A Novel Organic–Inorganic Hybrid Admixture for Increasing Flowability and Reducing Viscosity of Ultra-High Performance Paste
Abstract
:1. Introduction
2. Experimental
2.1. Materials
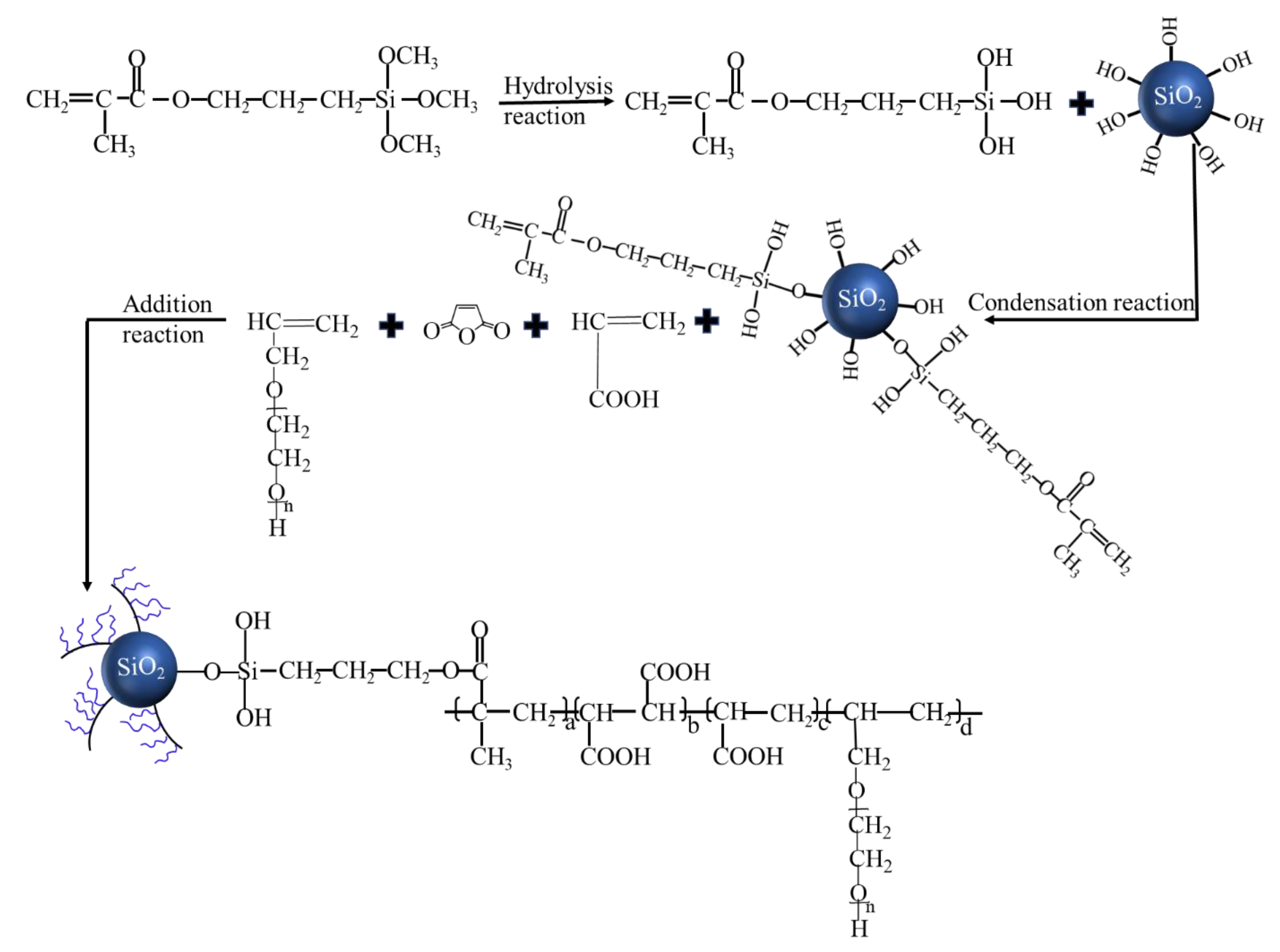
2.2. Preparation of the Organic–Inorganic Hybrid (OIH) Admixture
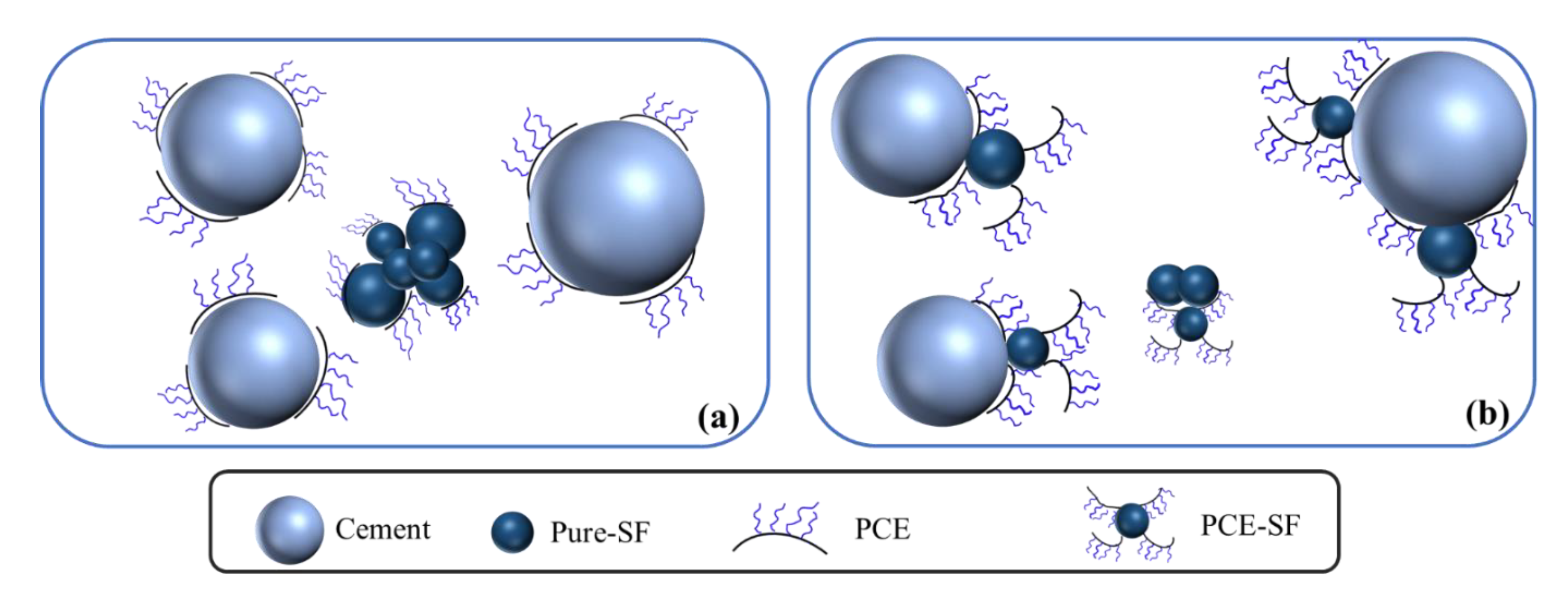
