Study on Preparation of Ultra-High-Molecular-Weight Polyethylene Pipe of Good Thermal-Mechanical Properties Modified with Organo-Montmorillonite by Screw Extrusion
Abstract
1. Introduction
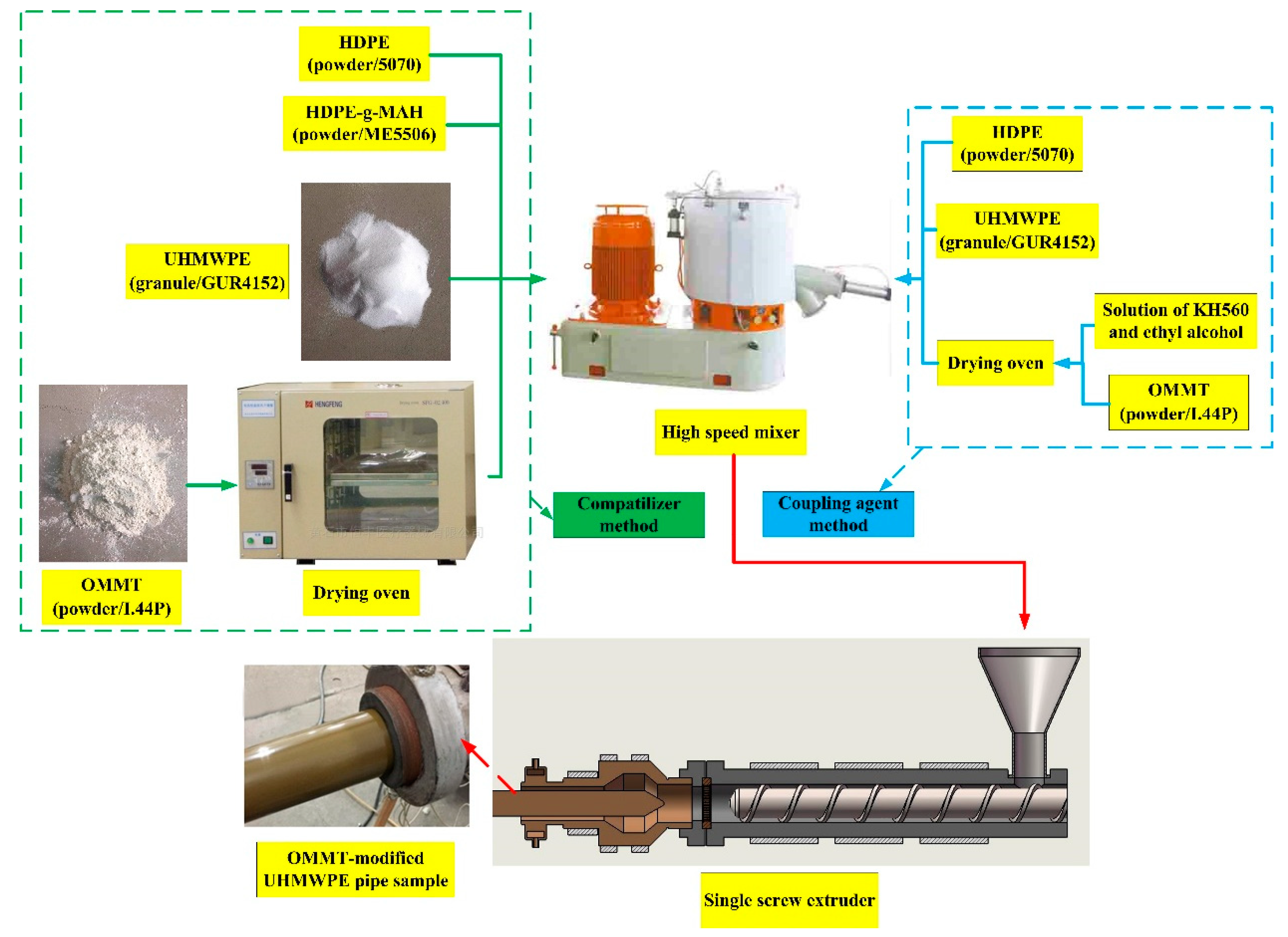
2. Preparation of OMMT-Modified UHMWPE Pipe Samples
2.1. Materials
2.2. Experimental Equipment
2.3. Preparation of OMMT-Modified UHMWPE Pipe Samples
- (a)
- For the compatibilizer method, the OMMT was kept in a drying oven at a constant temperature of 110 °C for four hours. Then, UHMWPE, HDPE, OMMT, HDPE-g-MAH, and other additives were added into a mixer for homogeneous mixing.
- (b)
- For the coupling agent method, a solution of KH560 and ethyl alcohol was prepared with a volume ratio of 1:10. Then, the solution was premixed uniformly with OMMT, and the mixture was kept in a drying oven at a constant temperature of 110 °C for four hours. Lastly, UHMWPE, HDPE, OMMT, KH560, and other additives were added into a mixer for homogeneous mixing.
