Microstructural Characterisation of Austenitic Heat Resistant Sanicro 25 Steel after Steam Oxidation
Abstract
:1. Introduction
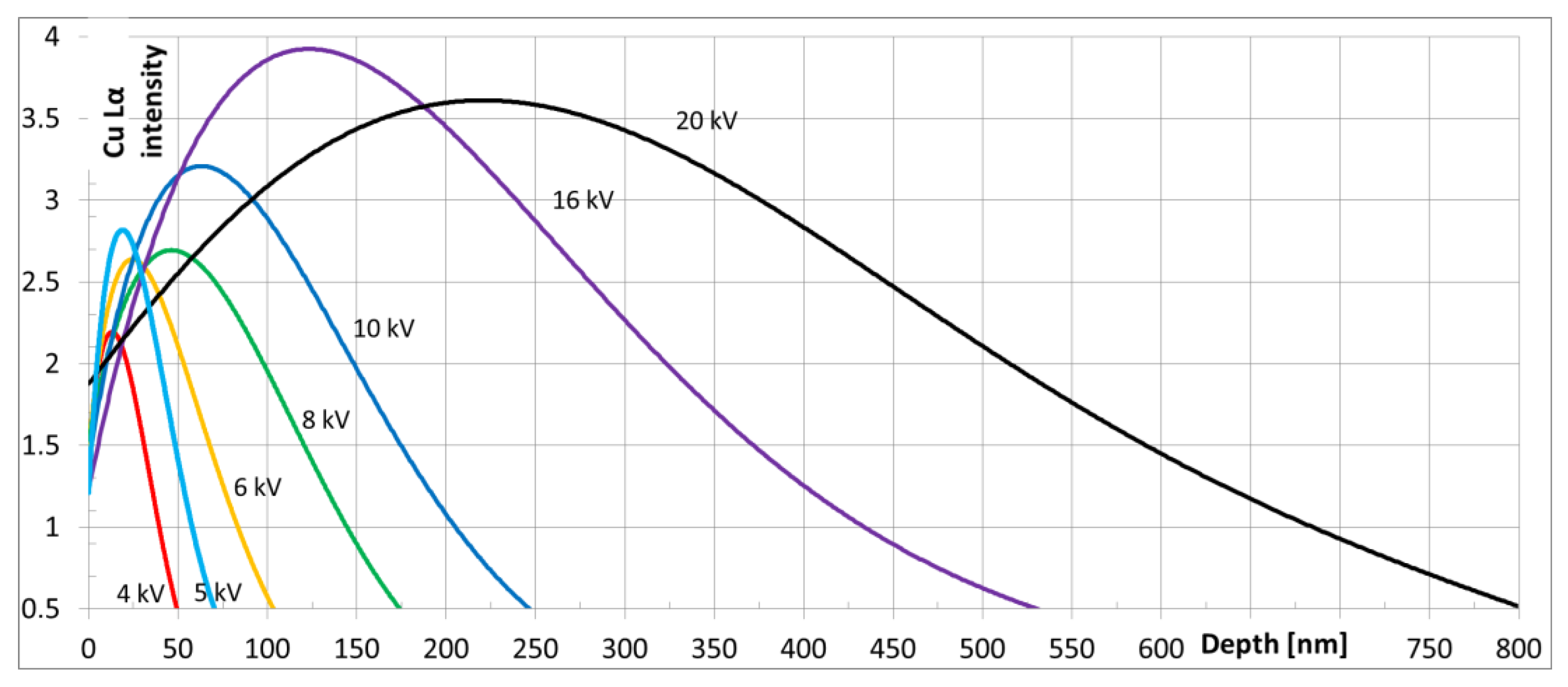
2. Materials and Methods
3. Results and Discussion
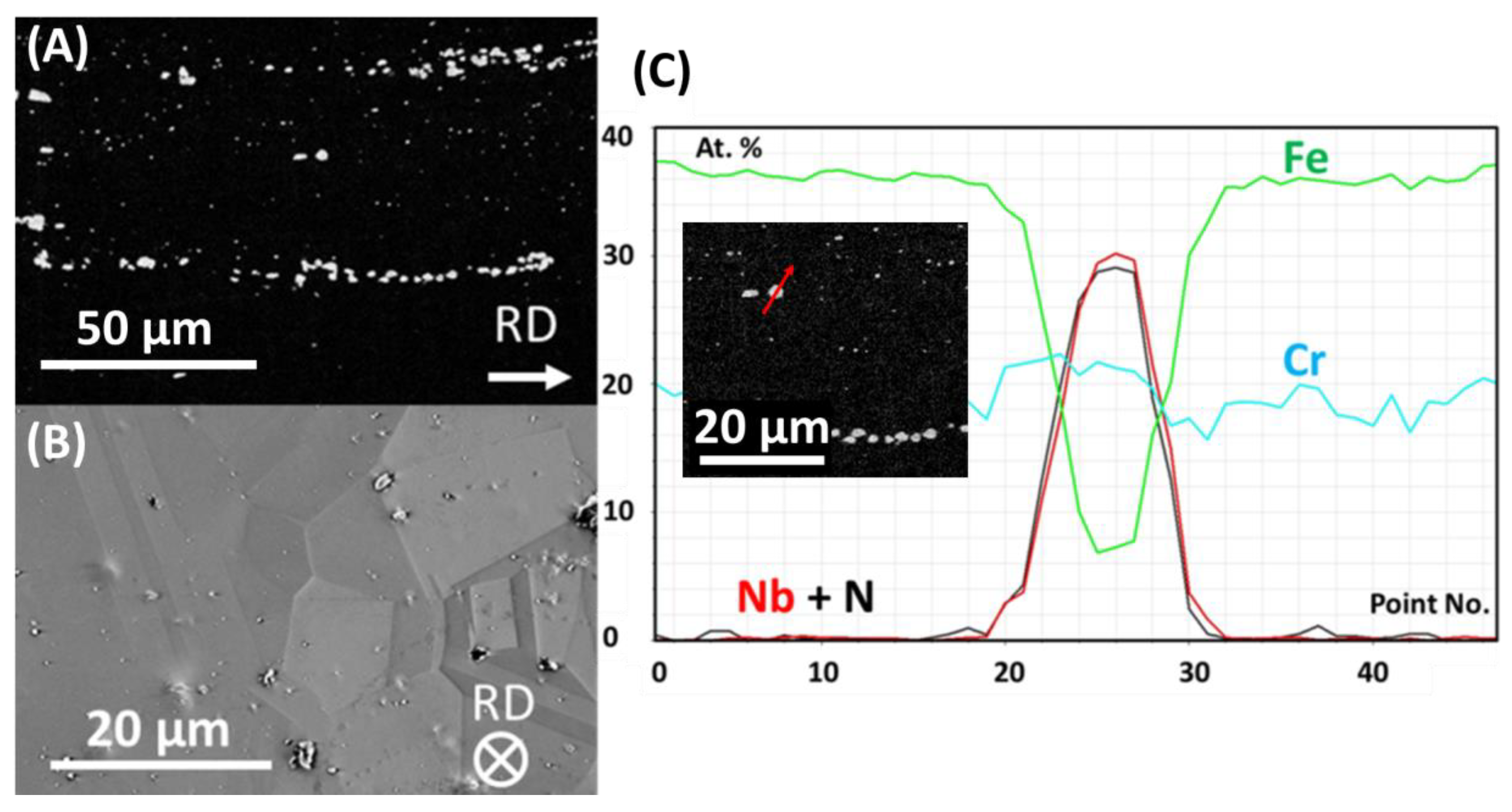
