Biomimetic Design for a Dual Concentric Porous Titanium Scaffold with Appropriate Compressive Strength and Cells Affinity
Abstract
:1. Introduction
2. Material and Methods
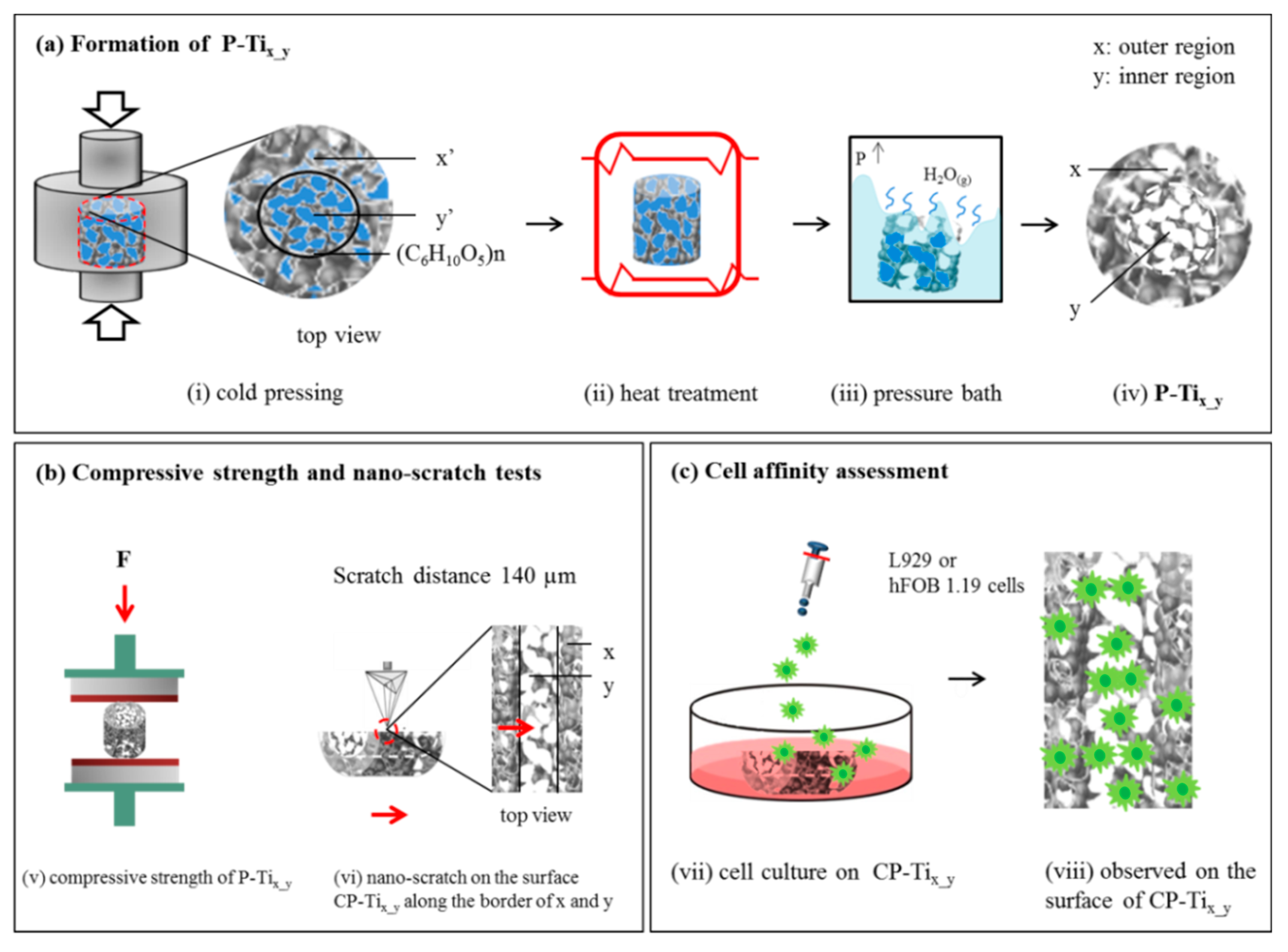
2.1. Preparation of Dual Concentric Porous Titanium
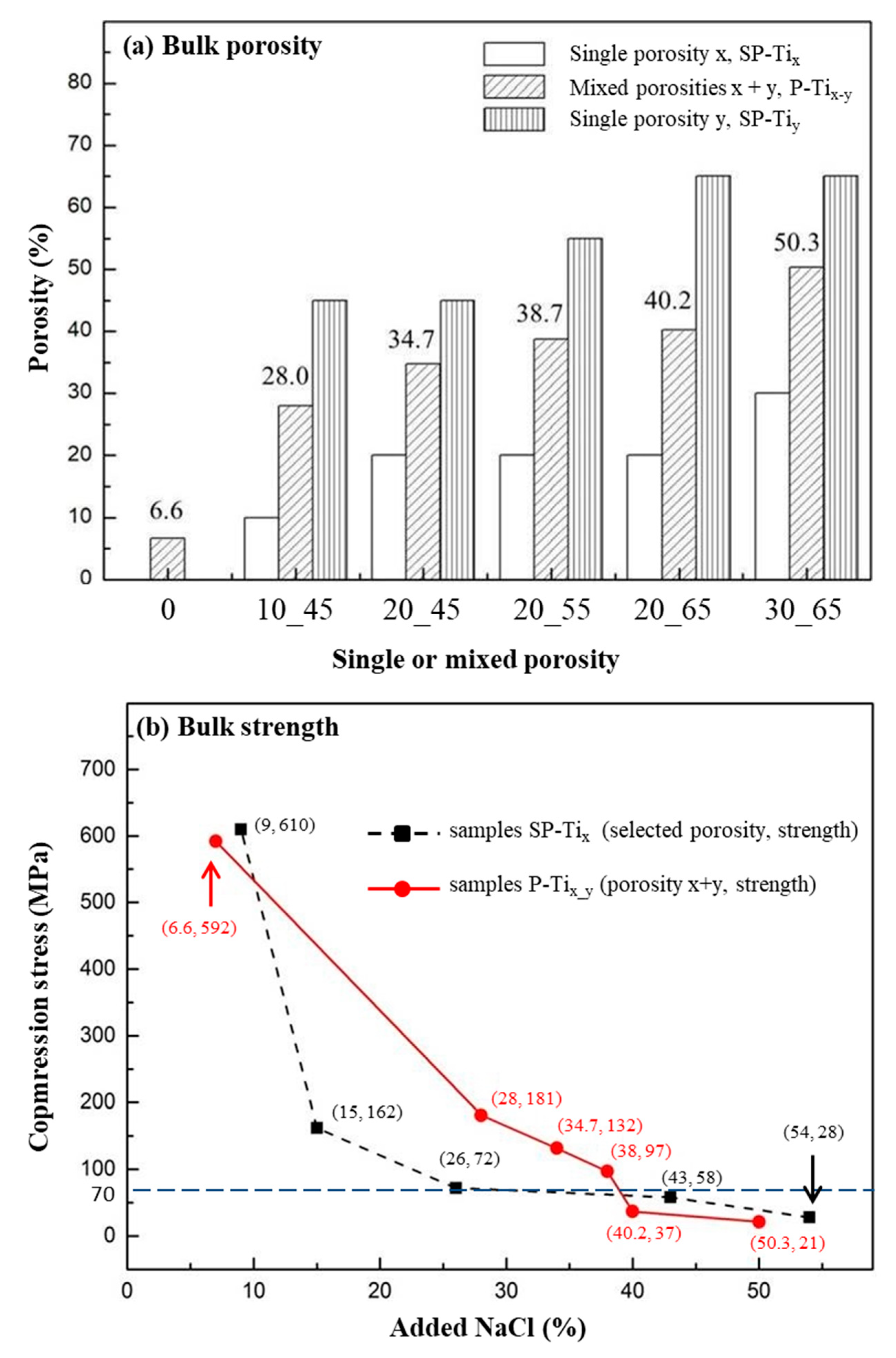
2.2. Compression Stress and Porosity of P-Tix_y
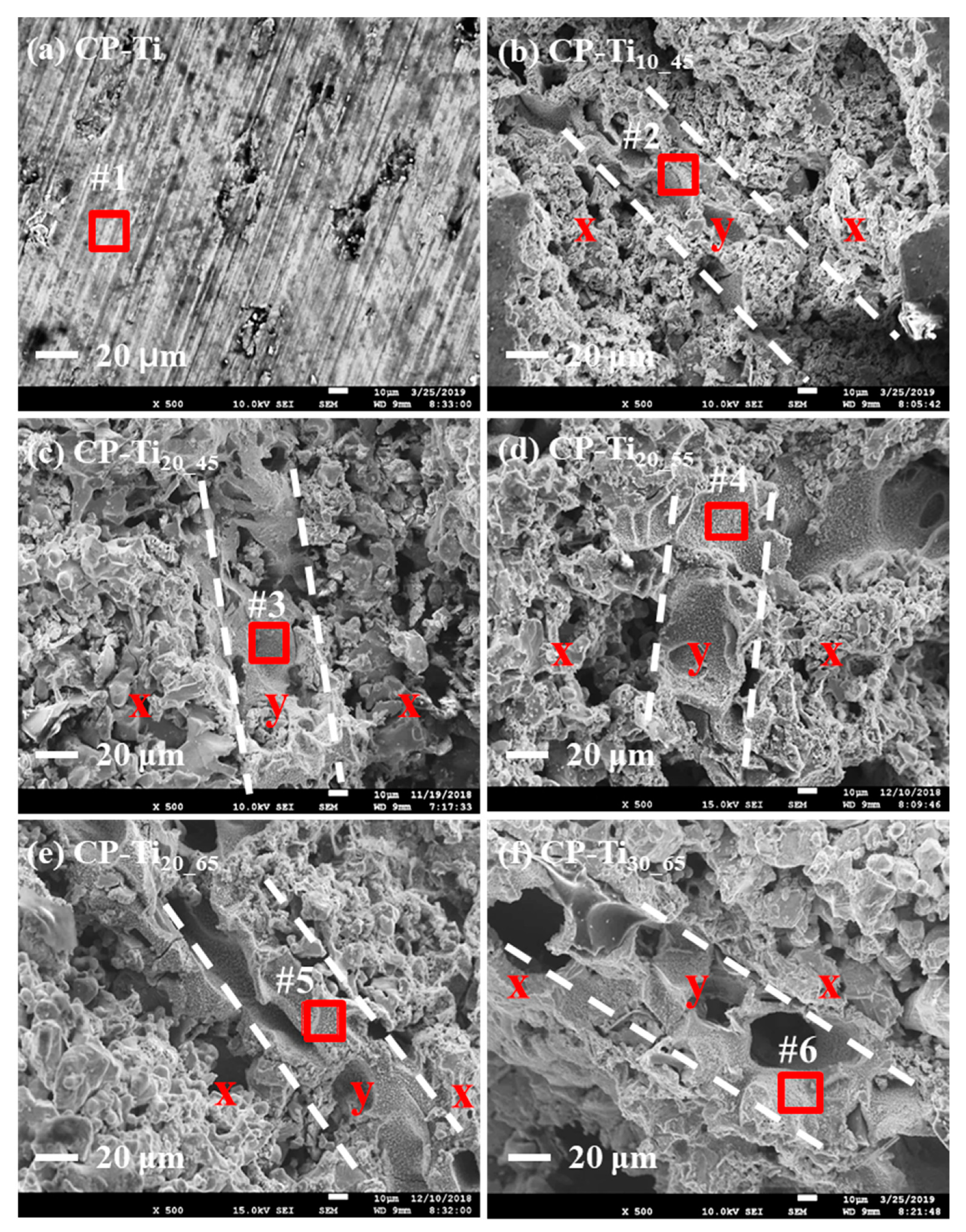
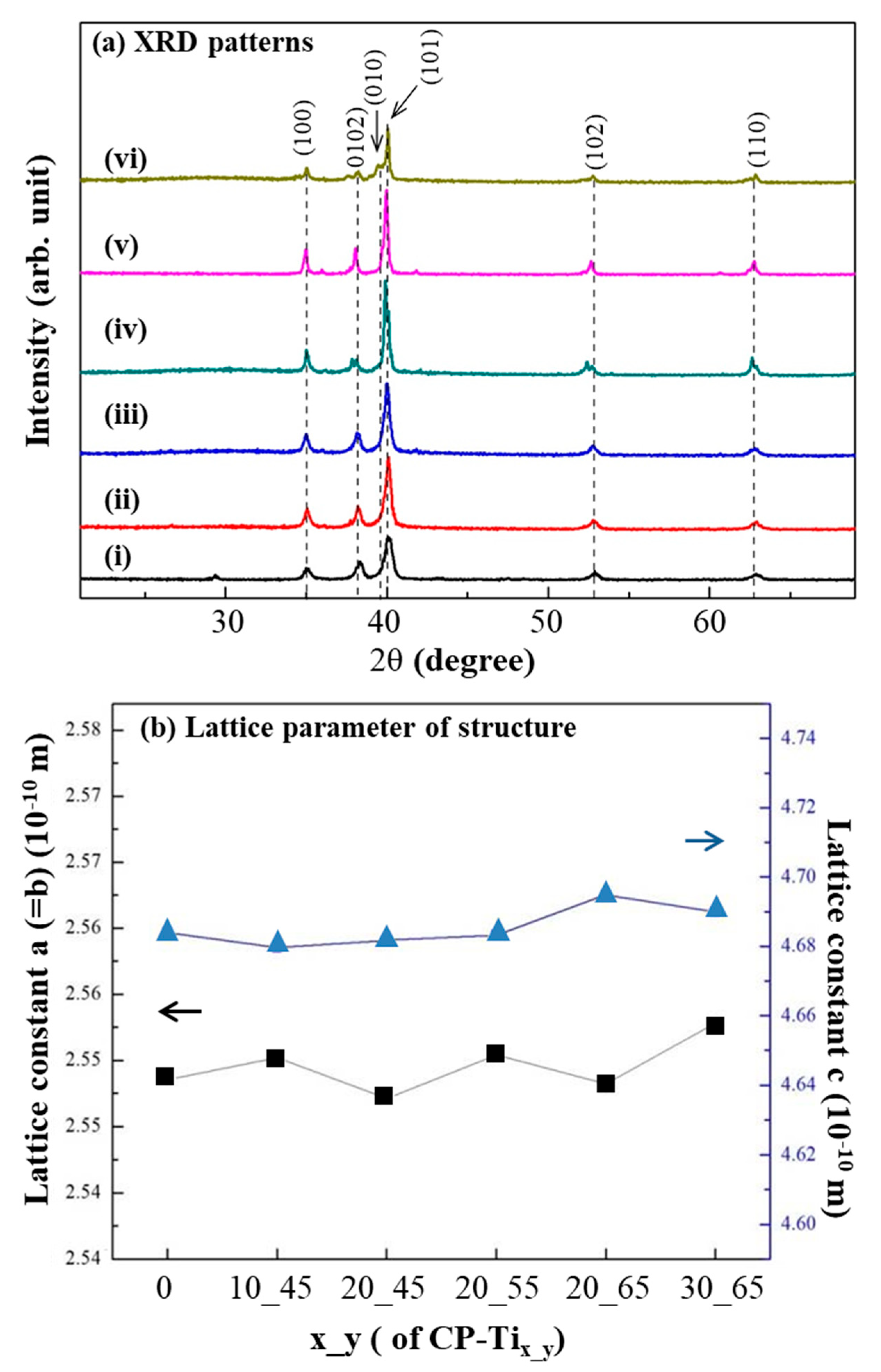
2.3. Surface Morphology and Crystalline Structure of the Cross-Sectioned P-Tix_y
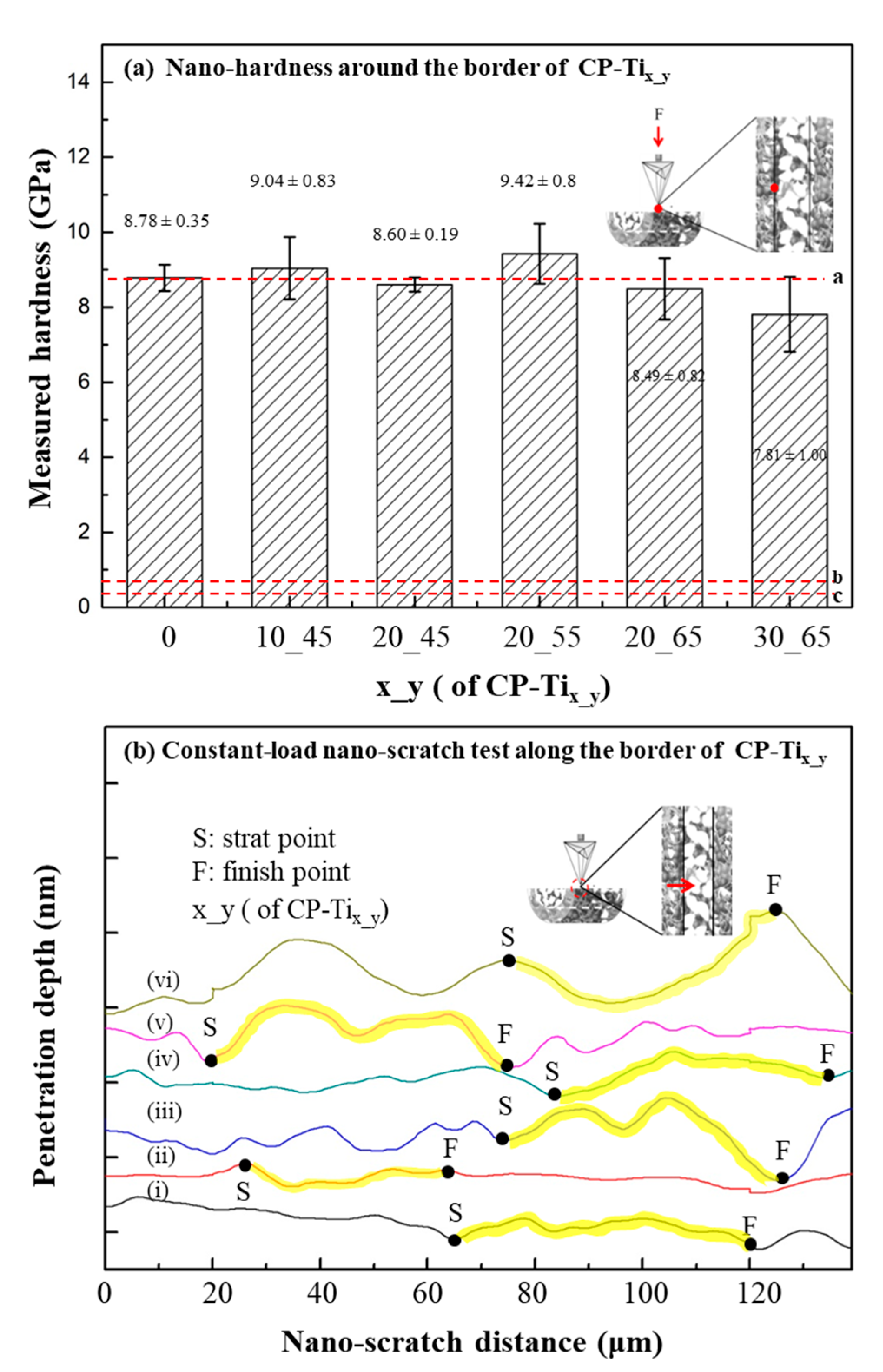
2.4. Nano-Hardness and Nano-Scratch Tests on the Surface of CP-Tix_y
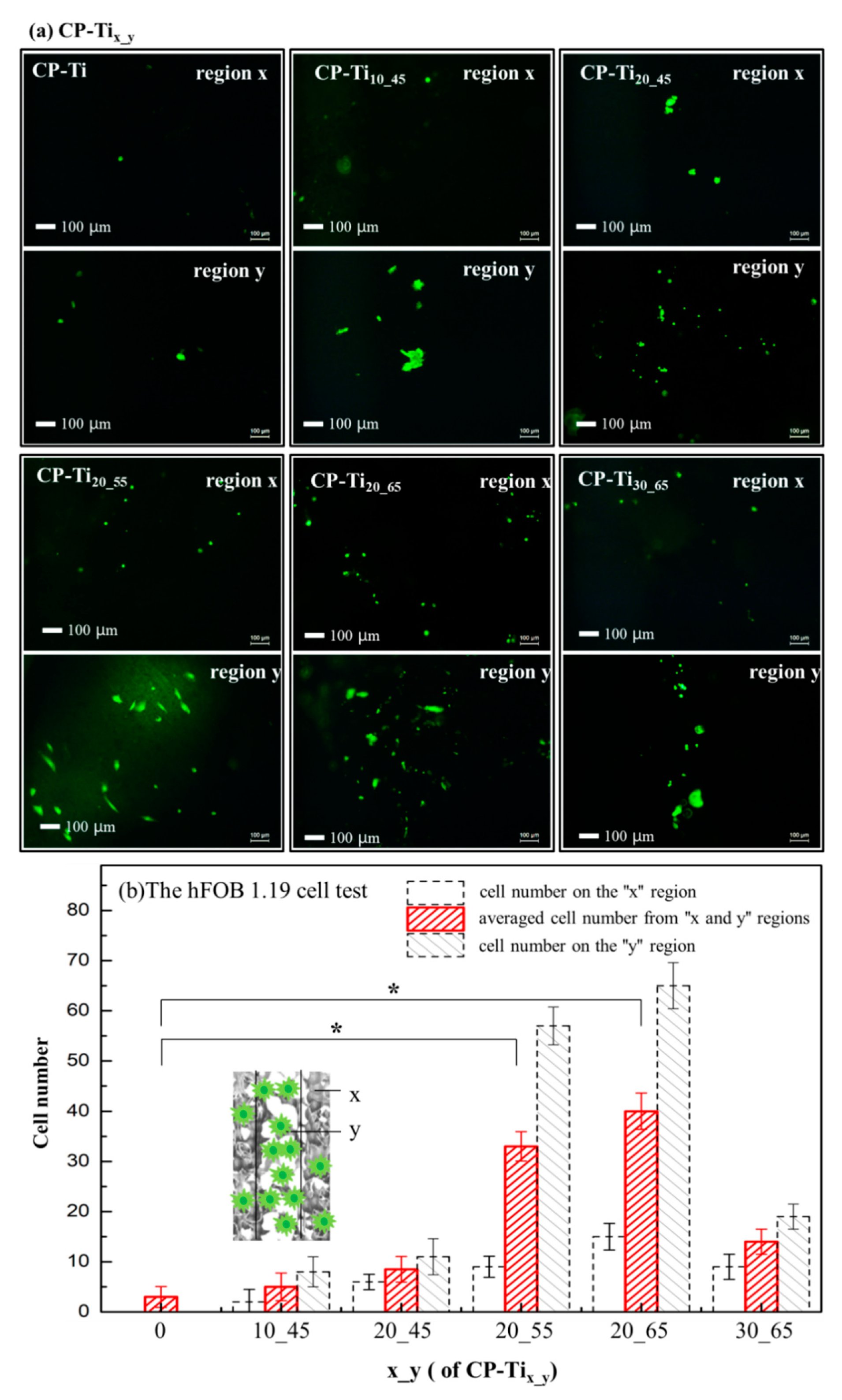
2.5. Cell Affinity on the Surface of CP-Tix_y
2.6. Statistical Analysis
3. Results and Discussion
3.1. Compression Stress of P-Tix_y
3.2. Surface Morphology and Crystalline Structure of CP-Tix_y
3.3. Nanomechanical Property of CP-Tix_y
3.4. Cells’ Affinity upon the Surface of CP-Tix_y
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Turner, C.H.; Wang, T.; Burr, D.B. Shear strength and fatigue properties of human cortical bone determined from pure shear tests. Calcif. Tissue Int. 2001, 69, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D. Biomechanical Aspects of Bone Repair, 2nd ed.; Elsevier Ltd: Barcelona, Spain, 2019; ISBN 9780081024515. [Google Scholar]
