Electrophoretic Co-deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy
Abstract
:1. Introduction
2. Materials and Methods
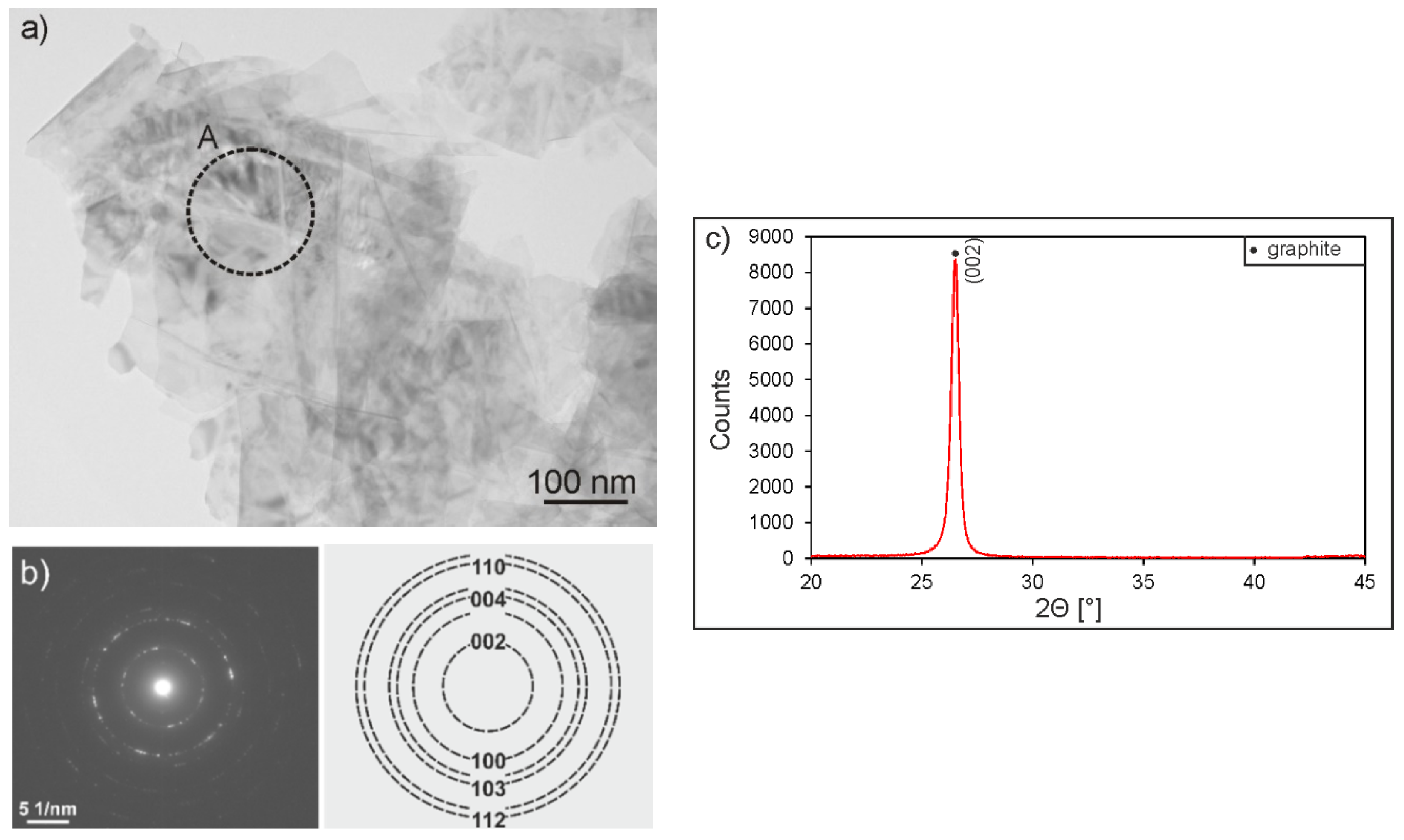
3. Results and Discussion
4. Conclusions
- Nanocomposite graphite/PEEK 708 coatings were electrophoretically deposited on titanium alloy substrates from an environmentally-friendly ethanol-based suspension containing 1 g/L of graphite. The optimal parameters to obtain homogeneous coatings were: voltage of 70 V and deposition time of 40 s. The EPD mechanism was investigated and discussed. The most probable mechanism of co-deposition of the particles consisted of the electrostatic interaction between them. As a result, graphite particles adsorbed on the surface of PEEK microparticles and graphite-PEEK complexes were deposited on the anode.