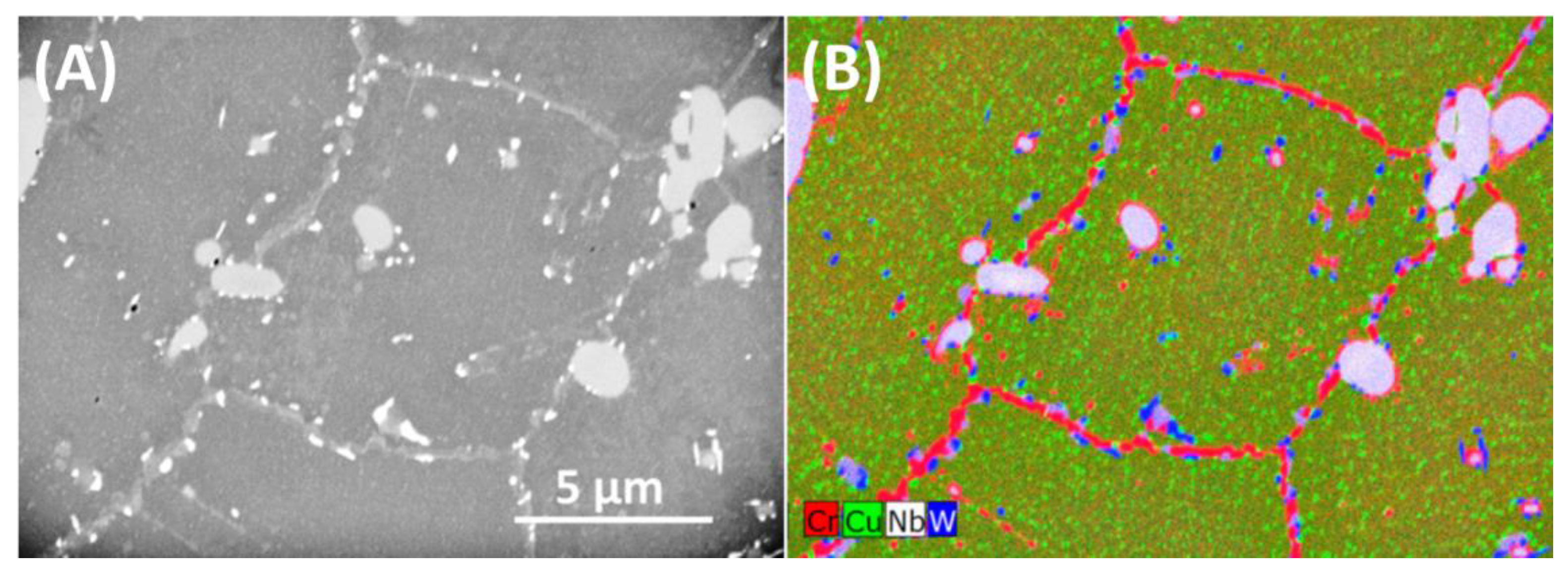
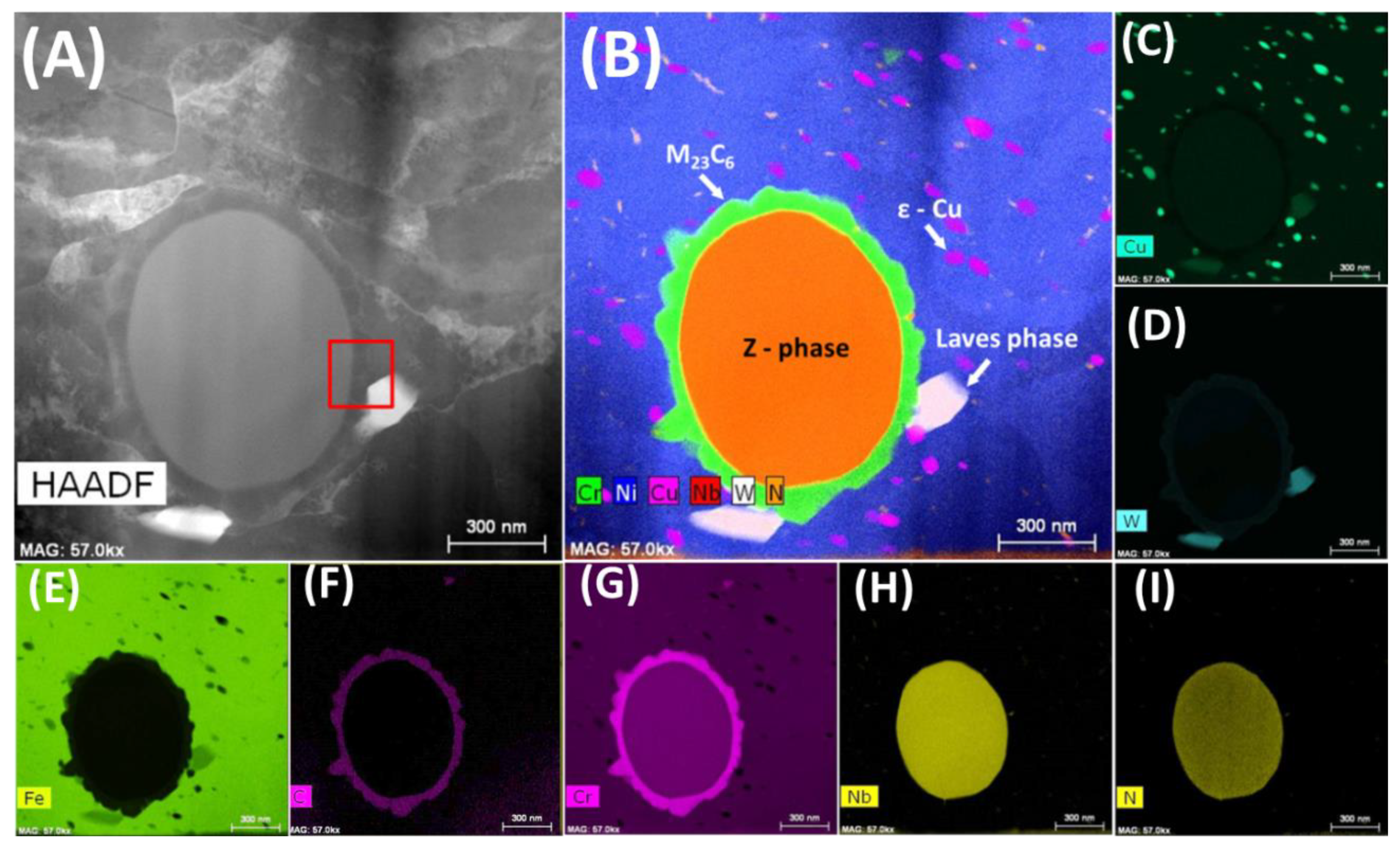
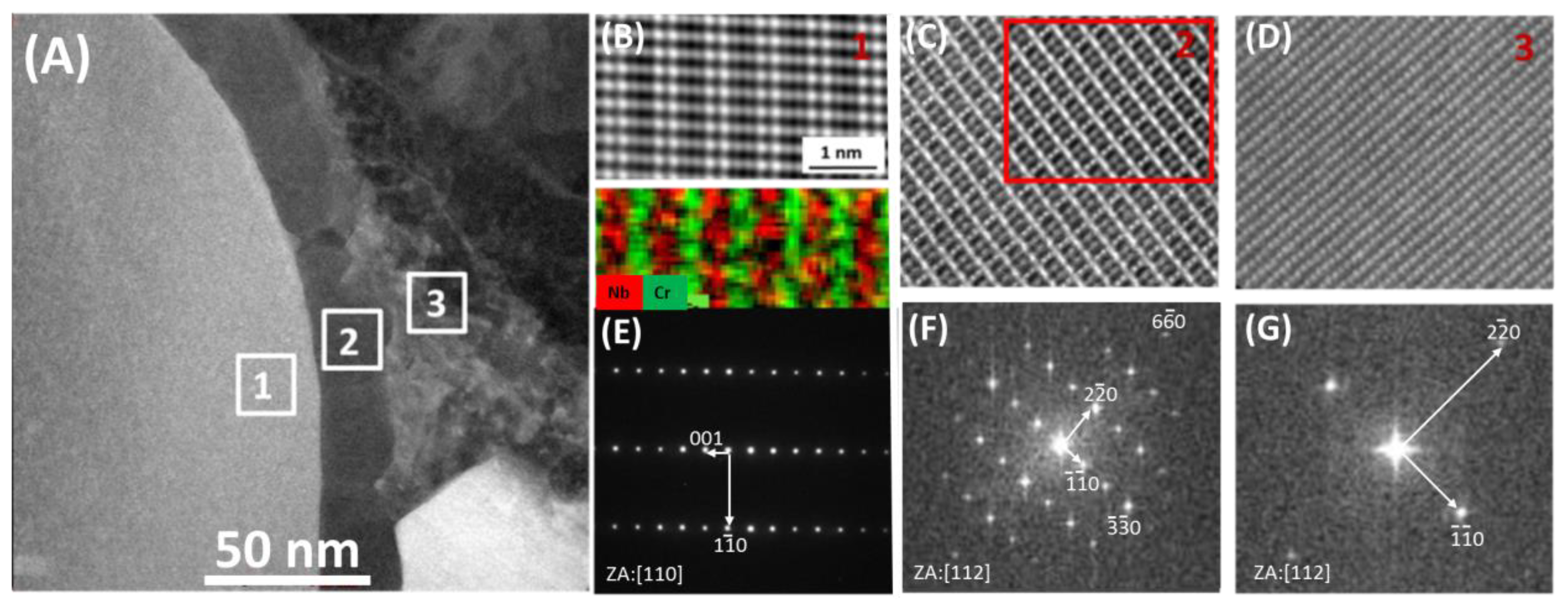
3.1. As-Received Material
