Nitrobenzene as Additive to Improve Reproducibility and Degradation Resistance of Highly Efficient Methylammonium-Free Inverted Perovskite Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hole Transporting Layers’ (HTLs) Preparation on ITO
2.3. Devices Fabrication
2.4. Characterizations
3. Results and Discussion
3.1. Perovskite Solar Cells
3.2. Perovskite Solutions and Films’ Characterization
3.3. Lifetime Testing of Perovskite Solar Cells
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- The National Renewable Energy Laboratory (NREL). Best Research-Cell Efficiencies. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 1 May 2019).
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, 6408. [Google Scholar] [CrossRef]
- Khenkin, M.V.; Katz, E.A.; Abate, A.; Bardizza, G.; Berry, J.J.; Brabec, C.; Brunetti, F.; Bulović, V.; Burlingame, Q.; Di Carlo, A.; et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 2020, 5, 35–49. [Google Scholar] [CrossRef]
- Koushik, D.; Verhees, W.J.H.; Kuang, Y.; Veenstra, S.; Zhang, D.; Verheijen, M.A.; Creatore, M.; Schropp, R.E.I. High-efficiency humidity-stable planar perovskite solar cells based on atomic layer architecture. Energy Environ.. Sci. 2017, 10, 91–100. [Google Scholar] [CrossRef]
- Papadas, I.T.; Galatopoulos, F.; Armatas, G.S.; Tessler, N.; Choulis, S.A. Nanoparticulate metal oxide top electrode interface modification improves the thermal stability of inverted perovskite photovoltaics. Nanomaterials 2019, 9, 1616. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fei, C.; Zheng, K.; Qu, X.; Pullerits, T.; Cao, G.; Tian, J. Constructing water-resistant CH3NH3PbI3 perovskite films via coordination interaction. J. Mater. Chem. A 2016, 4, 17018–17024. [Google Scholar] [CrossRef]
- Li, X.; Xue, Z.; Luo, D.; Huang, C.; Liu, L.; Qiao, X.; Liu, C.; Song, Q.; Yan, C.; Li, Y.; et al. A stable lead halide perovskite nanocrystals protected by PMMA. Sci. China Mater. 2018, 61, 363–370. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Z.; Dong, Q.; Li, J.; Yang, Z.; Chueh, C.-C.; Jen, A.K.-Y.; Wang, L. Enhanced Moisture Stability of Cesium-Containing Compositional Perovskites by a Feasible Interfacial Engineering. Adv. Mater. Interfaces 2017, 4. [Google Scholar] [CrossRef]