- The post-EPD heat treatment densified the coatings and increased their adhesion to the titanium alloy substrates. The coating had high scratch resistance and there was no adhesive damage. The first cohesive cracks appeared at the load LC1 = 9 N and their number grew with increasing load. The coating was characterized by high susceptibility to deformation, and plastic deformation of the coating after unloading was up to 40 μm.
- As a result of duplex treatment, the graphite particles embedded in the polymer were oriented parallel to the coating surface, which is the most advantageous arrangement in the context of friction processes. However, their amount was rather low and the further optimization of their content in the coating to achieve a stable and low COF is necessary.
- The coated alloy exhibited better corrosion resistance compared to the uncoated alloy in a sodium chloride solution at a temperature of 25 °C.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Simsiriwong, J.; Shrestha, R.; Shamsaei, N.; Lugo, M.; Moser, R.D. Effects of microstructural inclusions on fatigue life of polyether ether ketone (PEEK). J. Mech. Behav. Biomed. Mater. 2015, 51, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Dandy, L.O.; Oliveux, G.; Wood, J.; Jenkins, M.J.; Leeke, G.A. Accelerated degradation of Polyetheretherketone (PEEK) composite materials for recycling applications. Polym. Degrad. Stab. 2015, 112, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Li, E.Z.; Guo, W.L.; Wang, H.D.; Xu, B.S.; Liu, X.T. Research on tribological behavior of PEEK and glass fiber reinforced PEEK composite. Phys. Procedia 2013, 50, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.P.; Friedrich, K. On sliding friction and wear of PEEK and its composites. Wear 1995, 181–183, 624–631. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, H.; Coddet, C. Friction and wear behavior of PEEK and its composite coatings. In Tribology of Polymeric Nanocomposites, 2nd ed.; Friedrich, K., Schlarb, A.K., Eds.; Elsevier Ltd.: Oxford, UK, 2013; pp. 458–482. [Google Scholar]
- Baştan, F.E.; Atiq Ur Rehman, M.; Avcu, Y.Y.; Avcu, E.; Üstel, F.; Boccaccini, A.R. Electrophoretic co-deposition of PEEK-hydroxyapatite composite coatings for biomedical applications. Colloids Surf. B 2018, 169, 176–182. [Google Scholar] [CrossRef]
- Vail, J.R.; Krick, B.A.; Marchman, K.R.; Sawyer, W.G. Polytetrafluoroethylene (PTFE) fiber reinforced polyetheretherketone (PEEK) composites. Wear 2011, 270, 737–741. [Google Scholar] [CrossRef]
- Patel, K.; Doyle, C.S.; Yonekura, D.; James, B.J. Effect of surface roughness parameters on thermally sprayed PEEK coatings. Surf. Coat. Technol. 2010, 204, 3567–3572. [Google Scholar] [CrossRef]
- Zhang, G.; Li, W.-Y.; Cherigui, M.; Zhang, C.; Liao, H.; Bordes, J.-M.; Coddet, C. Structures and tribological performances of PEEK (poly-ether-ether-ketone)-based coatings designed for tribological application. Prog. Org. Coat. 2007, 60, 39–44. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Clavijo, S.; Membrives, F.; Boccaccini, A.R.; Santillán, M.J. Characterization of polyetheretherketone particle suspensions for electrophoretic deposition. J. Appl. Polym. Sci. 2014, 131, 2–7. [Google Scholar] [CrossRef]
- Wang, C.; Ma, J.; Cheng, W. Formation of polyetheretherketone polymer coating by electrophoretic deposition method. Surf. Coat. Technol. 2003, 173, 271–275. [Google Scholar] [CrossRef]