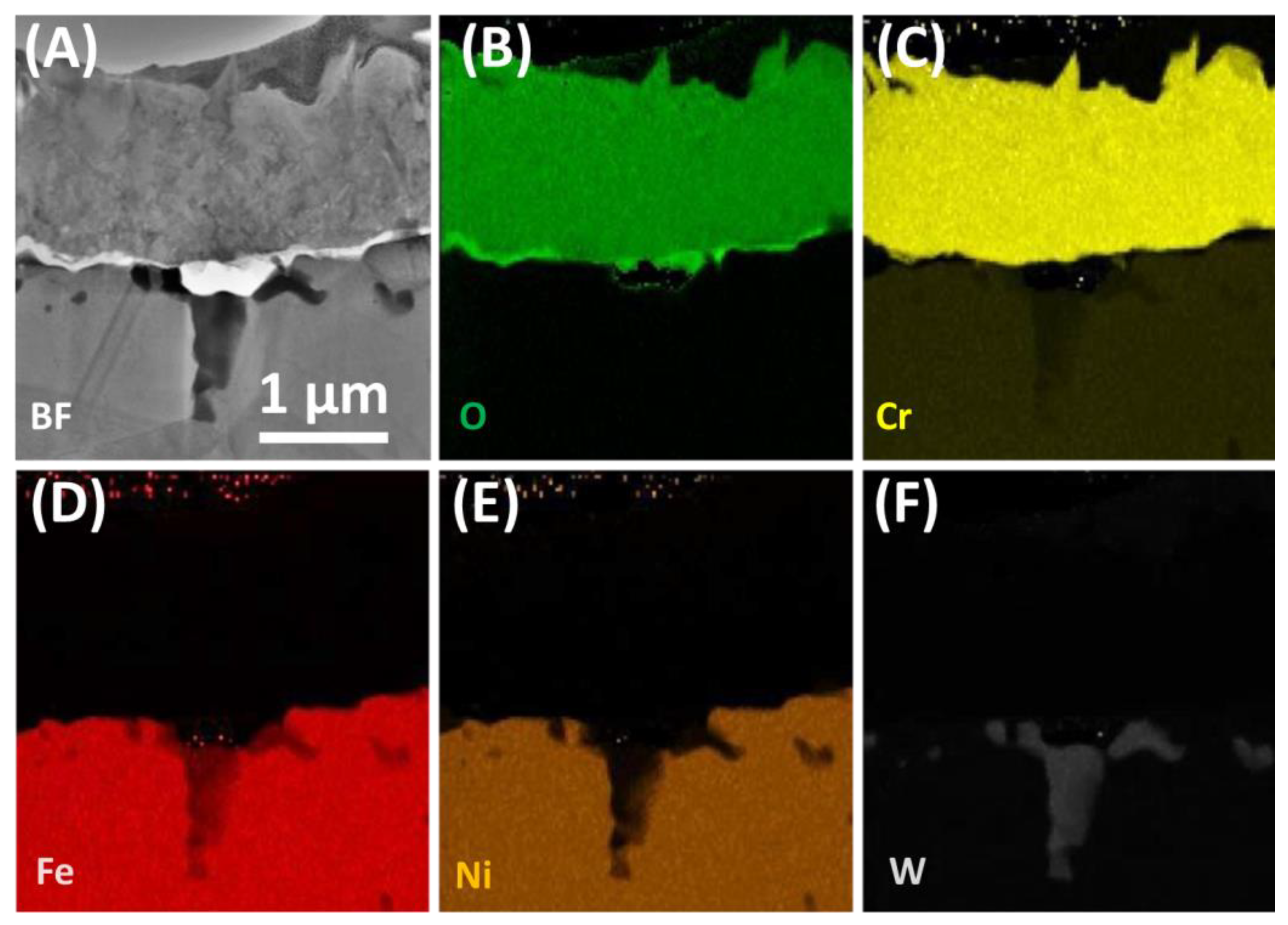
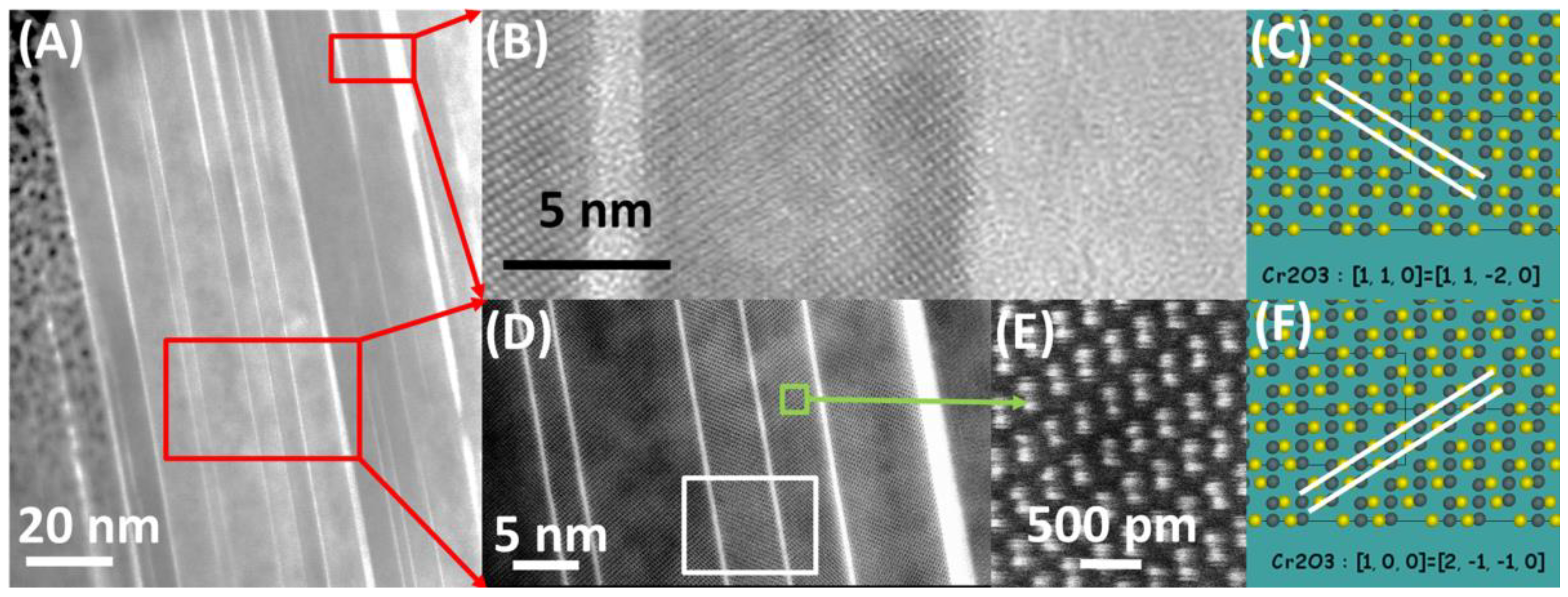
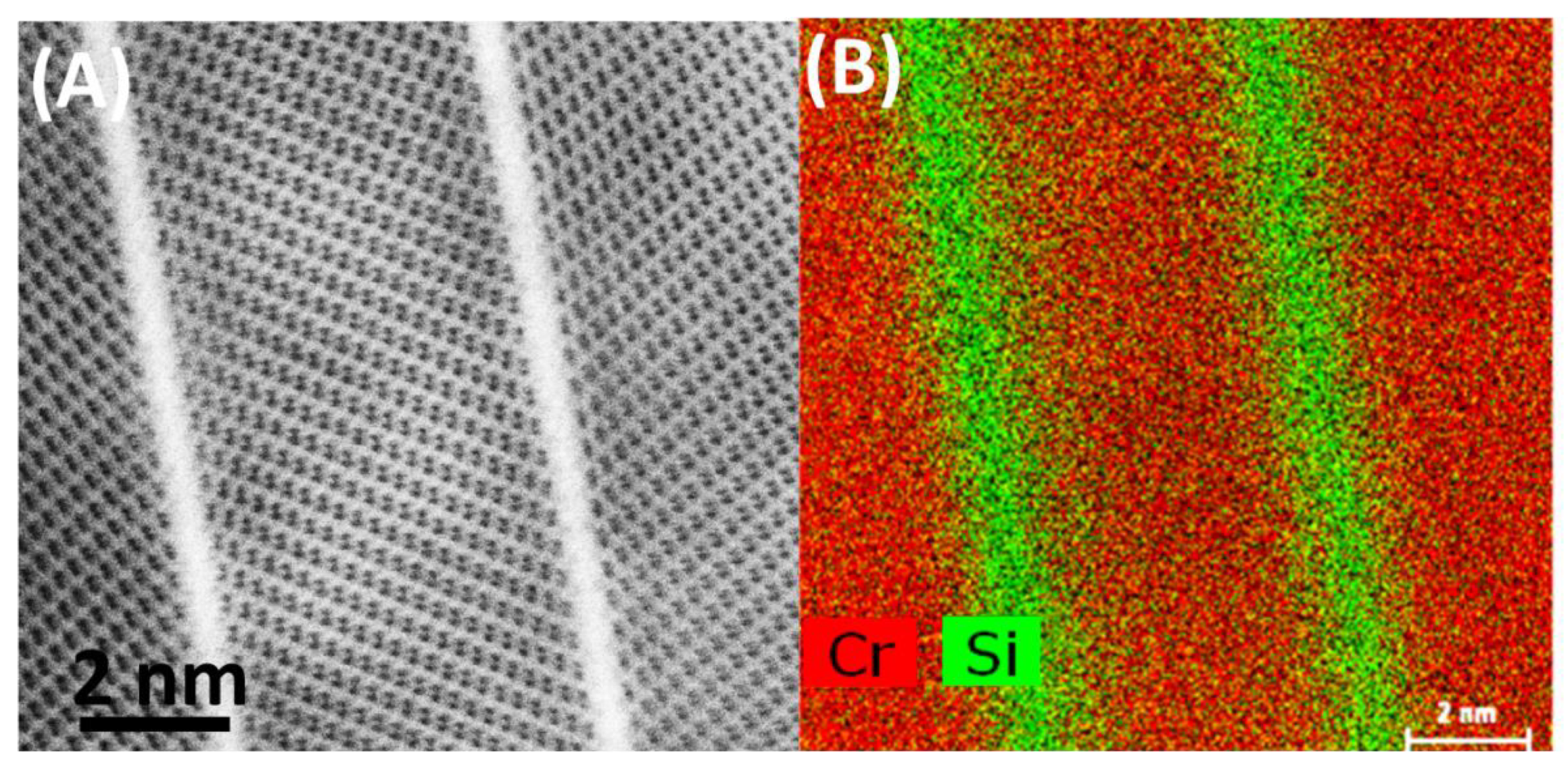
3.2. After Oxidation
4. Conclusions
- During heat treatment of Sanicro 25 alloy, the matrix semicoherent M23C6 carbides nucleate on the interface between the matrix and the Z-phase,
- Two-phase oxide scale developed on the steel surface is continuous and well- protective.