- Liu, K.; Dai, S.; Meng, F.; Shi, J.; Li, Y.; Wu, J.; Meng, Q.; Zhan, X. Fluorinated fused nonacyclic interfacial materials for efficient and stable perovskite solar cells. J. Mater. Chem. A 2017, 5, 21414–21421. [Google Scholar] [CrossRef]
- Leijtens, T.; Giovenzana, T.; Habisreutinger, S.N.; Tinkham, J.S.; Noel, N.K.; Kamino, B.A.; Sadoughi, G.; Sellinger, A.; Snaith, H.J. Hydrophobic Organic Hole Transporters for Improved Moisture Resistance in Metal Halide Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 5981–5989. [Google Scholar] [CrossRef]
- Hou, X.; Huang, S.; Ou-Yang, W.; Pan, L.; Sun, Z.; Chen, X. Constructing Efficient and Stable Perovskite Solar Cells via Interconnecting Perovskite Grains. ACS Appl. Mater. Interfaces 2017, 9, 35200–35208. [Google Scholar] [CrossRef]
- Yang, Z.; Dou, J.; Kou, S.; Dang, J.; Ji, Y.; Yang, G.; Wu, W.; Kuang, D.; Wang, M. Multifunctional Phosphorus-Containing Lewis Acid and Base Passivation Enabling Efficient and Moisture-Stable Perovskite Solar Cells. Adv. Funct. Mater. 2020, 13, 1910710. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Liu, S.; Guan, Y.; Sheng, Y.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. A Review on Additives for Halide Perovskite Solar Cells. Adv. Energy Mater. 2019, 10, 1–28. [Google Scholar] [CrossRef]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.; Jen, A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef]
- Li, X.; Ibrahim Dar, M.; Yi, C.; Luo, J.; Tschumi, M.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Han, H.; Grätzel, M. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω -ammonium chlorides. Nat. Chem. 2015, 7, 703–711. [Google Scholar] [CrossRef]
- Burgués-Ceballos, I.; Savva, A.; Georgiou, E.; Kapnisis, K.; Papagiorgis, P.; Mousikou, A.; Itskos, G.; Othonos, A.; Choulis, S.A. The influence of additives in the stoichiometry of hybrid lead halide perovskites. AIP Adv. 2017, 7. [Google Scholar] [CrossRef]