2.4. Characterization of OMMT-Modified UHMWPE Pipe
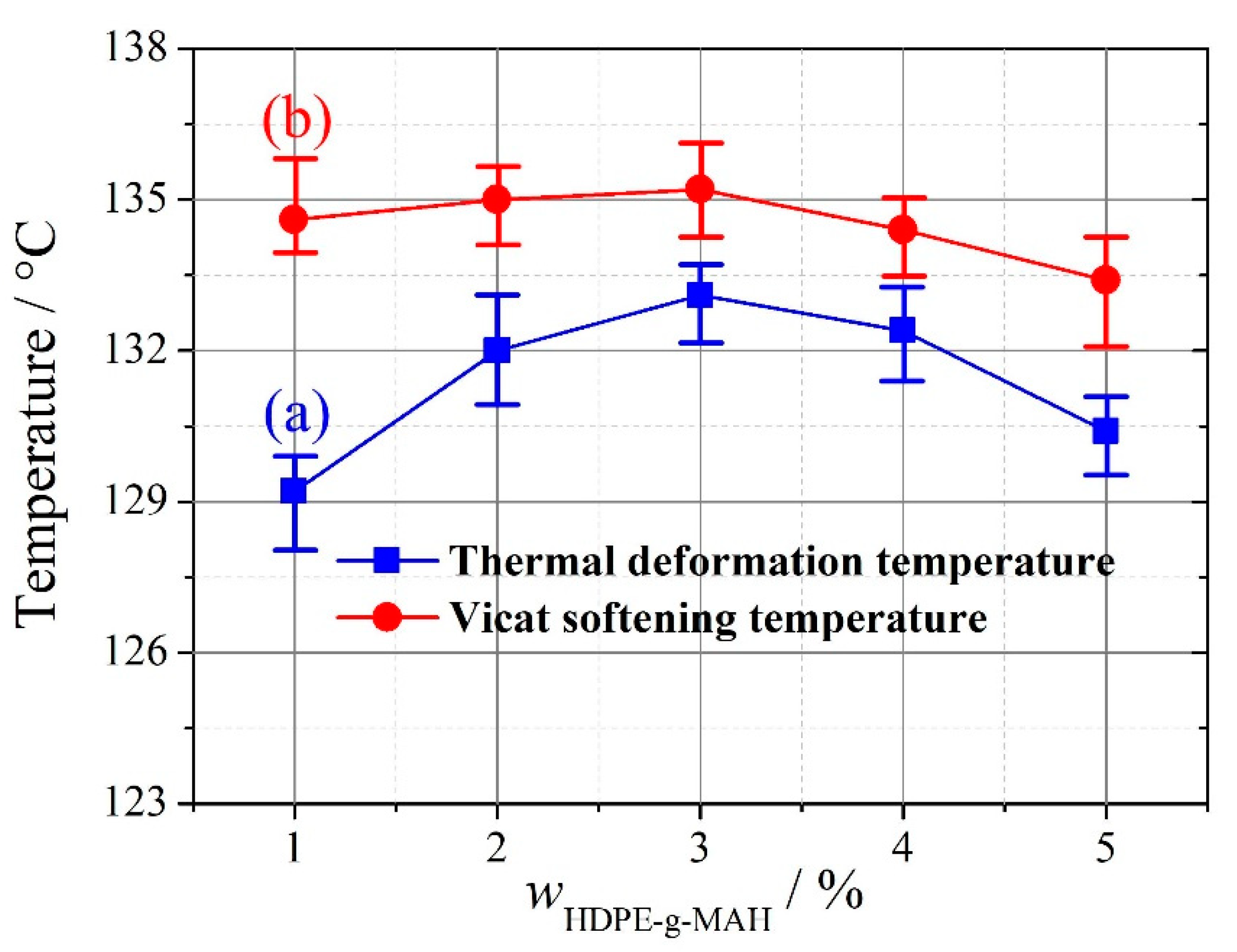
2.4.1. Vicat Softening Temperature and Thermal Deformation Temperature
2.4.2. Shore Hardness
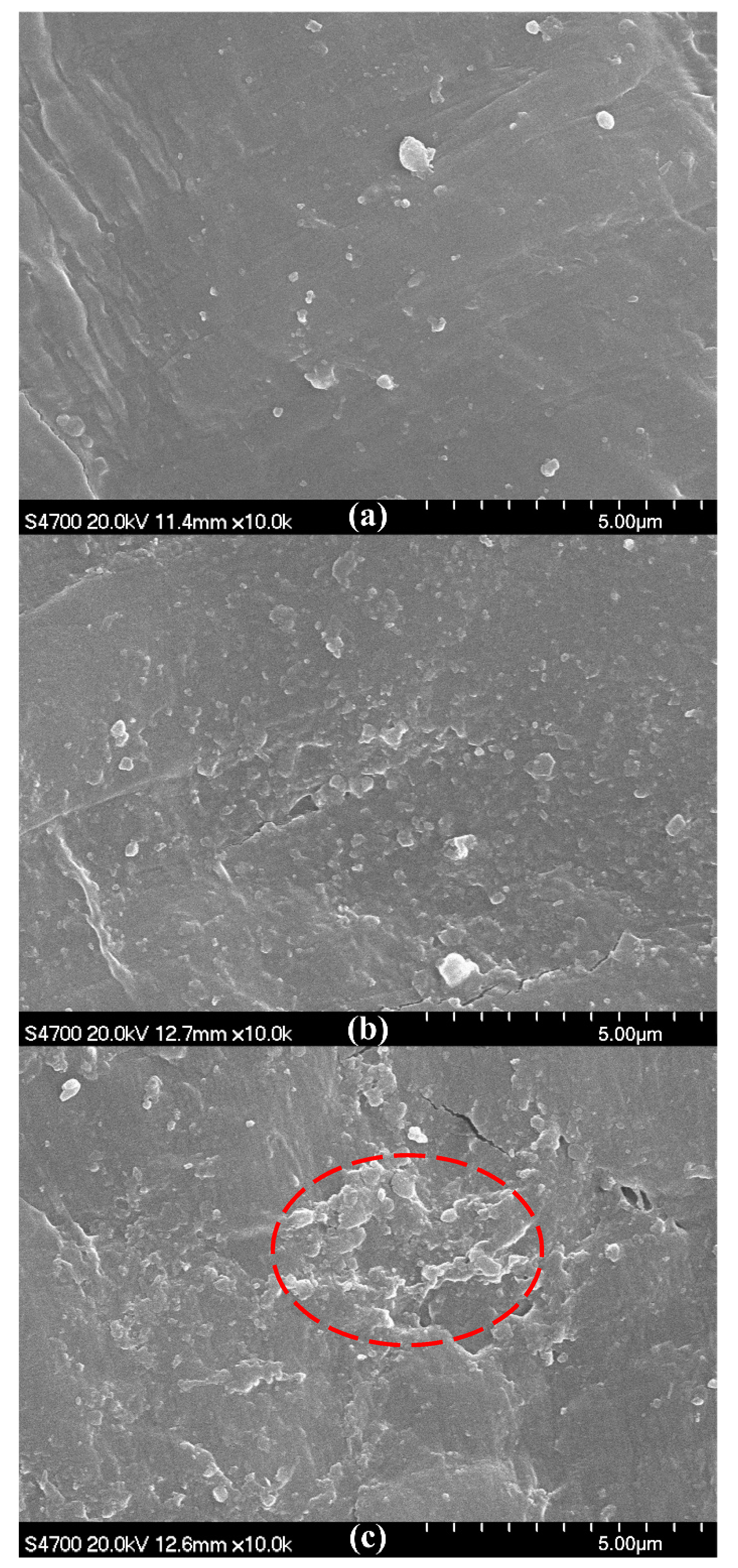
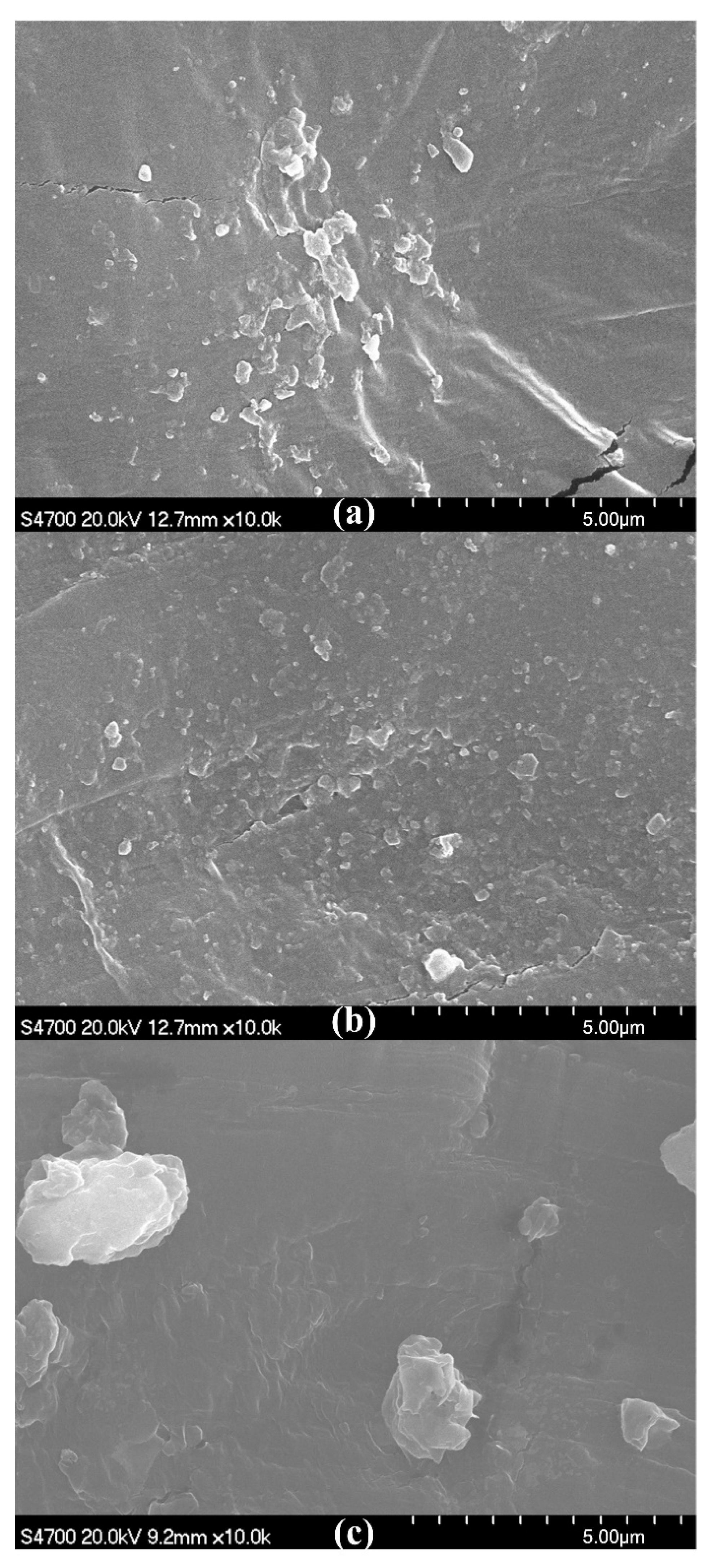
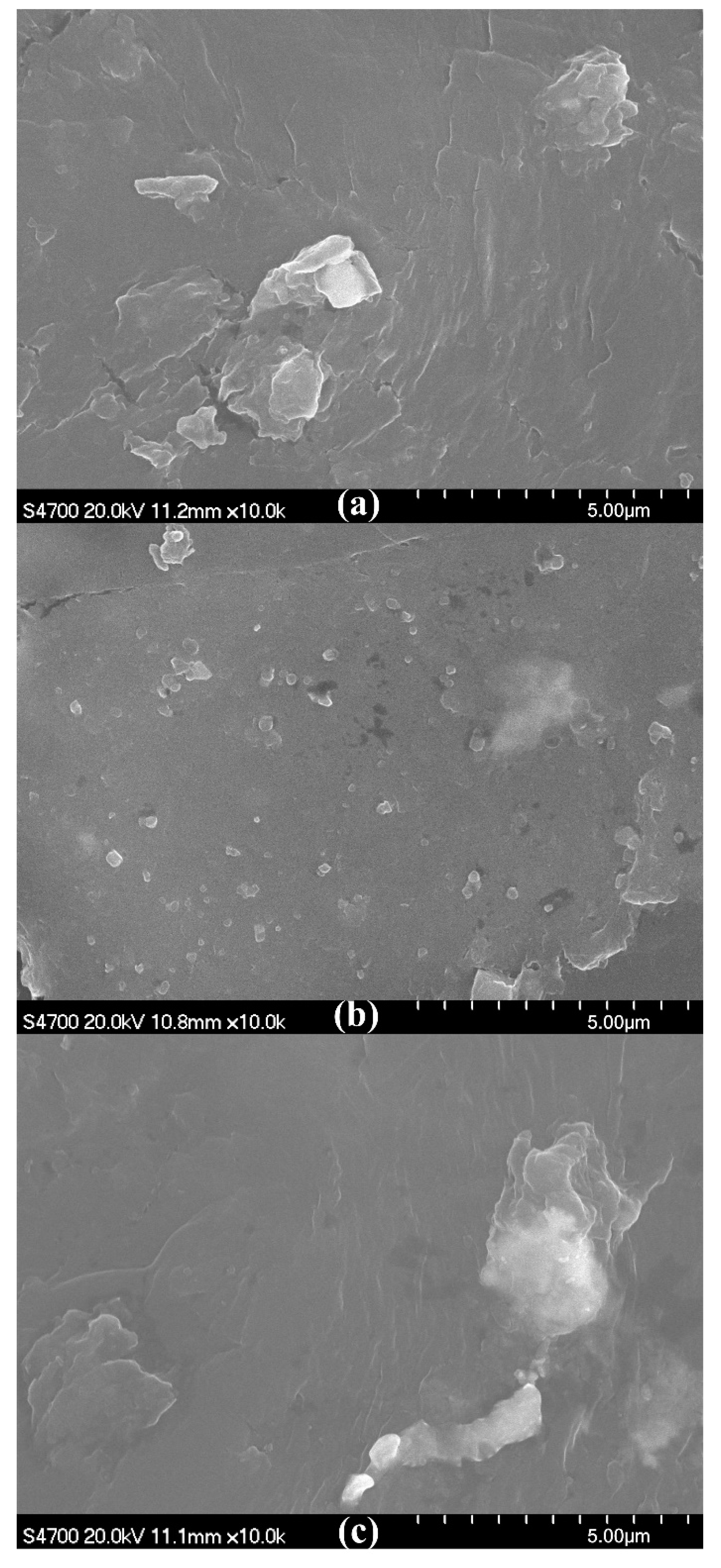
2.4.3. SEM
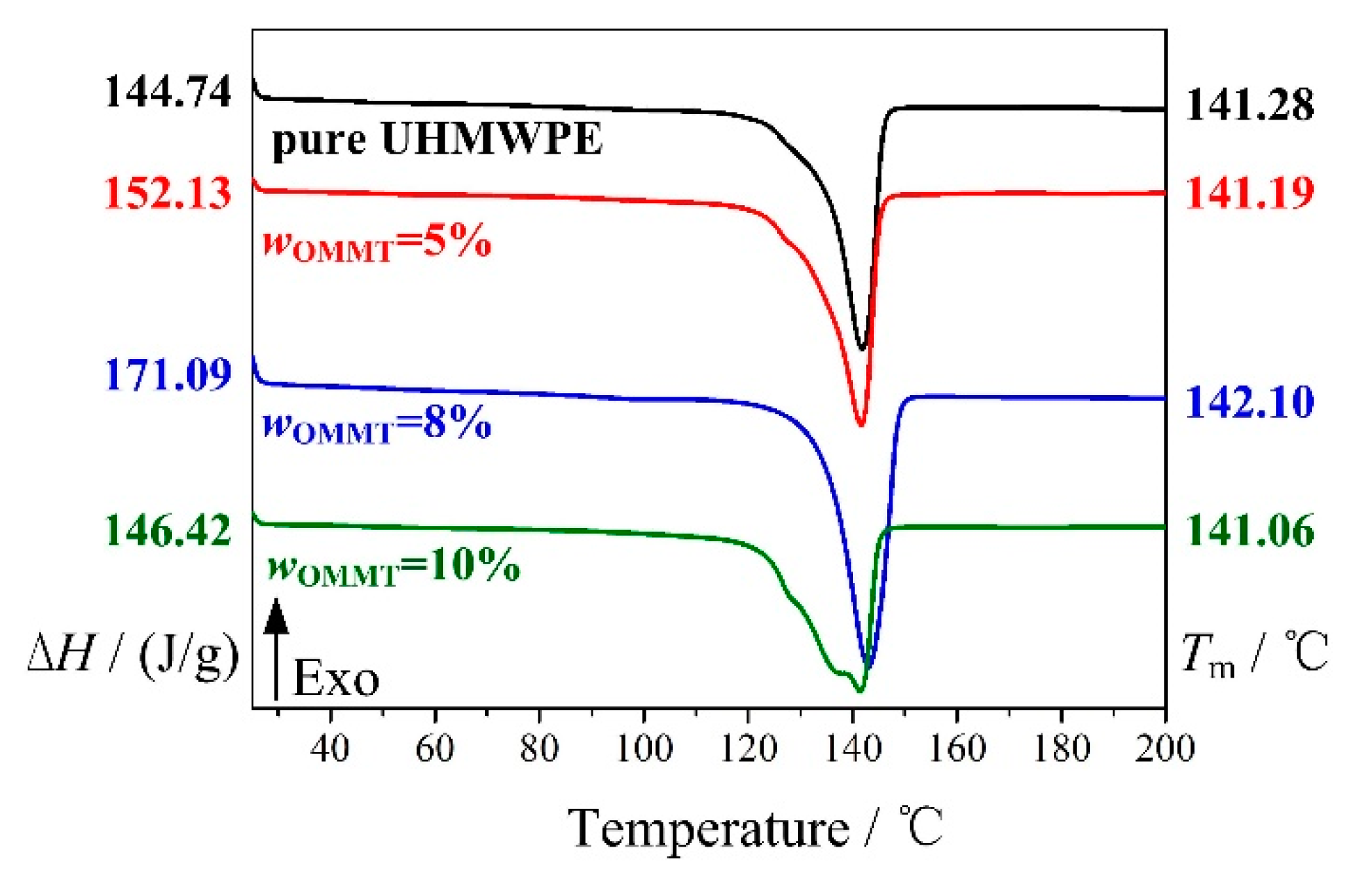
2.4.4. DSC
2.4.5. Bending Strength and Tensile Strength
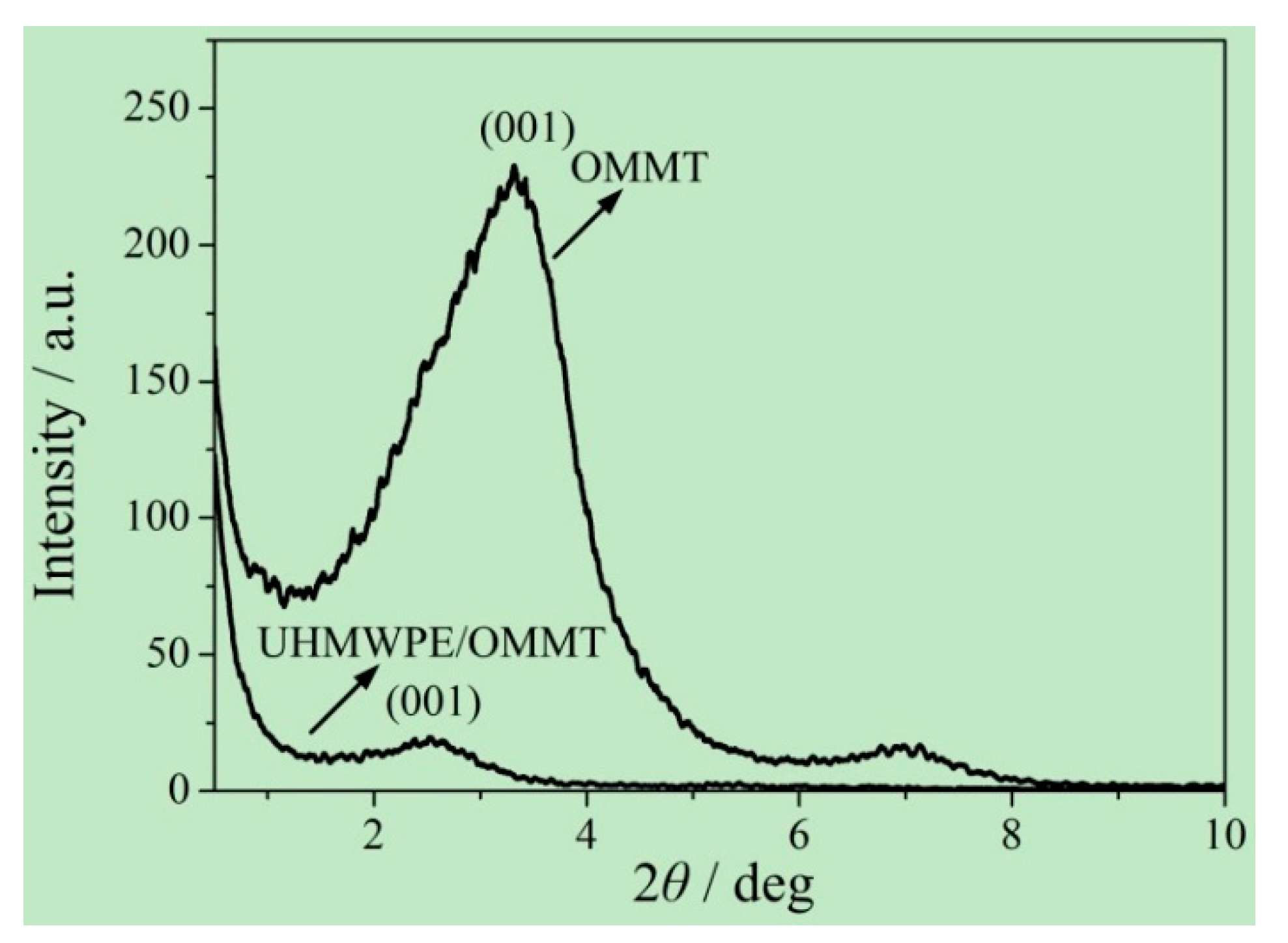
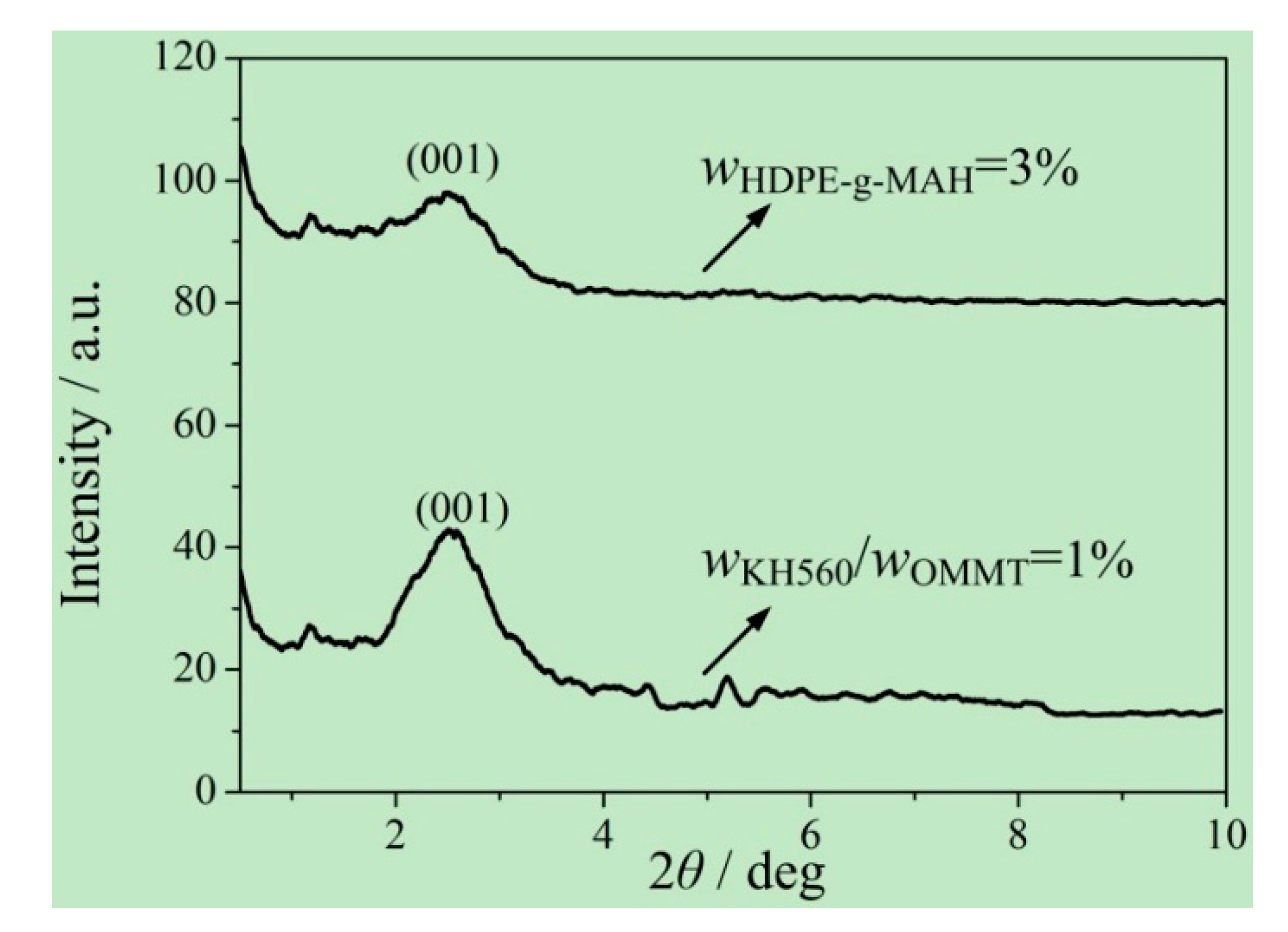
2.4.6. Small-Angle X-ray Diffraction
3. Thermal-Mechanical Property Management
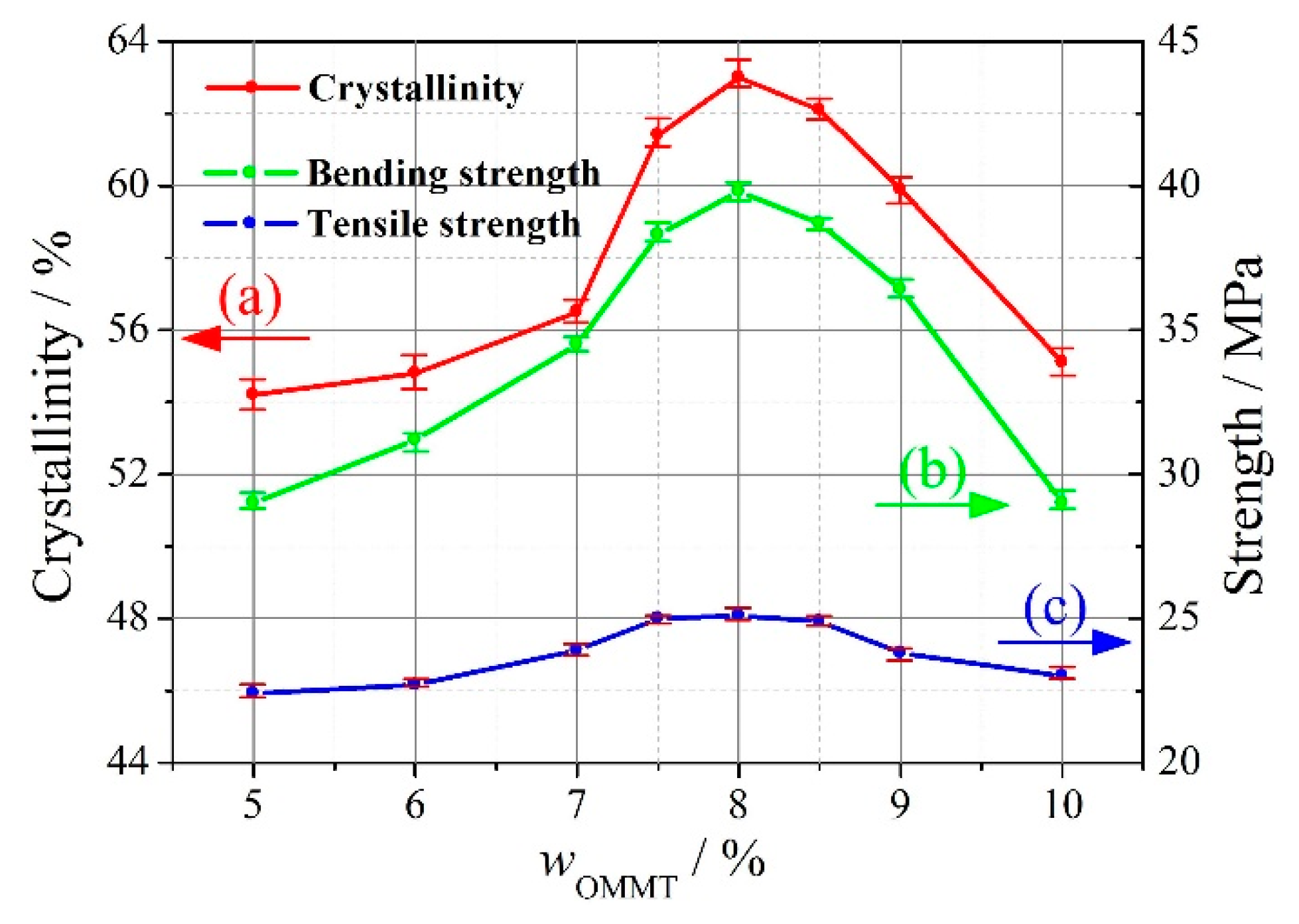
3.1. Addition Amount of OMMT
3.2. Addition Amount of HDPE-g-MAH
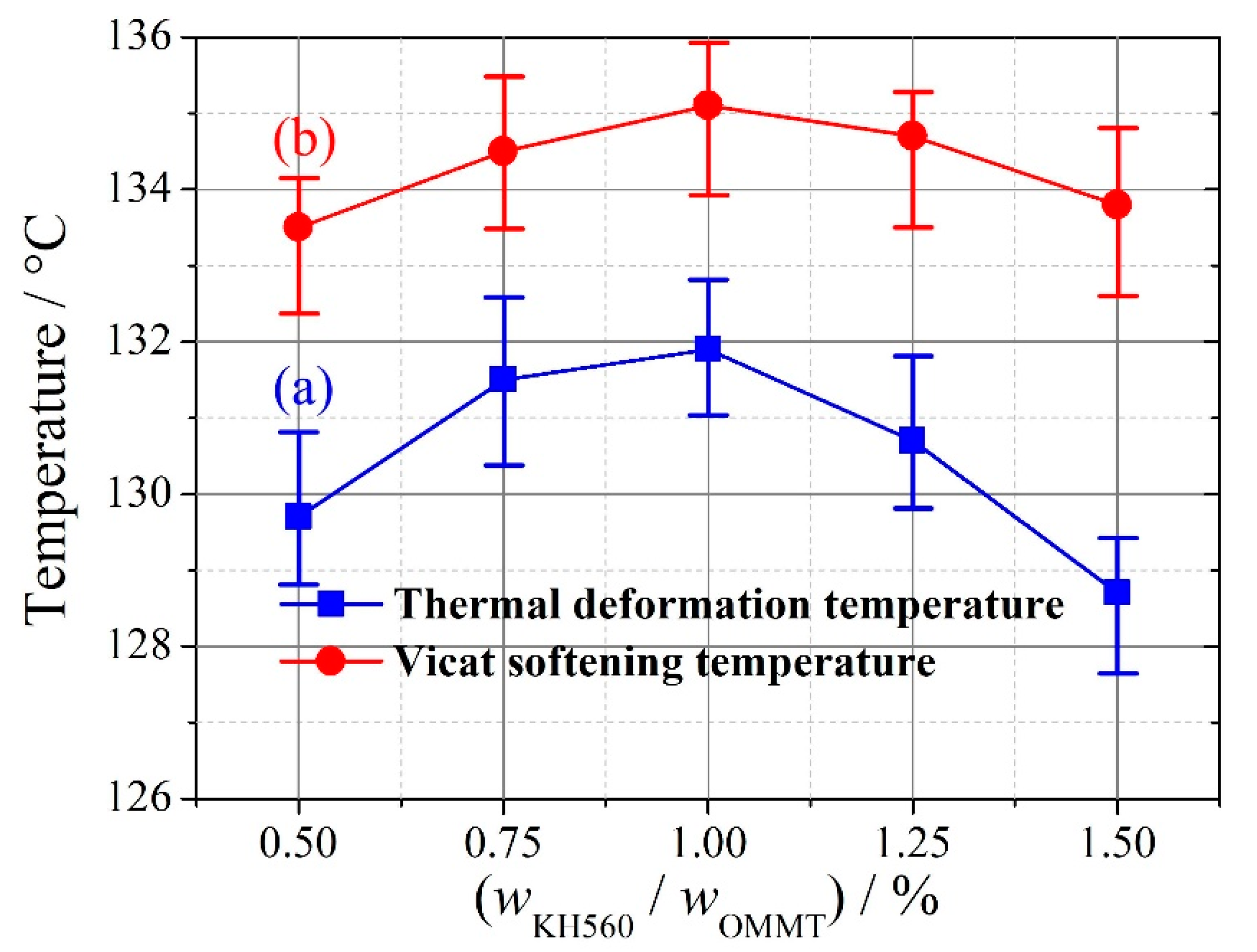
3.3. Addition Amount of KH560
4. Conclusions
- (1)
- screw extrusion molding of UHMWPE was feasible by the modification of UHMWPE;
- (2)
- for the process parameters in this study, the reasonable addition amounts of OMMT and HDPE-g-MAH were 8% and 3%, respectively, for the compatibilizer method, and the reasonable addition amount of KH560 was wKH560/wOMMT = 1% for the coupling agent method;
- (3)
- with the management of thermal-mechanical properties, OMMT-modified UHMWPE pipe with good thermal-mechanical properties was able to be prepared by extrusion for the first time; and
- (4)
- the compatibilizer method of HDPE-g-MAH was slightly more effective than the coupling agent method of KH560.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Biedroń, I.; Traczewska, T.; Konieczny, T.; Płaza, G. Characterization of biofilms from selected synthetic materials used in water distribution system. J. Ecol. Eng. 2017, 18, 284–293. [Google Scholar] [CrossRef]