- Havaldar, R.; Pilli, S.C.; Putti, B.B. Insights into the effects of tensile and compressive loadings on human femur bone. Adv. Biomed. Res. 2014, 3, 101. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47, S11–S20. [Google Scholar] [CrossRef] [Green Version]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef]
- Chan, G.K.; Duque, G. Age-related bone loss: Old bone, new facts. Gerontology 2002, 48, 62–71. [Google Scholar] [CrossRef]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Pioletti, D.P. Biomechanics in bone tissue engineering. Comput. Methods Biomech. Biomed. Eng. 2010, 13, 837–846. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F. Functional Tissue Engineering. Ann. N. Y. Acad. Sci. 2002, 961, 193–195. [Google Scholar] [CrossRef]
- Zivic, F.; Grujovic, N.; Pellicer, E.; Sort, J.; Mitrovic, S.; Adamovic, D.; Vulovic, M. Biodegradable Metals as Biomaterials for Clinical Practice: Iron-based Materials; Springer: Berlin, Germany, 2017; ISBN 9783319680255. [Google Scholar]
- Kiani, F.; Wen, C.; Li, Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites—A review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef]
- Li, Y.; Wen, C.; Mushahary, D.; Sravanthi, R.; Harishankar, N.; Pande, G.; Hodgson, P. Mg-Zr-Sr alloys as biodegradable implant materials. Acta Biomater. 2012, 8, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Xu, Y.K.; Ma, H.; Xu, J.; Ma, E. Mg-based bulk metallic glass composites with plasticity and gigapascal strength. Acta Mater. 