- Zuo, L.; Guo, H.; DeQuilettes, D.W.; Jariwala, S.; De Marco, N.; Dong, S.; DeBlock, R.; Ginger, D.S.; Dunn, B.; Wang, M.; et al. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chu, C.Y.; Huang, Y.C.; Huang, C.W.; Chang, S.Y.; Chen, C.A.; Chao, C.Y.; Su, W.F. Tuning perovskite morphology by polymer additive for high efficiency solar cell. ACS Appl. Mater. Interfaces 2015, 7, 4955–4961. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Nazeeruddin, M.K.; Gratzel, M.; Wu, C.-G. The synergistic effect of H2O and DMF towards stable and 20% efficiency inverted perovskite solar cells. Energy Environ. Sci. 2017, 10, 808–817. [Google Scholar] [CrossRef]
- Rong, Y.; Hou, X.; Hu, Y.; Mei, A.; Liu, L.; Wang, P.; Han, H. Synergy of ammonium chloride and moisture on perovskite crystallization for efficient printable mesoscopic solar cells. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Liu, Z.; Chen, Q.; Wang, X.; Zhou, H. The Additive Coordination Effect on Hybrids Perovskite Crystallization and High-Performance Solar Cell. Adv. Mater. 2016, 28, 9862–9868. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, H.; Xu, H.; Zhao, N. HPbI3: A New Precursor Compound for Highly Efficient Solution-Processed Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 1120–1126. [Google Scholar] [CrossRef]
- Gong, X.; Li, M.; Shi, X.-B.; Ma, H.; Wang, Z.-K.; Liao, L.-S. Controllable Perovskite Crystallization by Water Additive for High-Performance Solar Cells. Adv. Funct. Mater. 2015, 25, 6671–6678. [Google Scholar] [CrossRef]
- De Marco, N.; Zhou, H.; Chen, Q.; Sun, P.; Liu, Z.; Meng, L.; Yao, E.-P.; Liu, Y.; Schiffer, A.; Yang, Y. Guanidinium: A Route to Enhanced Carrier Lifetime and Open-Circuit Voltage in Hybrid Perovskite Solar Cells. Nano Lett. 2016, 16, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zheng, X.; Bai, Y.; Wang, Q.; Zhao, J.; Huang, J. Surfactant-controlled ink drying enables high-speed deposition of perovskite films for efficient photovoltaic modules. Nat. Energy 2018, 3, 560–566. [Google Scholar] [CrossRef]