- He, X.; Wang, Y.; Wang, Q.; Tang, Y.; Liu, B. Effects of addition of ultra-high molecular weight polyethylene on tie-molecule and crystallization behavior of unimodal PE-100 pipe materials. J. Macromol. Sci. 2016, 55, 1007–1021. [Google Scholar] [CrossRef]
- Li, W.; Hui, L.; Xue, B.; Dong, C.; Chen, Y.; Hou, L.; Jiang, B.; Wang, J.; Yang, Y. Facile high-temperature synthesis of weakly entangled polyethylene using a highly activated Ziegler-Natta catalyst. J. Catal. 2018, 360, 145–151. [Google Scholar] [CrossRef]
- Tuskaev, V.A.; Gagieva, S.C.; Kurmaev, D.A.; Fedyanin, I.V.; Zubkevich, S.V.; Bulychev, B.M. New titanium(IV) coordination compounds with 2-hydroxybenzyl alcohol derivatives used in the preparation of ultra-high molecular weight polyethylene. Russ. Chem. Bull. 2018, 67, 377–381. [Google Scholar] [CrossRef]
- Camacho, N.; Franco-Urquiza, E.A.; Stafford, S.W. Wear performance of multiwalled carbon nanotube-reinforced ultra-high molecular weight polyethylene composite. Adv. Polym. Technol. 2018, 37, 2261–2269. [Google Scholar] [CrossRef]
- Yilmaz, G.; Ellingham, T.; Turng, L.-S. Injection and injection compression molding of ultra-high-molecular weight polyethylene powder. Polym. Eng. Sci. 2019, 59, E170–E179. [Google Scholar] [CrossRef]