- Characteristic plates of Cr2O3 are present, indicating the presence of volatile compounds decomposing at the surface.
- Growth terraces are present at the surfaces of plates, suggesting the presence of surface diffusion processes.
- Plates are built of Cr2O3 crystals, separated by amorphous SiO2 phase.
Funding
Acknowledgments
Conflicts of Interest
References
- Viswanathan, R.; Henry, J.F.; Tanzosh, J.; Stanko, G.; Shingledecker, J.; Vitalis, B.; Purgert, R.U.S. Program on materials technology for ultra-supercritical coal power plants. J. Mater. Eng. Perform. 2013, 22, 2904–2915. [Google Scholar] [CrossRef]
- Hald, J. Microstructure and long-term creep properties of 9–12 % Cr steels. Int. J. Press. Vessel. Pip. 2008, 85, 30–37. [Google Scholar] [CrossRef]
- Rajendran Pillail, S. High Temperature Corrosion of Austenitic Stainless Steels. In Corrosion of Austenitic Stainless Steels; Alpha Science International Ltd.: Oxford, UK, 2002. [Google Scholar]
- Fuchs, R.; Heuser, H.; Jochum, C. Properties of matching filler metals for T91/P91. In Advances in Materials Technology for Fossil Power Plants; Viswanathan, R., Gandy, D.K.C., Eds.; ASM International: Hilton Head Island, SC, USA, 2005; pp. 950–965. [Google Scholar]
- SANDVIK. Available online: http://www.smt.sandvik.com (accessed on 16 April 2020).
- Swaminathan, J.; Das, C.R.; Baral, J.; Phaniraj, C.; Ghosh, R.N.; Albert, S.K.; Bhaduri, A.K. Creep strength behavior of boron added P91 steel and its weld in the temperature range of 600–650 °C. In Energy Materials; Springer: Cham, Swizerland, 2014; pp. 111–121. [Google Scholar]
- Isaac Samuel, E.; Choudhary, B.K.; Rao Palaparti, D.P.; Mathew, M.D. Creep Deformation and Rupture Behaviour of P92 Steel at 923 K. Procedia Eng. 2013, 55, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.; Yu, H.; Dong, J.; Liu, W.Q.; Cheng, S.C.; Liu, Z.D.; Xie, X. The precipitation strengthening behavior of Cu-rich phase in Nb contained advanced Fe-Cr-Ni type austenitic heat resistant steel for USC power plant application. Prog. Nat. Sci. Mater. Int. 2012, 22, 175–185. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, B.; Gil, A.; Aguero, A.; Gonzalez, V.; Czyrska-Filemonowicz, A. Microstructure, Chemical- and Phase Composition of Sanicro 25 Austenitic Steel after Oxidation in Steam at 700 °C. Oxid. Met. 2018, 89, 183–195. [Google Scholar] [CrossRef]
- Agüero, A.; González, V.; Gutiérrez, M.; Muelas, R. Oxidation under pure steam: Cr based protective oxides and coatings. Surf. Coat. Technol. 2013, 237, 30–38. [Google Scholar] [CrossRef]
- Casino—Monte CArlo SImulation of electroN trajectory in sOlids. Available online: https://www.gel.usherbrooke.ca/casino/index.html (accessed on 16 April 2020).
- Stadelmann, P. JEMS Java Electron Microscopy Software. Available online: https://www.jems-swiss.ch/ (accessed on 16 April 2020).