- Corni, I.; Neumann, N.; Eifler, D.; Boccaccini, A.R. Polyetheretherketone (PEEK) coatings on stainless steel by electrophoretic deposition. Adv. Eng. Mater. 2008, 10, 559–564. [Google Scholar] [CrossRef]
- Iveković, A.; Novak, S.; Lukek, M.; Kalin, M. Aqueous electrophoretic deposition of bulk polyether ether ketone (PEEK). J. Mater. Process. Technol. 2015, 223, 58–64. [Google Scholar] [CrossRef]
- Gonzalez-Castillo, E.I.; Costantini, T.; Shaffer, M.S.P.; Boccaccini, A.R. Nanocomposite coatings obtained by electrophoretic co-deposition of poly( etheretherketone)/graphene oxide suspensions. J. Mater. Sci. 2020, 55, 8881–8899. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Peters, C.; Roether, J.A.; Eifler, D.; Misra, S.K.; Minay, E.J. Electrophoretic deposition of polyetheretherketone (PEEK) and PEEK/Bioglass® coatings on NiTi shape memory alloy wires. J. Mater. Sci. 2006, 41, 8152–8159. [Google Scholar] [CrossRef]
- Virk, R.S.; Atiq Ur Rehman, M.; Boccaccini, A.R. PEEK Based Biocompatible Coatings Incorporating h-BN and Bioactive Glass by Electrophoretic Deposition. ECS Trans. 2018, 82, 89–95. [Google Scholar] [CrossRef]
- Atiq Ur Rehman, M.; Baştan, F.E.; Haider, B.; Boccaccini, A.R. Electrophoretic deposition of PEEK/bioactive glass composite coatings for orthopedic implants: A design of experiments (DoE) study. Mater. Des. 2017, 130, 223–230. [Google Scholar] [CrossRef]
- Atiq Ur Rehman, M.; Ferraris, S.; Goldmann, W.; Perero, S.; Baştan, F.E.; Nawaz, Q.; Gautier di Confiengo, G.; Ferraris, M.; Boccaccini, A.R. Antibacterial and Bioactive Coatings Based on Radio Frequency Co-Sputtering of Silver Nanocluster-Silica Coatings on PEEK/Bioactive Glass Layers Obtained by Electrophoretic Deposition. ACS Appl. Mater. Interfaces 2017, 9, 32489–32497. [Google Scholar] [CrossRef]
- Kruk, A.; Zimowski, S.; Łukaszczyk, A.; Cieniek, Ł.; Moskalewicz, T. The influence of heat treatment on the microstructure, surface topography and selected properties of PEEK coatings electrophoretically deposited on the Ti-6Al-4V alloy. Prog. Org. Coat. 2019, 133, 180–190. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Zimowski, S.; Fiołek, A.; Łukaszczyk, A.; Dubiel, B.; Cieniek, Ł. The Effect of the Polymer Structure in Composite Alumina/Polyetheretherketone Coatings on Corrosion Resistance, Micro-mechanical and Tribological Properties of the Ti-6Al-4V Alloy. J. Mater. Eng. Perform. 2019, 29, 1426–1438. [Google Scholar] [CrossRef] [Green Version]
- Fiołek, A.; Zimowski, S.; Kopia, A.; Moskalewicz, T. The influence of electrophoretic deposition parameters and heat treatment on the microstructure and tribological properties of nanocomposite Si3N4/PEEK 708 coatings on titanium alloy. Coatings 2019, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Moskalewicz, T.; Zych, A.; Kruk, A.; Kopia, A.; Zimowski, S.; Sitarz, M.; Cieniek, Ł. Electrophoretic deposition and microstructure development of Si3N4/polyetheretherketone coatings on titanium alloy. Surf. Coat. Technol. 2018, 350, 633–647. [Google Scholar] [CrossRef]
- Moskalewicz, T.; Warcaba, M.; Zimowski, S.; Łukaszczyk, A. Improvement of the Ti-6Al-4V Alloy’s Tribological Properties and Electrochemical Corrosion Resistance by Nanocomposite TiN/PEEK708 Coatings. Metall. Mater. Trans. A 2019, 50, 5914–5924. [Google Scholar] [CrossRef] [Green Version]