2.3. Dispersion Mechanism of OIH Admixture in UHPC System
2.4. Mix Design of UHPC Paste Formulation
2.5. Characterization of the OIH Admixture
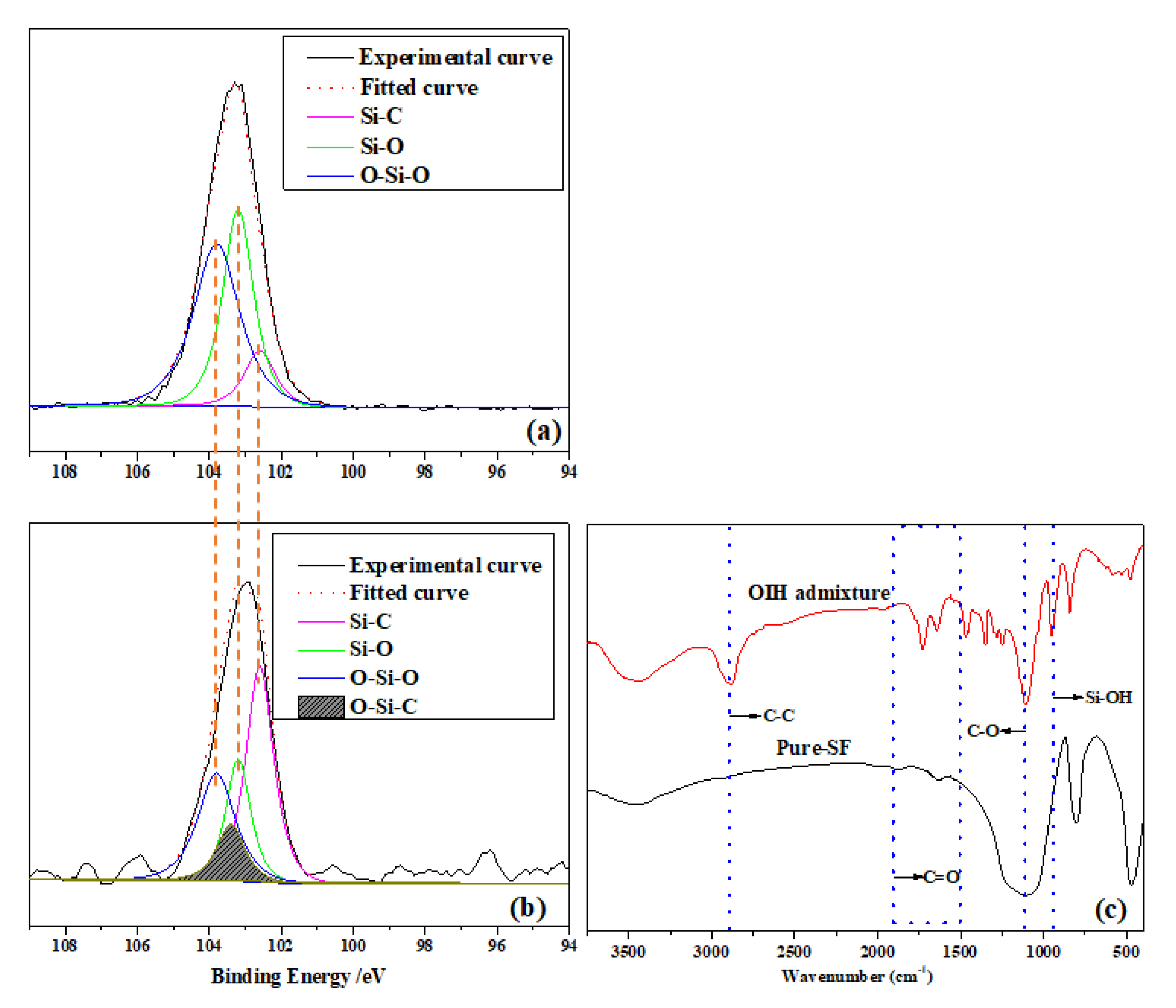
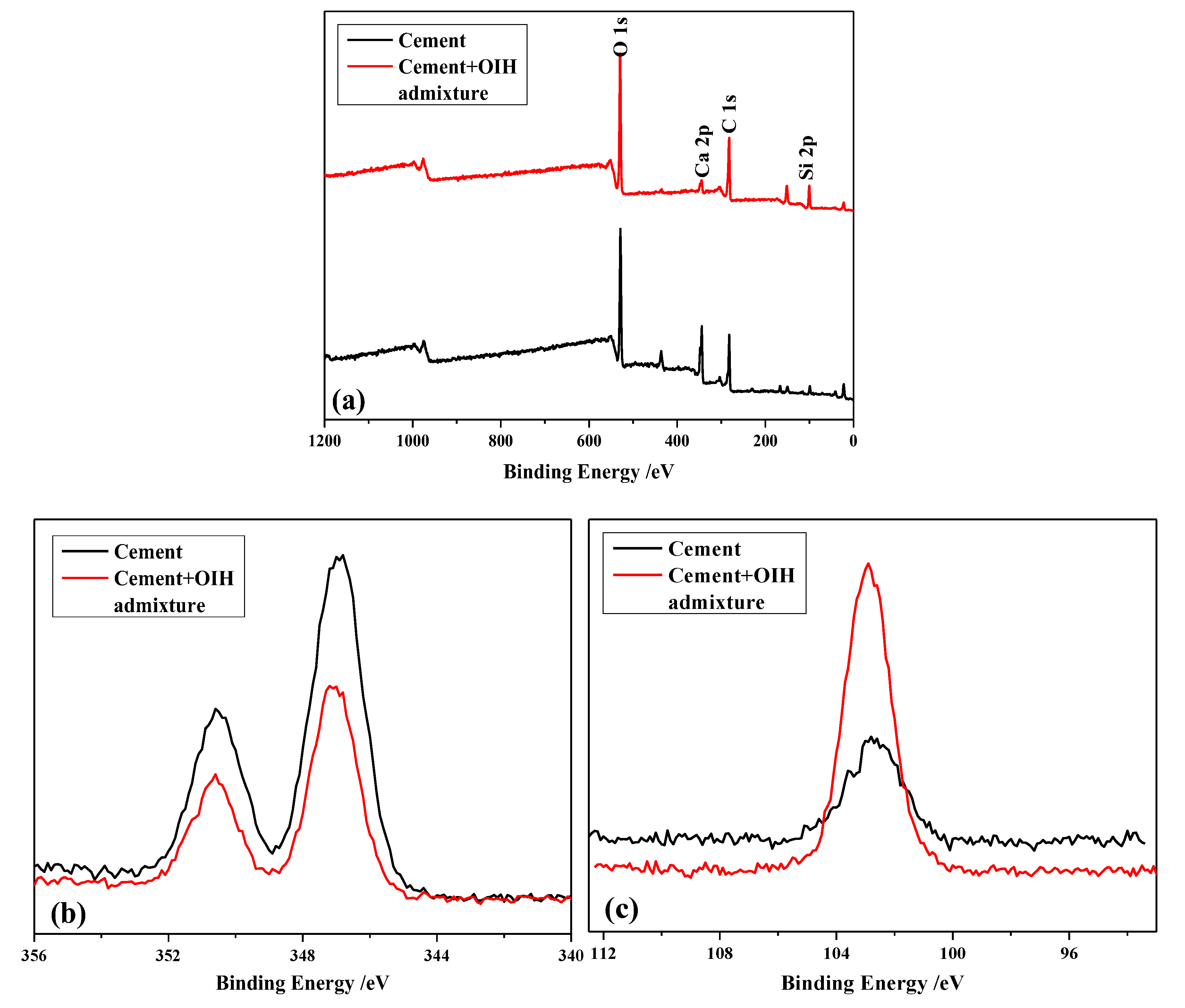
2.5.1. Characterization of the Chemical Structure of OIH Admixture