- Shen, C.; Wu, Y.; Zhang, S.; Wu, T.; Tian, H.; Zhu, W.-H.; Han, L. Stabilizing Formamidinium Lead Iodide Perovskite by Sulfonyl-Functionalized Phenethylammonium Salt via Crystallization Control and Surface Passivation. Sol. RRL 2020, 5, 2000069. [Google Scholar] [CrossRef]
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; van Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 2, 1–35. [Google Scholar] [CrossRef]
- Turren-Cruz, S.-H.; Hagfeldt, A.; Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 2018, 362, 449–453. [Google Scholar] [CrossRef]
- Schutt, K.; Nayak, P.K.; Ramadan, A.J.; Wenger, B.; Lin, Y.-H.; Snaith, H.J. Overcoming Zinc Oxide Interface Instability with a Methylammonium-Free Perovskite for High-Performance Solar Cells. Adv. Funct. Mater. 2019, 29, 1900466. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Z.; Jia, X.; Wu, Y.; Yuan, N.; Ding, J.; Zhang, W.-H.; Liu, S. (Frank) Thermally stable methylammonium-free inverted perovskite solar cells with Zn2+ doped CuGaO2 as efficient mesoporous hole-transporting layer. Nano Energy 2019, 61, 148–157. [Google Scholar] [CrossRef]
- Zhou, Y.; Xue, H.; Jia, Y.-H.; Brocks, G.; Tao, S.; Zhao, N. Enhanced Incorporation of Guanidinium in Formamidinium-Based Perovskites for Efficient and Stable Photovoltaics: The Role of Cs and Br. Adv. Funct. Mater. 2019, 29, 1905739. [Google Scholar] [CrossRef]
- Gao, X.-X.; Luo, W.; Zhang, Y.; Hu, R.; Zhang, B.; Züttel, A.; Feng, Y.; Nazeeruddin, M.K. Stable and High-Efficiency Methylammonium-Free Perovskite Solar Cells. Adv. Mater. 2020, 32, 1905502. [Google Scholar] [CrossRef]
- Bush, K.A.; Frohna, K.; Prasanna, R.; Beal, R.E.; Leijtens, T.; Swifter, S.A.; McGehee, M.D. Compositional Engineering for Efficient Wide Band Gap Perovskites with Improved Stability to Photoinduced Phase Segregation. ACS Energy Lett. 2018, 3, 428–435. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.S.; Wei, S.H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2016, 28, 284–292. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D.H.; Kim, H.S.; Seo, S.W.; Cho, S.M.; Park, N.G. Formamidinium and cesium hybridization for photo- and moisture-stable perovskite solar cell. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Yi, C.; Luo, J.; Meloni, S.; Boziki, A.; Ashari-Astani, N.; Grätzel, C.; Zakeeruddin, S.M.; Röthlisberger, U.; Grätzel, M. Entropic stabilization of mixed A-cation ABX3 metal halide perovskites for high performance perovskite solar cells. Energy Environ. Sci. 2016, 9, 656–662. [Google Scholar] [CrossRef]
- Tremblay, M.H.; Thouin, F.; Leisen, J.; Bacsa, J.; Srimath Kandada, A.R.; Hoffman, J.M.; Kanatzidis, M.G.; Mohite, A.D.; Silva, C.; Barlow, S.; et al. (4NPEA)2PbI4 (4NPEA = 4-Nitrophenylethylammonium): Structural, NMR, and Optical Properties of a 3 × 3 Corrugated 2D Hybrid Perovskite. J. Am. Chem. Soc. 2019, 141, 4521–4525. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef]
- Jung, J.W.; Chueh, C.C.; Jen, A.K.Y. A Low-Temperature, Solution-Processable, Cu-Doped Nickel Oxide Hole-Transporting Layer via the Combustion Method for High-Performance Thin-Film Perovskite Solar Cells. Adv. Mater. 2015, 27, 7874–7880. [Google Scholar] [CrossRef]
- McMeekin, D.P.; Wang, Z.; Rehman, W.; Pulvirenti, F.; Patel, J.B.; Noel, N.K.; Johnston, M.B.; Marder, S.R.; Herz, L.M.; Snaith, H.J. Crystallization Kinetics and Morphology Control of Formamidinium–Cesium Mixed-Cation Lead Mixed-Halide Perovskite via Tunability of the Colloidal Precursor Solution. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Kovalenko, A.; Forés, S.M.; Aranda, C.; Guerrero, A. Coordination Chemistry Dictates the Structural Defects in Lead Halide Perovskites. Chem. Phys. Chem 2016, 17, 2795–2798. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; McCleese, C.; Kolodziej, C.; Samia, A.C.S.; Zhao, Y.; Burda, C. Identification and characterization of the intermediate phase in hybrid organic-inorganic MAPbI3 perovskite. Dalt. Trans. 2016, 45, 3806–3813. [Google Scholar] [CrossRef]