2005, 53, 1857–1866. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.; Weng, S.L.; Juang, Y.D.; Yao, C.K. Dual properties of zirconia coated porous titanium for a stiffness enhanced bio-scaffold. Mater. Des. 2017, 132, 13–21. [Google Scholar] [CrossRef]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Grill, A. Diamond-like carbon coatings as biocompatible materials—An overview. Diam. Relat. Mater. 2003, 12, 166–170. [Google Scholar] [CrossRef]
- Subramanian, B.; Muraleedharan, C.V.; Ananthakumar, R.; Jayachandran, M. A comparative study of titanium nitride (TiN), titanium oxy nitride (TiON) and titanium aluminum nitride (TiAlN), as surface coatings for bio implants. Surf. Coat. Technol. 2011, 205, 5014–5020. [Google Scholar] [CrossRef]
- Cruz, A.M.; Casañ-Pastor, N. Graded conducting titanium-iridium oxide coatings for bioelectrodes in neural systems. Thin Solid Films 2013, 534, 316–324. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.C.; Su, Y.H.; Yao, C.K.; Juang, Y.D. Hydrothermal fabrication of highly porous titanium bio-scaffold with a load-bearable property. Materials (Basel) 2017, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, B.; Sarhan, A.A.D.; Basirun, W.J.; Abas, W.A.B.W. Ceramic tantalum oxide thin film coating to enhance the corrosion and wear characteristics of Ti-6Al-4V alloy. J. Alloys Compd. 2016, 676, 369–376. [Google Scholar] [CrossRef]