- Dvilis, E.S.; Panin, S.V.; Alexenko, V.O.; Kornienko, L.A.; Buslovich, D.G.; Tolkachev, O.S.; Ovechkin, B.B.; Alishin, T.R. Structure and tribomechanical properties of polymer compacts fabricated by ultrasonic consolidation and compression moulding of UHMWPE powder. AIP Conf. Proc. 2018, 2051, 020070. [Google Scholar]
- Sun, P.; Qian, T.Y.; Ji, X.Y.; Wu, C.; Yan, Y.S.; Qi, R.R. HDPE/UHMWPE composite foams prepared by compression molding with optimized foaming capacity and mechanical properties. J. Appl. Polym. Sci. 2018, 135, 46768. [Google Scholar] [CrossRef]
- Feng, Y.; Gao, Y.; Chen, J.; Jiang, J.; Yin, X.; He, G.; Zeng, Y.; Kuang, Q.; Qu, J. Properties of compression molded ultra-high molecular weight polyethylene products pretreated by eccentric rotor extrusion. Polym. Int. 2019, 68, 862–870. [Google Scholar] [CrossRef]
- Smith, P.; Visjager, J.; Tervoort, T. Sintering Ultrahigh Molecular Weight Polyethylene. U.S. Patent 7,550,555, 14 April 2011. [Google Scholar]
- Jaberzadeh, M. Fabrication and characterization of UHMWPE reinforced by forsterite nano crystallites as an implant biocomposite. Ceram. Silik. 2016, 60, 1–10. [Google Scholar] [CrossRef][Green Version]
- Panin, S.V.; Kornienko, L.A.; Alexenko, V.O.; Buslovich, D.G.; Dontsov, Y.V. Extrudable polymer-polymer composites based on ultra-high molecular weight polyethylene. AIP Conf. Proc. 2017, 1915, 020005. [Google Scholar]
- da Silva, B.C.; dos Santos, C.M.; de Oliveira Couto, C.A.; Backes, E.H.; Passador, F.R. Evaluation of aging resistance in UHMWPE/LLDPE blend-based carbon nanotubes nanocomposites. Macromol. Symp. 2019, 383, 1700079. [Google Scholar] [CrossRef]