- Yan, K.; Long, M.; Zhang, T.; Wei, Z.; Chen, H.; Yang, S.; Xu, J. Hybrid Halide Perovskite Solar Cell Precursors: Colloidal Chemistry and Coordination Engineering behind Device Processing for High Efficiency. J. Am. Chem. Soc. 2015, 137, 4460–4468. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wu, J.; Tu, Y.; He, X.; Lan, Z.; Huang, M.; Lin, J. Solvent engineering for high-quality perovskite solar cell with an efficiency approaching 20%. J. Power Sources 2017, 365, 1–6. [Google Scholar] [CrossRef]
- Fei, C.; Guo, L.; Li, B.; Zhang, R.; Fu, H.; Tian, J.; Cao, G. Controlled growth of textured perovskite films towards high performance solar cells. Nano Energy 2016, 27, 17–26. [Google Scholar] [CrossRef]
- Cao, X.; Zhi, L.; Li, Y.; Fang, F.; Cui, X.; Ci, L.; Ding, K.; Wei, J. Fabrication of Perovskite Films with Large Columnar Grains via Solvent-Mediated Ostwald Ripening for Efficient Inverted Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 868–875. [Google Scholar] [CrossRef]
- Fei, C.; Li, B.; Zhang, R.; Fu, H.; Tian, J.; Cao, G. Highly Efficient and Stable Perovskite Solar Cells Based on Monolithically Grained CH3NH3PbI3 Film. Adv. Energy Mater. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Z.; Hu, X.; Meng, X.; Huang, L.; Xiong, J.; Tan, L.; Chen, Y. Grain Boundary Modification via F4TCNQ to Reduce Defects of Perovskite Solar Cells with Excellent Device Performance. ACS Appl. Mater. Interfaces 2018, 10, 1909–1916. [Google Scholar] [CrossRef]
- Chaudhary, B.; Koh, T.M.; Febriansyah, B.; Bruno, A.; Mathews, N.; Mhaisalkar, S.G.; Soci, C. Mixed-Dimensional Naphthylmethylammoinium-Methylammonium Lead Iodide Perovskites with Improved Thermal Stability. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, X.; Chen, X.; Mao, J.; Cheng, J.; Zhao, Y.; Liu, Y.; Milic, J.; Yin, W.J.; Grätzel, M.; et al. Improving the stability and performance of perovskite solar cells: Via off-the-shelf post-device ligand treatment. Energy Environ. Sci. 2018, 11, 2253–2262. [Google Scholar] [CrossRef]