- Gronostajski, Z.; Bandoła, P.; Skubiszewski, T. Argon-shielded hot pressing of titanium alloy (TI6AL4V) powders. Acta Bioeng. Biomech. 2010, 12, 41–46. [Google Scholar] [PubMed]
- Yan, M.; Xu, W.; Dargusch, M.S.; Tang, H.P.; Brandt, M.; Qian, M. Review of effect of oxygen on room temperature ductility of titanium and titanium alloys. Powder Metall. 2014, 57, 251–257. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Abboud, M.; Ramirez-Fernández, M.P.; Maté-Sánchez, J.E.; Negri, B.; Won, A.; Romanos, G. Porous titanium granules in critical size defects of rabbit tibia with or without membranes. Int. J. Oral Sci. 2014, 6, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunand, D.C. Processing of titanium foams. Adv. Eng. Mater. 2004, 6, 369–376. [Google Scholar] [CrossRef]
- Makena, I.M.; Shongwe, M.B.; Machaka, R.; Masete, M.S. Effect of spark plasma sintering temperature on the pore characteristics, porosity and compression strength of porous titanium foams. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Shbeh, M.; Oner, E.; Al-Rubaye, A.; Goodall, R. Production and Digital Image Correlation Analysis of Titanium Foams with Different Pore Morphologies as a Bone-Substitute Material. Adv. Mater. Sci. Eng. 2019, 2019, 1670837. [Google Scholar] [CrossRef] [Green