- Chaudhuri, K.; Poddar, S.; Pol, H.; Lele, A.; Mathur, A.; Srinivasa Rao, G.S.; Jasra, R. The effect of processing conditions on the rheological properties of blends of ultra high molecular weight polyethylene with high-density polyethylene. Polym. Eng. Sci. 2019, 59, 821–829. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Ma, Y.; Geng, Y.; Tan, J. Rheological and mechanical properties of ultrahigh molecular weight polyethylene/high density polyethylene/polyethylene glycol blends. Adv. Ind. Eng. Polym. Res. 2019, 2, 51–60. [Google Scholar] [CrossRef]
- Liu, L.; Wang, F.; Xue, P.; Wang, S. Influence of interfacial condition on rheological instability behavior of UHMWPE/HDPE/nano-SiO2 blends in capillary extrusion. Rheol. Acta 2019, 58, 3–4. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, Y.; Li, Y.; Zhang, G. Effect of OMMT on microstructure, crystallisation and rheological behaviour of UHMWPE/PP nanocomposites under elongation flow. Plast. Rubber Compos. 2018, 47, 315–323. [Google Scholar] [CrossRef]
- Kuang, T.; Chang, L.; Fu, D.; Yang, J.; Zhong, M.; Chen, F.; Peng, X. Improved crystallizability and processability of ultra high molecular weight polyethylene modified by poly(amido amine) dendrimers. Polym. Eng. Sci. 2017, 57, 153–160. [Google Scholar] [CrossRef]
- Ma, H.; Chen, X.; Hsiao, B.S.; Chu, B. Improving toughness of ultra-high molecular weight polyethylene with ionic liquid modified carbon nanofiber. Polymer 2014, 55, 160–165. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Li, R.; Xing, Z.; Xu, L.; Wang, M.; Guo, X.; Wu, G. Radiation synthesis of a new amidoximated UHMWPE fibrous adsorbent with high adsorption selectivity for uranium over vanadium in simulated seawater. Radiat. Phys. Chem. 2016, 122, 1–8. [Google Scholar] [CrossRef]
- Shitz, E.Y.; Koriakina, V.V. Polyolefins structure formation effected by addition of industrial natural diamond powders. Int. J. Abras. Technol. 2015, 7, 14. [Google Scholar] [CrossRef]
- Chunzheng, P. Improved interfacial properties of carbon fiber/UHMWPE composites through surface coating on carbon fiber surface. Surf. Interface Anal. 2018, 50, 558–563. [Google Scholar] [CrossRef]
- Benkocká, M.; Lupínková, S.; Knapová, T.; Kolářová, K.; Matoušek, J.; Slepička, P.; Švorčík, V.; Kolská, Z. Antimicrobial and photophysical properties of chemically grafted ultra-high-molecular-weight polyethylene. Mater. Sci. Eng. 2019, 96, 479–486. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Domínguez-Díaz, M.; Paniagua, R.; Hernández-Pérez, J.A.; Martínez, H. Microhardness modification of ultrahigh-molecular-weight polyethylene by oxygen plasma: Effect of the polymer crosslinking. Nucl. Instrum. Methods Phys. Res. Sect. B: Beam Interact. Mater. At. 2019, 445, 8–12. [Google Scholar]
- Van Vrekhem, S.; Vloebergh, K.; Asadian, M.; Vercruysse, C.; Declercq, H.; Van Tongel, A.; De Wilde, L.; De Geyter, N.; Morent, R. Improving the surface properties of an UHMWPE shoulder implant with an atmospheric pressure plasma jet. Sci. Rep. 2018, 8, 4720. [Google Scholar] [CrossRef] [PubMed]