- Moskalewicz, T.; Kruk, A.; Sitarz, M.; Kopia, A. Effect of the processing and heat treatment route on the microstructure of MoS2/polyetheretherketone coatings obtained by electrophoretic deposition. J. Electrochem. Soc. 2019, 166, 151–161. [Google Scholar] [CrossRef]
- Burris, D.L.; Sawyer, W.G. A low friction and ultra low wear rate PEEK/PTFE composite. Wear 2006, 261, 410–418. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Wang, H. Graphite Solid Lubrication Materials. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y., Eds.; Springer: Boston, MA, USA, 2013; pp. 1550–1555. [Google Scholar]
- Ozkan, C. Handbook of Graphene, Volume 4: Composites, 1st ed.; John Wiley & Sons Inc.: New York, NY, USA, 2019. [Google Scholar]
- Berman, D.; Erdemir, A.; Sumant, V. Graphene: A new emerging lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Raza, M.A.; Ali, A.; Ghauri, F.A.; Aslam, A.; Yaqoob, J.; Wasay, A.; Raffi, M.; Ahmad, R. Electrochemical behavior of graphene coatings deposited on copper metal by electrophoretic deposition and chemical vapor deposition. Surf. Coat. Technol. 2017, 332, 112–119. [Google Scholar] [CrossRef]
- Chen, X.; Chen, S.; Liang, L.; Hong, H.; Zhang, Z.; Shen, B. Electrochemical behaviour of EPD synthesized graphene coating on titanium alloys for orthopedic implant application. Procedia CIRP 2018, 71, 322–328. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Ye, X.; Liu, X.; Yang, Z.; Wang, Z.; Wang, H.; Wang, J.; Yang, S. Tribological properties of fluorinated graphene reinforced polyimide composite coatings under different lubricated conditions. Compos. Part A Appl. Sci. Manuf. 2016, 81, 282–288. [Google Scholar] [CrossRef]
- Bhargava, S.; Koratkar, N.; Blanchet, T.A. Effect of platelet thickness on wear of graphene-polytetrafluoroethylene (PTFE) composites. Tribol. Lett. 2015, 59, 17. [Google Scholar] [CrossRef]
- Mo, Y.; Yang, M.; Lu, Z.; Huang, F. Preparation and tribological performance of chemically-modified reduced graphene oxide/polyacrylonitrile composites. Compos. Part A Appl. Sci. Manuf. 2013, 54, 153–158. [Google Scholar] [CrossRef]
- Kalin, M.; Zalaznik, M.; Novak, S. Wear and friction behaviour of poly-ether-ether-ketone (PEEK) filled with graphene, WS2 and CNT nanoparticles. Wear 2015, 332–333, 855–862. [Google Scholar] [CrossRef]
- Tewatia, A.; Hendrix, J.; Dong, Z.; Taghon, M.; Tse, S.; Chiu, G.; Mayo, W.E.; Kear, B.; Nosker, T.; Lynch, J. Characterization of melt-blended grapheme—Poly(ether ether ketone) nanocomposite. Mater. Sci. Eng. B 2017, 216, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Ma, R.; Wang, Y.; Cao, W.; Liu, C.; Shen, C. Comparative study of fullerenes and graphene nanoplatelets on the mechanical and thermomechanical properties of poly(ether ether ketone). Mater. Lett. 2019, 249, 180–184. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Liu, M.; Li, Z.; Vallés, C.; Young, R.J.; Kinloch, I.A. Hybrid poly(ether ether ketone) composites reinforced with a combination of carbon fibres and graphene nanoplatelets. Compos. Sci. Technol. 2019, 175, 60–68. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Castro, M.; Morris, J.A.; Ríos, R.; Ansón-Casaos, A. Tribological and mechanical properties of graphene nanoplatelet/PEEK composites. Carbon 2019, 141, 107–122. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Berretta, S.; Evans, K.; Smith, K.; Ghita, O. A primary study into graphene/polyether ether ketone (PEEK) nanocomposite for laser sintering. Appl. Surf. Sci. 2018, 428, 1018–1028. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Gómez, A.; Quiles-Díaz, S.; Enrique-Jimenez, P.; Flores, A.; Ania, F.; Gómez-Fatou, M.A.; Salavagione, H.J. Searching for effective compatibilizing agents for the preparation of poly(ether ether ketone)/graphene nanocomposites with enhanced properties. Compos. Part A Appl. Sci. Manuf. 2018, 113, 180–188. [Google Scholar] [CrossRef]