Version]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan-Manshadi, A.; StJohn, D.H.; Dargusch, M.S.; Qian, M. Fabrication of highly porous titanium scaffolds using metal injection moulding and space holder. Euro PM 2018 Congr. Exhib. 2020. [Google Scholar]
- Malahias, M.A.; Kostretzis, L.; Greenberg, A.; Nikolaou, V.S.; Atrey, A.; Sculco, P.K. Highly Porous Titanium Acetabular Components in Primary and Revision Total Hip Arthroplasty: A Systematic Review. J. Arthroplast. 2020, 6, 1737–1749. [Google Scholar] [CrossRef]
- Solutions, S.; Classification, D. Gription TF®. 2015. Available online: http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_DePuy_PDFs/DSEM-JRC-0315-0282_LR.pdf?fbclid=IwAR0zh8WmU4eYdCXrWNHkvo8G97H4KrIBuvo-O-GG40dBZ8EV1vJR56jUL6o (accessed on 7 May 2020).
- Ghouse, S.; Babu, S.; Nai, K.; Hooper, P.A.; Jeffers, J.R.T. The influence of laser parameters, scanning strategies and material on the fatigue strength of a stochastic porous structure. Addit. Manuf. 2018, 22, 290–301. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Brooks, W.K.; Jones, E.; Sutcliffe, C.J. Selective laser melting: A regular unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 325–334. [Google Scholar] [CrossRef]
- Wauthle, R.; Van Der Stok, J.; Yavari, S.A.; Van Humbeeck, J.; Kruth, J.P.; Zadpoor, A.A.; Weinans, H.; Mulier, M.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef]