- Scolaro, C.; Visco, A.; Torrisi, L.; Restuccia, N.; Pedullà, E. Modification induced by laser irradiation on physical features of plastics materials filled with nanoparticles. EPJ Web Conf. 2018, 167, 5008. [Google Scholar] [CrossRef][Green Version]
- Panin, S.V.; Kornienko, L.A.; Huang, Q.; Buslovich, D.G.; Bochkareva, S.A.; Alexenko, V.O.; Berto, F. Effect of adhesion on mechanical and tribological properties of glass fiber composites, based on ultra-high molecular weight polyethylene powders with various initial particle sizes. Materials 2020, 13, 1601–1626. [Google Scholar] [CrossRef] [PubMed]

| Machine Barrel Temperature/°C | Mold Temperature/°C | Water Jacket | Screw Speed | ||||
|---|---|---|---|---|---|---|---|
| Zone #1 | Zone #2 | Zone #3 | Zone #1 | Zone #2 | Zone #3 | Temperature/°C | /rpm |
| 120 | 220 | 270 | 245 | 160 | 120 | 20 | 3 |
| Parameter | Value |
|---|---|
| Vicat Softening Temperature/°C | 128 |
| Thermal Deformation Temperature/°C | 84.4 |
| Shore Hardness/HD | 69 |
| Melting Enthalpy/(J/g) | 144.74 |
| Bending Strength/MPa | 27.3 |
| Tensile Strength/MPa | 20.8 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| OMMT | 5 | 6 | 7 | 7.5 | 8 | 8.5 | 9 | 10 |
| HDPE-g-MAH | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| UHMWPE | 59 | 58 | 57 | 56.5 | 56 | 55.5 | 55 | 54 |
| HDPE | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Other Additives | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| No. | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|