- Shen, B.; Hong, H.; Chen, S.; Chen, X.; Zhang, Z. Cathodic electrophoretic deposition of magnesium nitrate modified graphene coating as a macro-scale solid lubricant. Carbon 2019, 145, 297–310. [Google Scholar] [CrossRef]
- Sak, A.; Moskalewicz, T.; Zimowski, S.; Cieniek, Ł.; Dubiel, B.; Radziszewska, A.; Kot, M.; Łukaszczyk, A. Influence of polyetheretherketone coatings on the Ti-13Nb-13Zr titanium alloy’s bio-tribological properties and corrosion resistance. Mater. Sci. Eng. C 2016, 63, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; Liang, C.H. Colloidal and electrophoretic behavior of polymer particulates in suspension. Mater. Sci. Eng. C 2007, 27, 886–889. [Google Scholar] [CrossRef]
- Seuss, S.; Heinloth, M.; Boccaccini, A.R. Development of bioactive composite coatings based on combination of PEEK, bioactive glass and Ag nanoparticles with antibacterial properties. Surf. Coat. Technol. 2016, 301, 100–105. [Google Scholar] [CrossRef]
- Vallar, S.; Houivet, D.; El Fallah, J.; Kervadec, D.; Haussonne, J.-M. Oxide slurries stability and powders dispersion: Optimization with zeta potential and rheological measurements. J. Eur. Ceram. Soc. 1999, 19, 1017–1021. [Google Scholar] [CrossRef]
- Yousefpour, M.; Afshar, A.; Chen, J.; Zhang, X. Electrophoretic deposition of porous hydroxyapatite coatings using polytetrafluoroethylene particles as templates. Mater. Sci. Eng. C 2007, 27, 1482–1486. [Google Scholar] [CrossRef]
- Castro, R.H.R.; Marcos, P.J.B.; Sakamoto, E.K.; Gouvêa, D. Surface reactivity and electrophoretic deposition of ZrO2-MgO mechanical mixture. J. Mater. Sci. 2007, 42, 6946–6950. [Google Scholar] [CrossRef]
- McCafferty, E.; Hubler, G.K. Electrochemical Behavior of Palladium-Implanted Titanium. J. Electrochem. Soc. 1978, 125, 1892–1893. [Google Scholar] [CrossRef]
- Li, J.; Liao, H.; Coddet, C. Friction and wear behavior of flame-sprayed PEEK coatings. Wear 2002, 252, 824–831. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, H.; Li, H.; Mateus, C.; Bordes, J.-M.; Coddet, C. On dry sliding friction and wear behaviour of PEEK and PEEK/SiC-composite coatings. Wear 2006, 260, 594–600. [Google Scholar] [CrossRef]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiołek, A.; Zimowski, S.; Kopia, A.; Łukaszczyk, A.; Moskalewicz, T. Electrophoretic Co-deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials 2020, 13, 3251. https://doi.org/10.3390/ma13153251
Fiołek A, Zimowski S, Kopia A, Łukaszczyk A, Moskalewicz T. Electrophoretic Co-deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials. 2020; 13(15):3251. https://doi.org/10.3390/ma13153251
Chicago/Turabian StyleFiołek, Aleksandra, Sławomir Zimowski, Agnieszka Kopia, Alicja Łukaszczyk, and Tomasz Moskalewicz. 2020. "Electrophoretic Co-deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy" Materials 13, no. 15: 3251. https://doi.org/10.3390/ma13153251