- Wu, Y.; Islam, A.; Yang, X.; Qin, C.; Liu, J.; Zhang, K.; Peng, W.; Han, L. Retarding the crystallization of PbI 2 for highly reproducible planar-structured perovskite solar cells via sequential deposition. Energy Environ. Sci. 2014, 7, 2934. [Google Scholar] [CrossRef]
- Lee, J.W.; Bae, S.H.; Hsieh, Y.T.; De Marco, N.; Wang, M.; Sun, P.; Yang, Y. A Bifunctional Lewis Base Additive for Microscopic Homogeneity in Perovskite Solar Cells. Chem 2017, 3, 290–302. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y.; et al. A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.M.; Tennyson, E.M.; Barik, S.; Szostak, R.; Waks, E.; Toney, M.F.; Nogueira, A.F.; Neves, B.R.A.; Leite, M.S. Humidity-Induced Photoluminescence Hysteresis in Variable Cs/Br Ratio Hybrid Perovskites. J. Phys. Chem. Lett. 2018, 9, 3463–3469. [Google Scholar] [CrossRef]
- Barbé, J.; Newman, M.; Lilliu, S.; Kumar, V.; Lee, H.K.H.; Charbonneau, C.; Rodenburg, C.; Lidzey, D.; Tsoi, W.C. Localized effect of PbI2 excess in perovskite solar cells probed by high-resolution chemical-optoelectronic mapping. J. Mater. Chem. A 2018, 6, 23010–23018. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Liu, Y.; Deng, Y.; Bai, Y.; Dong, Q.; Huang, J. Scaling behavior of moisture-induced grain degradation in polycrystalline hybrid perovskite thin films. Energy Environ. Sci. 2017, 10, 516–522. [Google Scholar] [CrossRef]
- Yun, J.S.; Kim, J.; Young, T.; Patterson, R.J.; Kim, D.; Seidel, J.; Lim, S.; Green, M.A.; Huang, S.; Ho-Baillie, A. Humidity-Induced Degradation via Grain Boundaries of HC(NH2)2PbI3 Planar Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1–8. [Google Scholar] [CrossRef]
- Wang, M.; Fu, Q.; Yan, L.; Guo, P.; Zhou, L.; Wang, G.; Zheng, Z.; Luo, W. Improving the Performance and Reproducibility of Inverted Planar Perovskite Solar Cells Using Tetraethyl Orthosilicate as the Antisolvent. ACS Appl. Mater. Interfaces 2019, 11, 3909–3916. [Google Scholar] [CrossRef]