| OMMT | 8 | 8 | 8 | 8 | 8 |
| HDPE-g-MAH | 1 | 2 | 3 | 4 | 5 |
| UHMWPE | 58 | 57 | 56 | 55 | 54 |
| HDPE | 30 | 30 | 30 | 30 | 30 |
| Other Additives | 3 | 3 | 3 | 3 | 3 |
| No. | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|
| OMMT | 8 | 8 | 8 | 8 | 8 |
| KH560 | 0.04 | 0.06 | 0.08 | 0.10 | 0.12 |
| UHMWPE | 58.96 | 58.94 | 58.92 | 58.90 | 58.88 |
| HDPE | 30 | 30 | 30 | 30 | 30 |
| Other Additives | 3 | 3 | 3 | 3 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Xu, R.; Xue, P. Study on Preparation of Ultra-High-Molecular-Weight Polyethylene Pipe of Good Thermal-Mechanical Properties Modified with Organo-Montmorillonite by Screw Extrusion. Materials 2020, 13, 3342. https://doi.org/10.3390/ma13153342
Guo Z, Xu R, Xue P. Study on Preparation of Ultra-High-Molecular-Weight Polyethylene Pipe of Good Thermal-Mechanical Properties Modified with Organo-Montmorillonite by Screw Extrusion. Materials. 2020; 13(15):3342. https://doi.org/10.3390/ma13153342
Chicago/Turabian StyleGuo, Zhouchao, Rui Xu, and Ping Xue. 2020. "Study on Preparation of Ultra-High-Molecular-Weight Polyethylene Pipe of Good Thermal-Mechanical Properties Modified with Organo-Montmorillonite by Screw Extrusion" Materials 13, no. 15: 3342. https://doi.org/10.3390/ma13153342
APA StyleGuo, Z., Xu, R., & Xue, P. (2020). Study on Preparation of Ultra-High-Molecular-Weight Polyethylene Pipe of Good Thermal-Mechanical Properties Modified with Organo-Montmorillonite by Screw Extrusion. Materials, 13(15), 3342. https://doi.org/10.3390/ma13153342