- Zurek, J.; Yang, S.-M.; Lin, D.-Y.; Hüttel, T.; Singheiser, L.; Quadakkers, W.J. Microstructural stability and oxidation behavior of Sanicro 25 during long-term steam exposure in the temperature range 600–750 °C. Mater. Corros. 2015, 66, 315–327. [Google Scholar] [CrossRef]
- Zhou, R.; Zhu, L.; Liu, Y.; Lu, Z.; Chen, L.; Ma, X. Microstructural evolution and the effect on hardness of Sanicro 25 welded joint base metal after creep at 973 K. J. Mater. Sci. 2017, 52, 6158–6169. [Google Scholar] [CrossRef]
- Suo, J.; Peng, Z.; Yang, H.; Chai, G.; Yu, M. Formation of Laves Phase in Sanicro 25 Austenitic Steel During Creep—Rupture Test at 700 °C. Metallogr. Microstruct. Anal. 2019, 8, 281–286. [Google Scholar] [CrossRef]
- Burke, M.G. Electron Diffraction and Phase Identification. In Transmission Electron Microscopy; Carter, C., Williams, D., Eds.; Springer: Cham, Swizerland, 2016. [Google Scholar]
- Uehara, T.; Toji, A.; Komatsubara, S.; Fujita, T. Improvement of creep rupture strength of high strength 12Cr ferritic heat-resistant steel. In Proceedings of the Conference on Materials for Advanced power Engineering, Liege, Belgium, 30 September–2 October 2002; p. 3206. [Google Scholar]
- Danielsen, H.K.; Di Nunzio, P.E.; Hald, J. Kinetics of Z-Phase Precipitation in 9 to 12 pct Cr Steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 2445–2452. [Google Scholar] [CrossRef] [Green Version]
- Chyrkin, A.; Pillai, R.; Ackermann, H.; Hattendorf, H.; Richter, S.; Nowak, W.; Grüner, D.; Quadakkers, W.J. Modeling carbide dissolution in alloy 602 CA during high temperature oxidation. Corros. Sci. 2015, 96, 32–41. [Google Scholar] [CrossRef]
- Durham, R.N.; Gleeson, B.; Young, D.J. Factors affecting chromium carbide precipitate dissolution during alloy oxidation. Oxid. Met. 1998, 50, 139–165. [Google Scholar] [CrossRef]
- Rutkowski, B.; Galanis, A.S.; Gil, A.; Czyrska-Filemonowicz, A. A novel approach to the characterization of thin oxide layers. Mater. Lett. 2016, 173, 235–238. [Google Scholar] [CrossRef]
- Webb, W.W.; Forgeng, W.D. Growth and defect structure of sapphire microcrystals. J. Appl. Phys. 1957, 28, 1449–1454. [Google Scholar] [CrossRef]
- Asteman, H.; Svensson, J.-E.; Norell, M.; Johansson, L.-G. Influence of Water Vapor and Flow Rate on the High-Temperature Oxidation of 304L; Effect of Chromium Oxide Hydroxide. Oxid. Met. 2000, 54, 11–26. [Google Scholar] [CrossRef]
- Rutkowski, B.; Gil, A.; Czyrska-Filemonowicz, A. Microstructure and chemical composition of the oxide scale formed on the sanicro 25 steel tubes after fireside corrosion. Corros. Sci. 2016, 102, 373–383. [Google Scholar] [CrossRef]
- Yoo, K.B.; He, Y.; Lee, H.S.; Bae, S.Y.; Kim, D.S.; Shin, K. Study of the scale formed on super 304H boiler tube steels after long-term steam oxidation at high temperatures. Mater. Charact. 2018, 146, 71–80. [Google Scholar] [CrossRef]

| Element | Fe | Ni | Cr | W | Cu | Co | Mn | Nb | N | Si | C | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 42.8 | 25.4 | 22.4 | 3.4 | 3 | 1.5 | 0.5 | 0.5 | 0.2 | 0.2 | 0.06 | 0.04 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowski, B. Microstructural Characterisation of Austenitic Heat Resistant Sanicro 25 Steel after Steam Oxidation. Materials 2020, 13, 3382. https://doi.org/10.3390/ma13153382
Rutkowski B. Microstructural Characterisation of Austenitic Heat Resistant Sanicro 25 Steel after Steam Oxidation. Materials. 2020; 13(15):3382. https://doi.org/10.3390/ma13153382
Chicago/Turabian StyleRutkowski, Bogdan. 2020. "Microstructural Characterisation of Austenitic Heat Resistant Sanicro 25 Steel after Steam Oxidation" Materials 13, no. 15: 3382. https://doi.org/10.3390/ma13153382