- Torres, Y.; Trueba, P.; Pavón, J.J.; Chicardi, E.; Kamm, P.; García-Moreno, F.; Rodríguez-Ortiz, J.A. Design, processing and characterization of titanium with radial graded porosity for bone implants. Mater. Des. 2016, 110, 179–187. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; Suzuki, J.; Kokubo, T.; Nakamura, T. Mechanical properties and osteoconductivity of porous bioactive titanium. Biomaterials 2005, 26, 6014–6023. [Google Scholar] [CrossRef]
- Itl, A.I.; Ylnen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone ingrowth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef]
- Takemoto, M.; Fujibayashi, S.; Otsuki, B.; Matsushita, T.; Kokubo, T.; Nakamura, T. 3-D analysis of pore structure of porous biomaterials using micro focus X-ray computed tomography. Key Eng. Mater. 2006, 309, 1095–1098. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Morejón, L.; Delgado, J.A.; Ribeiro, A.A.; de Oliveira, M.V.; Mendizábal, E.; García, I.; Alfonso, A.; Poh, P.; van Griensven, M.; Balmayor, E.R. Development, characterization and in vitro biological properties of scaffolds fabricated from calcium phosphate nanoparticles. Int. J. Mol. Sci. 2019, 20, 1790. [Google Scholar] [CrossRef] [Green Version]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite Scaffolds for Bone Tissue Engineering. Bioceram. Dev. Appl. 2017, 7, 5025. [Google Scholar]
