900291 | 24.61993 | 6.098782 |
| % Contamination after cleaning | 14 | 0.192341 | 0.149575 | 0.025887 | 0.42001 | 0.136643 |
| % Difference before/after | 14 | 4.669040 | 2.741231 | 0.532721 | 24.59404 | 6.141301 |
| Hybrid-Abutments: IPSemax-/Zirconia and Ti-Base | ||||||
| N | Mean | Median | Min. | Max. | St. Dev. | |
| % Contamination before cleaning | 14 | 0.030569 | 0.012648 | 0.006283 | 0.097961 | 0.030976 |
| % Contamination after cleaning | 14 | 0.050087 | 0.030263 | 0.005599 | 0.147662 | 0.044470 |
| % Difference before/after | 14 | −0.019518 | −0.008203 | −0.109325 | 0.028793 | 0.043838 |
| Mann–Whitney U-Test | ||||||
| Rank Sum | Rank Sum | U | N | N | p-value | |
| Monotype | Hybrid | Monotype | Hybrid | |||
| % Contamination before | 301.0000 | 105.0000 | 0.00000 | 14 | 14 | 0.000000 |
| % Contamination after | 276.0000 | 130.0000 | 25.00000 | 14 | 14 | 0.000421 |
| % Difference before/after | 301.0000 | 105.0000 | 0.00000 | 14 | 14 | 0.000000 |
| box-plot graphic | 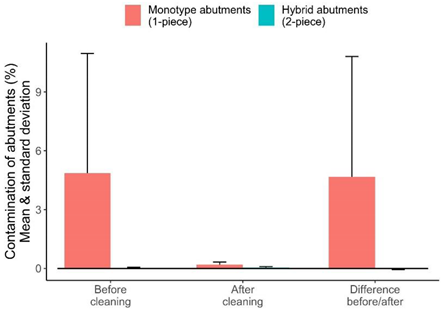 | |||||
| N | Mean | Median | Min. | Max. | St. Dev. | |
| Titanium Monotype | ||||||
| % Contamination before cleaning | 7 | 4.020973 | 2.924533 | 1.573535 | 8.789632 | 2.609841 |
| % Contamination after cleaning | 7 | 0.165439 | 0.111186 | 0.053941 | 0.379537 | 0.109002 |
| % Difference before/after | 7 | −3.86 | −2.82 | −8.60 | −1.47 | 2.55 |
| % Total contamination change | 7 | −95.4 | −96.5 | −97.8 | −92.6 | 2.2 |
| Zirconia Monotype | ||||||
| % Contamination before cleaning | 7 | 5.701788 | 2.251034 | 0.900291 | 24.61993 | 8.492950 |
| % Contamination after cleaning | 7 | 0.219242 | 0.187964 | 0.025887 | 0.42001 | 0.163965 |
| % Difference before/after | 7 | −5.48 | −2.06 | −24.59 | −0.53 | 8.58 |
| % Total contamination change | 7 | −87.1 | −89.5 | −99.9 | −57.5 | 14.3 |
| N | Mean | Median | Min. | Max. | St. Dev. | |
| Titanium and Zirconia Monotype | ||||||
| % Difference before/after | 14 | −4.67 | −2.74 | −24.59 | −0.53 | 6.14 |
| % Total contamination change | 14 | −91.2 | −93.5 | −99.9 | −57.5 | 10.7 |
| box-plot graphic. | 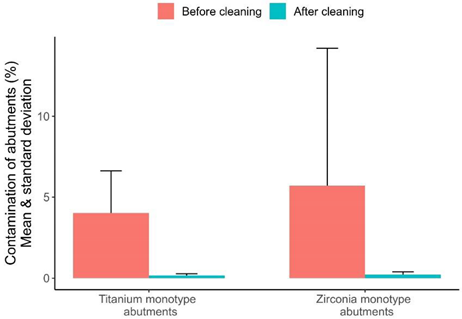 | |||||
| N | Mean | Median | Min. | Max. | St. Dev. | |
| IPSemax and Ti-Base | ||||||
| % Contamination before cleaning | 7 | 0.013165 | 0.007598 | 0.006283 | 0.040418 | 0.012325 |
| % Contamination after cleaning | 7 | 0.017373 | 0.015093 | 0.005599 | 0.031881 | 0.008056 |
| % Difference before/after | 7 | 0.004 | 0.008 | −0.020 | 0.023 | 0.013 |
| % Total contamination change | 7 | 82.6 | 104.3 | −48.5 | 252.4 | 110.4 |
| Zirconia and Ti-Base | ||||||
| % Contamination before cleaning | 7 | 0.047973 | 0.050000 | 0.010228 | 0.097961 | 0.034933 |
| % Contamination after cleaning | 7 | 0.082802 | 0.071295 | 0.028646 | 0.147662 | 0.041504 |
| % Difference before/after | 7 | 0.035 | 0.023 | −0.029 | 0.109 | 0.059 |
| % Total contamination change | 7 | 286.6 | 97.3 | −42.7 | 1007.6 | 443.2 |
| N | Mean | Median | Min. | Max. | St. Dev. | |
| IPSemax/Zirconia and Ti-Base | ||||||
| % Difference before/after | 14 | 0.020 | 0.008 | −0.029 | 0.109 | 0.044 |
| % Total contamination change | 14 | 184.6 | 100.8 | −48.5 | 1007.6 | 327.8 |
| box-plot graphic | 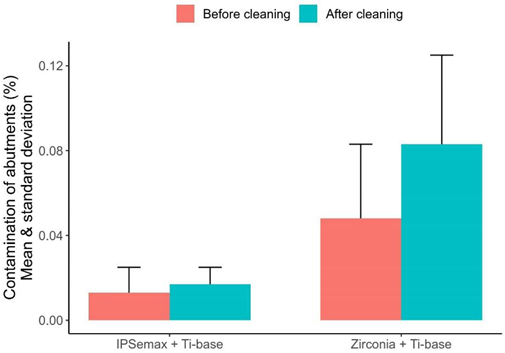 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehrke, P.; Abazari, C.; Schlichter, K.; Fischer, C.; Duddeck, D.; Romanos, G.E.; Weigl, P. Qualitative and Semi-Quantitative Assessment of Processing-Related Surface Contamination of One- and Two-Piece CAD/CAM Abutments before and after Ultrasonic Cleaning. Materials 2020, 13, 3225. https://doi.org/10.3390/ma13143225
Gehrke P, Abazari C, Schlichter K, Fischer C, Duddeck D, Romanos GE, Weigl P. Qualitative and Semi-Quantitative Assessment of Processing-Related Surface Contamination of One- and Two-Piece CAD/CAM Abutments before and after Ultrasonic Cleaning. Materials. 2020; 13(14):3225. https://doi.org/10.3390/ma13143225
Chicago/Turabian StyleGehrke, Peter, Cyrus Abazari, Kai Schlichter, Carsten Fischer, Dirk Duddeck, Georgios E. Romanos, and Paul Weigl. 2020. "Qualitative and Semi-Quantitative Assessment of Processing-Related Surface Contamination of One- and Two-Piece CAD/CAM Abutments before and after Ultrasonic Cleaning" Materials 13, no. 14: 3225. https://doi.org/10.3390/ma13143225