- Yun, S.C.; Ma, S.; Kwon, H.C.; Kim, K.; Jang, G.; Yang, H.; Moon, J. Amino acid salt-driven planar hybrid perovskite solar cells with enhanced humidity stability. Nano Energy 2019, 59, 481–491. [Google Scholar] [CrossRef]
- Wu, T.; Liu, X.; He, X.; Wang, Y.; Meng, X.; Noda, T.; Yang, X.; Han, L. Efficient and stable tin-based perovskite solar cells by introducing π-conjugated Lewis base. Sci. China Chem. 2020, 63, 107–115. [Google Scholar] [CrossRef]
- Wang, F.; Geng, W.; Zhou, Y.; Fang, H.H.; Tong, C.J.; Loi, M.A.; Liu, L.M.; Zhao, N. Phenylalkylamine Passivation of Organolead Halide Perovskites Enabling High-Efficiency and Air-Stable Photovoltaic Cells. Adv. Mater. 2016, 28, 9986–9992. [Google Scholar] [CrossRef] [PubMed]
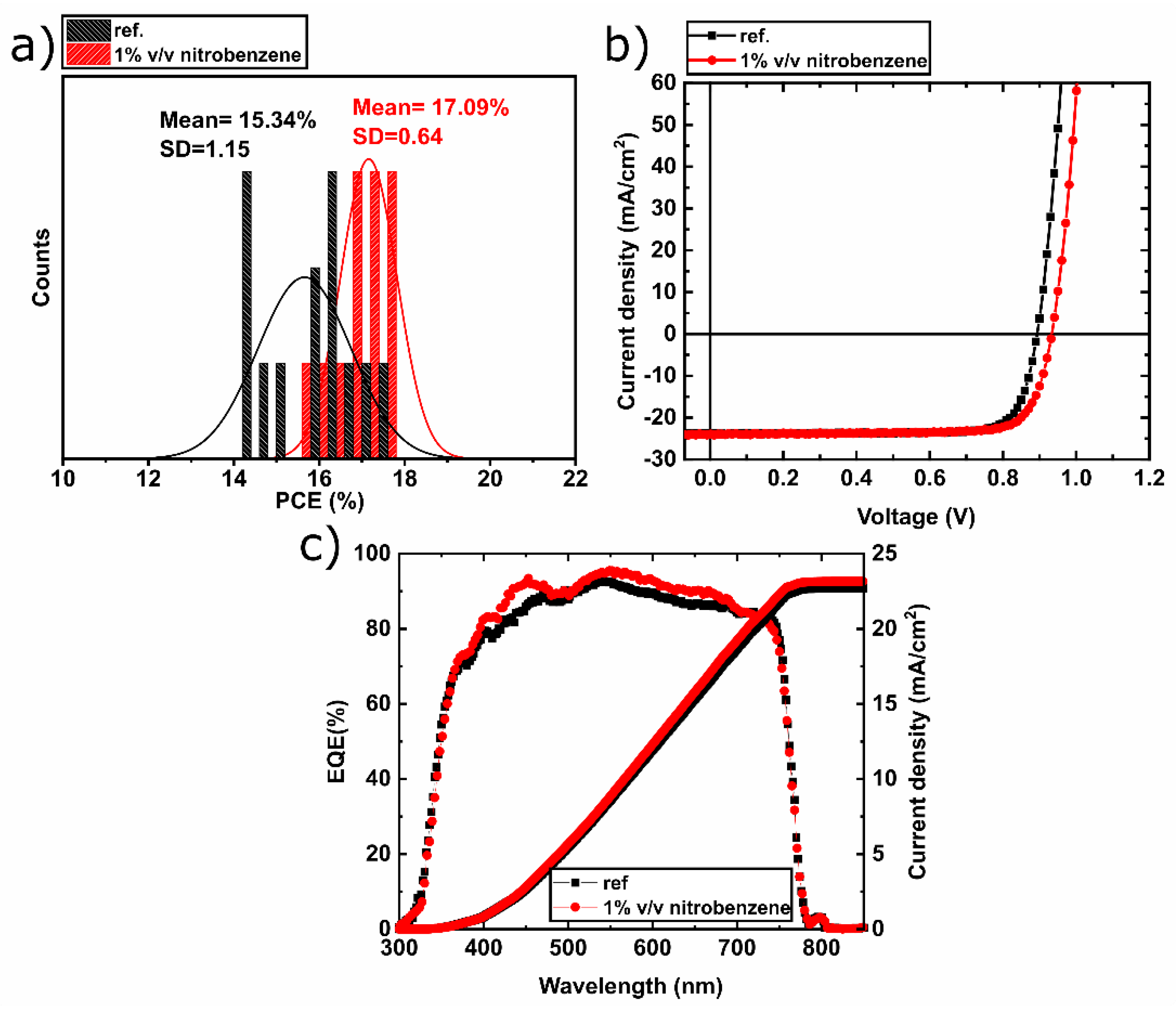
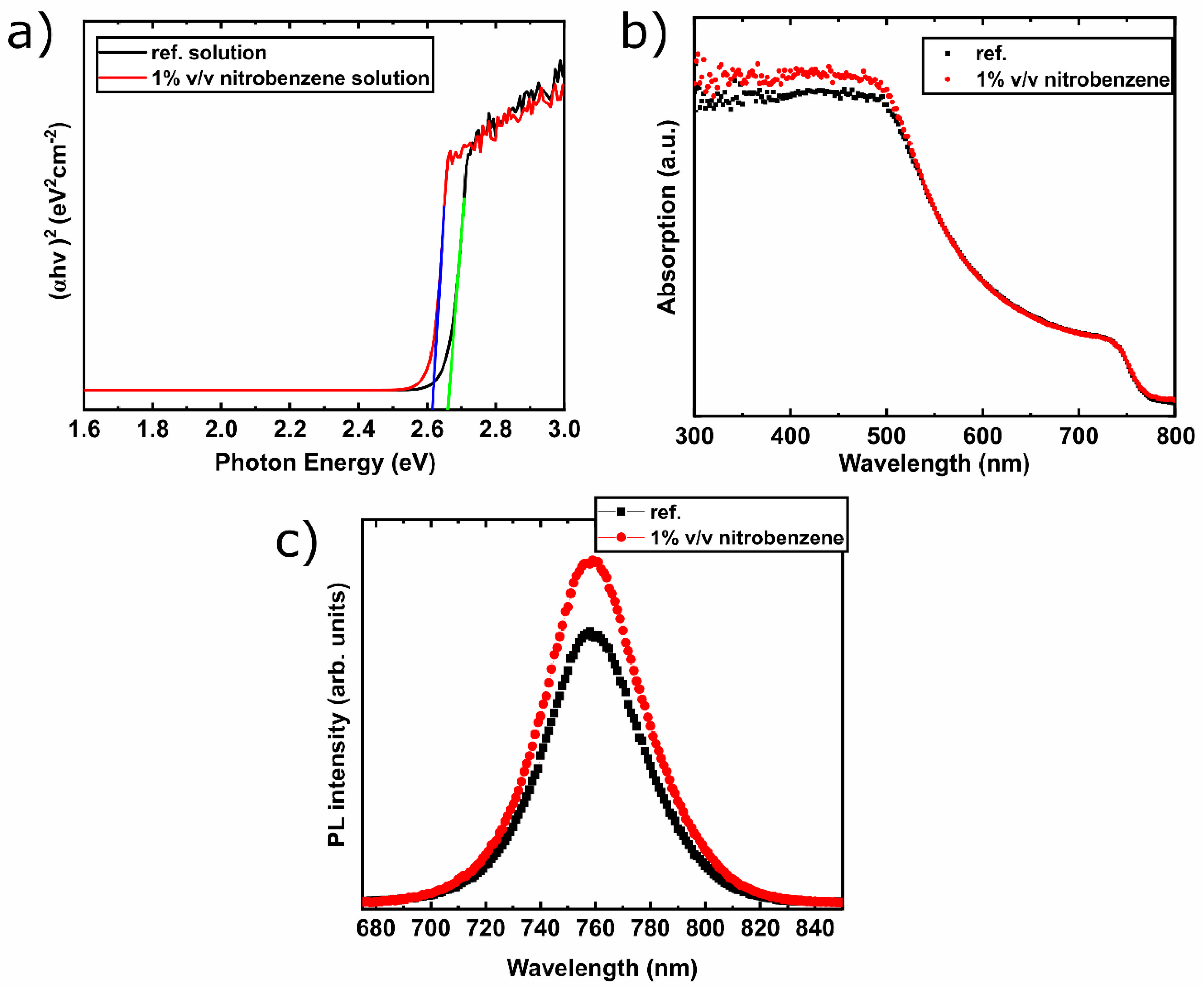

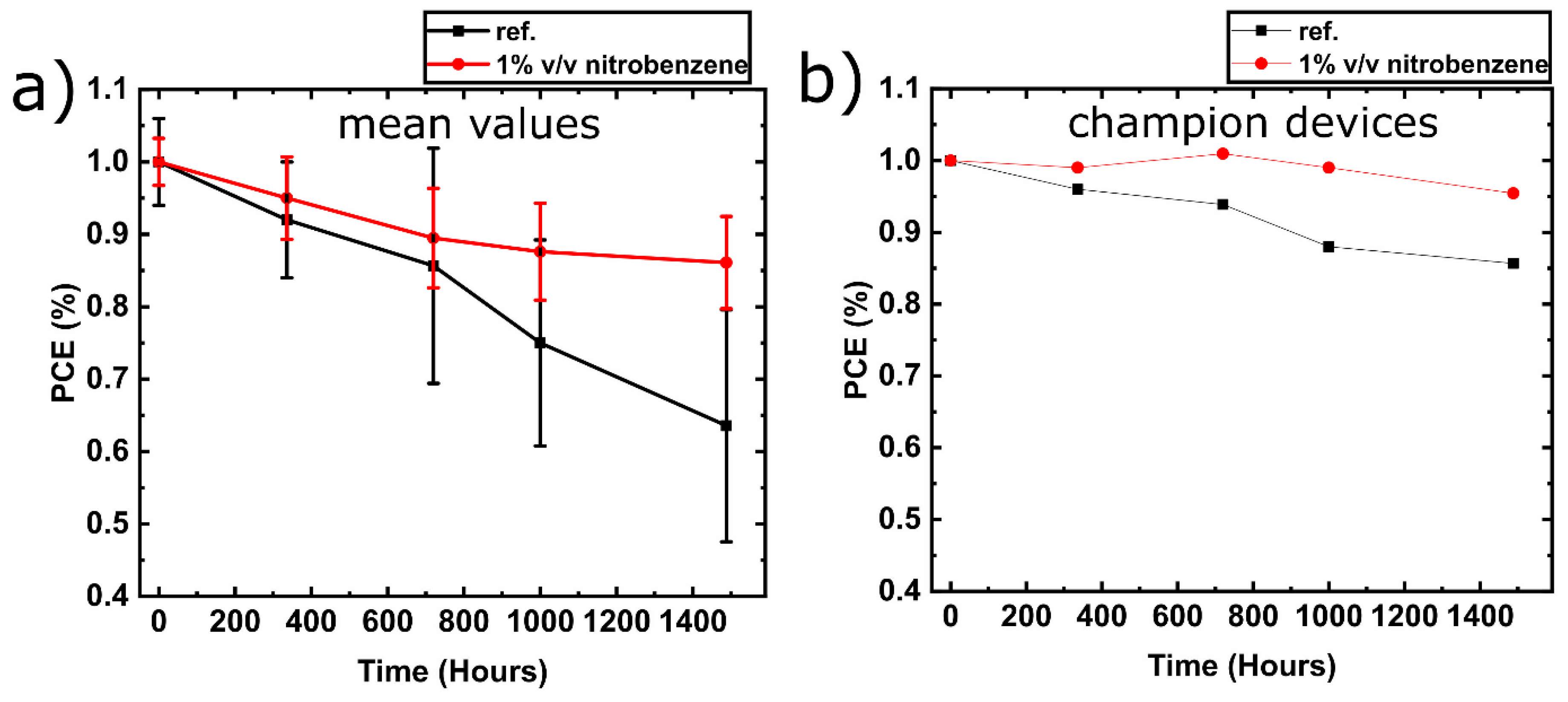

| Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) | |
|---|---|---|---|---|
| reference | 0.89 | 23.99 | 81.3 | 17.35 |
| 1% v/v Nitrobenzene | 0.92 | 24.36 | 80.3 | 18.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioakeimidis, A.; Choulis, S.A. Nitrobenzene as Additive to Improve Reproducibility and Degradation Resistance of Highly Efficient Methylammonium-Free Inverted Perovskite Solar Cells. Materials 2020, 13, 3289. https://doi.org/10.3390/ma13153289
Ioakeimidis A, Choulis SA. Nitrobenzene as Additive to Improve Reproducibility and Degradation Resistance of Highly Efficient Methylammonium-Free Inverted Perovskite Solar Cells. Materials. 2020; 13(15):3289. https://doi.org/10.3390/ma13153289
Chicago/Turabian StyleIoakeimidis, Apostolos, and Stelios A. Choulis. 2020. "Nitrobenzene as Additive to Improve Reproducibility and Degradation Resistance of Highly Efficient Methylammonium-Free Inverted Perovskite Solar Cells" Materials 13, no. 15: 3289. https://doi.org/10.3390/ma13153289
APA StyleIoakeimidis, A., & Choulis, S. A. (2020). Nitrobenzene as Additive to Improve Reproducibility and Degradation Resistance of Highly Efficient Methylammonium-Free Inverted Perovskite Solar Cells. Materials, 13(15), 3289. https://doi.org/10.3390/ma13153289