- Chen, C.; Li, Y.; Zhang, M.; Wang, X.; Zhang, C.; Jing, H. Effect of laser processing parameters on mechanical properties of porous tantalum fabricated by laser multi-layer micro-cladding. Rapid Prototyp. J. 2017, 23, 758–770. [Google Scholar] [CrossRef]
- Kuromoto, N.K.; Simão, R.A.; Soares, G.A. Titanium oxide films produced on commercially pure titanium by anodic oxidation with different voltages. Mater. Charact. 2007, 58, 114–121. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Lv, K.; Zhang, W.; Ding, X.; Yang, G.; Liu, X.; Jiang, X. Surface thermal oxidation on titanium implants to enhance osteogenic activity and in vivo osseointegration. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nouri, A.; Hodgson, P.D.; Wen, C. Biomimetic Porous Titanium Scaffolds for Orthopaedic and Dental Applications; InTech: Rijek, Croatia, 2010; pp. 415–450. [Google Scholar]
- Khoda, A.K.M.B.; Koc, B. Functionally heterogeneous porous scaffold design for tissue engineering. CAD Comput. Aided Des. 2013, 45, 1276–1293. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D. Heterogeneous minimal surface porous scaffold design using the distance field and radial basis functions. Med. Eng. Phys. 2012, 34, 625–639. [Google Scholar] [CrossRef] [PubMed]
- ISO-5833. Implants for Surgery-Acrylic Resin Cements; International Organization for Standardization: Geneva, Switzerland, 2002.
- ISO-10993-5. Biological Evaluation of Medical Devices—Part 5: Tests for in vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009.
- Cimatti, B.; Engel, E.E.; Nogueira-Barbosa, M.H.; Frighetto, P.D.; Volpon, J.B. Physical and mechanical characterization of a porous cement for metaphyseal bone repair. Acta Orthop. Bras. 2015, 23, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Graaf, G.M.M.; de Zoppa, A.L.D.V.; Moreira, R.C.; Maestrelli, S.C.; Marques, R.F.C.; Campos, M.G.N. Morphological and mechanical characterization of chitosan-calcium phosphate composites for potential application as bone-graft substitutes. Rev. Bras. Eng. Biomed. 2015, 31, 334–342. [Google Scholar] [CrossRef]
- Edward Hoffler, C.; Edward Guo, X.; Zysset, P.K.; Goldstein, S.A. An application of nanoindentation technique to measure bone tissue lamellae properties. J. Biomech. Eng. 2005, 127, 1046–1053. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials (Basel) 2017, 10, 884. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, U.; Ellis, J.K.; Wagh, V.; Nemade, H.; Hescheler, J.; Keun, H.C.; Sachinidis, A. Metabolite signatures of doxorubicin induced toxicity in human induced pluripotent stem cell-derived cardiomyocytes. Amino Acids 2017, 49, 1955–1963. [Google Scholar] [CrossRef]
- Grinnell, F. Cellular Adhesiveness and Extracellular Substrata. Int. Rev. Cytol. 1978, 53, 65–144. [Google Scholar]
- Altankov, G.; Grinnell, F.; Groth, T. Studies on the biocompatibility of materials: Fibroblast reorganization of substratum-bound fibronectin on surfaces varying in wettability. J. Biomed. Mater. Res. 1996, 30, 385–391. [Google Scholar] [CrossRef]
- Liu, X.; Lim, J.Y.; Donahue, H.J.; Dhurjati, R.; Mastro, A.M.; Vogler, E.A. Influence of substratum surface chemistry/energy and topography on the human fetal osteoblastic cell line hFOB 1.19: Phenotypic and genotypic responses observed in vitro. Biomaterials 2007, 28, 4535–4550. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.; Liu, X.; Vogler, E.A.; Donahue, H.J. Systematic variation in osteoblast adhesion and phenotype with substratum surface characteristics. J. Biomed. Mater. Res. Part A 2004, 68, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, M.; Jalal, S.M.; Rickard, D.J.; Harris, S.A.; Bolander, M.E.; Spelsberg, T.C. Further characterization of human fetal osteoblastic hFOB 1.19 and hFOB/ERα cells: Bone formation in vivo and karyotype analysis using multicolor fluorescent in situ hybridization. J. Cell. Biochem. 2002, 87, 9–15. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Liao, J.-D.; Guo, Y.-S.; Juang, Y.-D. Biomimetic Design for a Dual Concentric Porous Titanium Scaffold with Appropriate Compressive Strength and Cells Affinity. Materials 2020, 13, 3316. https://doi.org/10.3390/ma13153316
Lee H, Liao J-D, Guo Y-S, Juang Y-D. Biomimetic Design for a Dual Concentric Porous Titanium Scaffold with Appropriate Compressive Strength and Cells Affinity. Materials. 2020; 13(15):3316. https://doi.org/10.3390/ma13153316
Chicago/Turabian StyleLee, Han, Jiunn-Der Liao, Yao-Sheng Guo, and Yung-Der Juang. 2020. "Biomimetic Design for a Dual Concentric Porous Titanium Scaffold with Appropriate Compressive Strength and Cells Affinity" Materials 13, no. 15: 3316. https://doi.org/10.3390/ma13153316
APA StyleLee, H., Liao, J. -D., Guo, Y. -S., & Juang, Y. -D. (2020). Biomimetic Design for a Dual Concentric Porous Titanium Scaffold with Appropriate Compressive Strength and Cells Affinity. Materials, 13(15), 3316. https://doi.org/10.3390/ma13153316