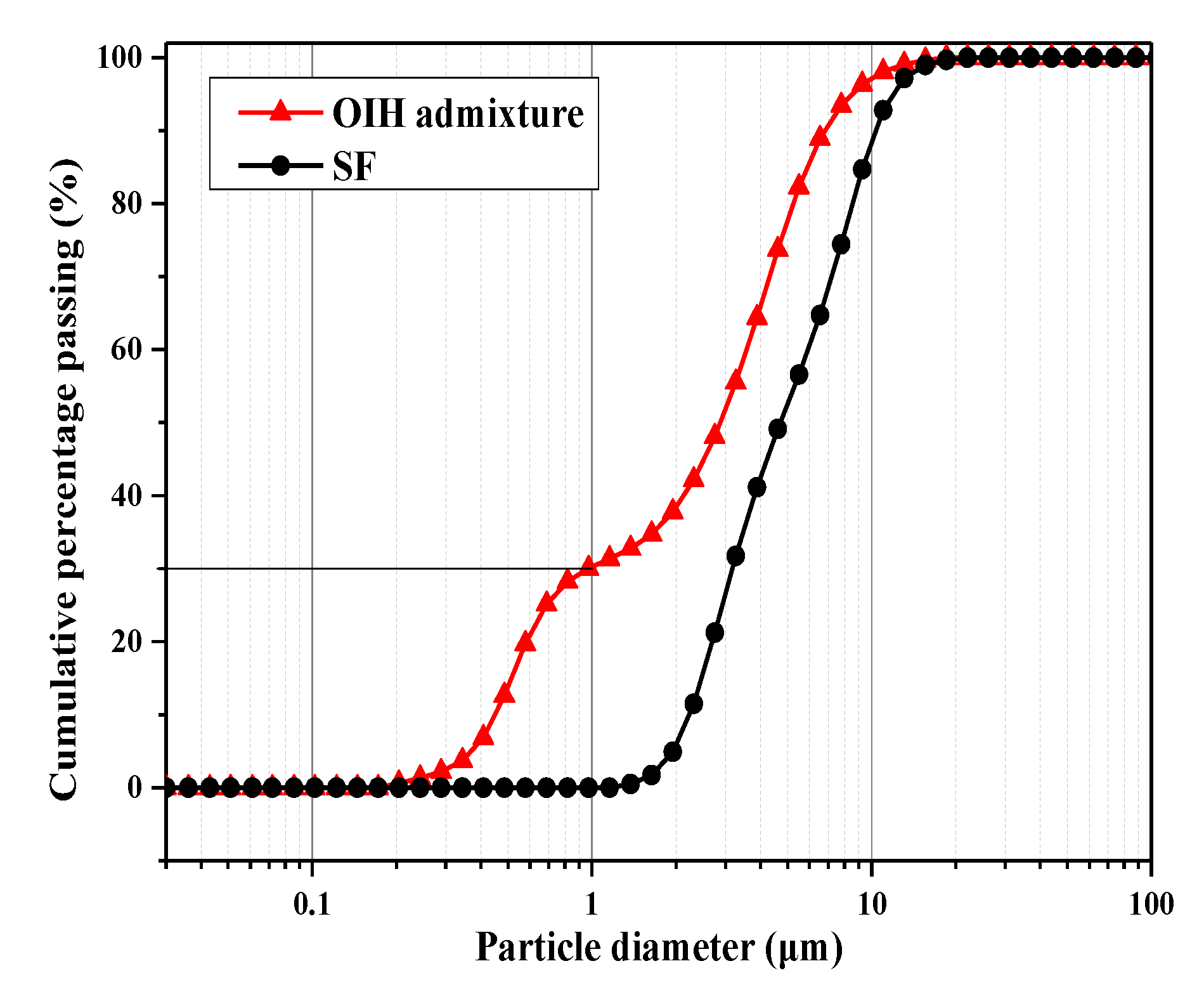
2.5.2. Characterization of the Particle Distribution of OIH Admixture
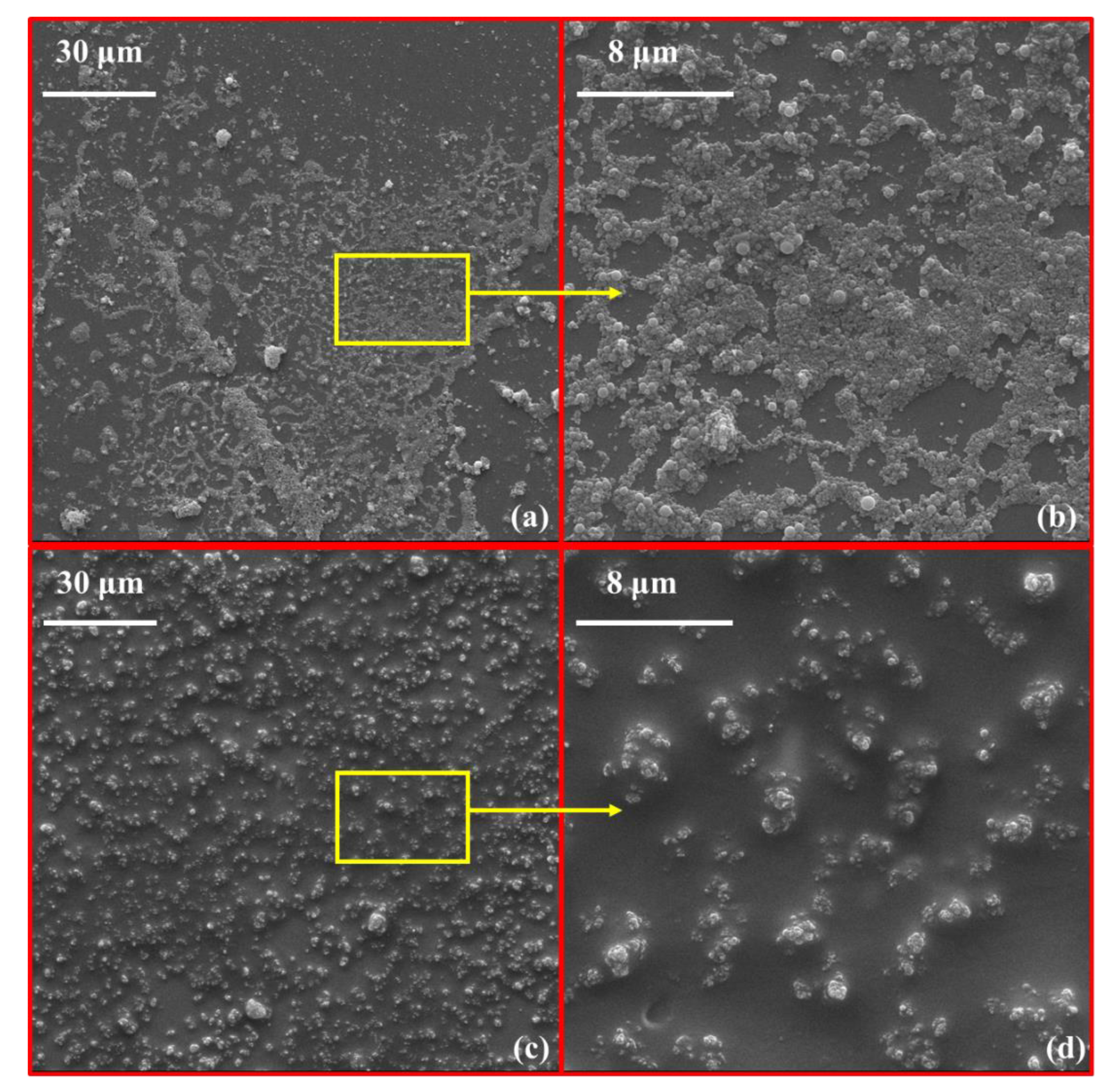
2.5.3. Micro-Morphologies of OIH Admixture
2.6. Characterization of the Dispersion Mechanism
2.6.1. Adsorption Behavior of OIH Admixture onto Cement and SF Surface
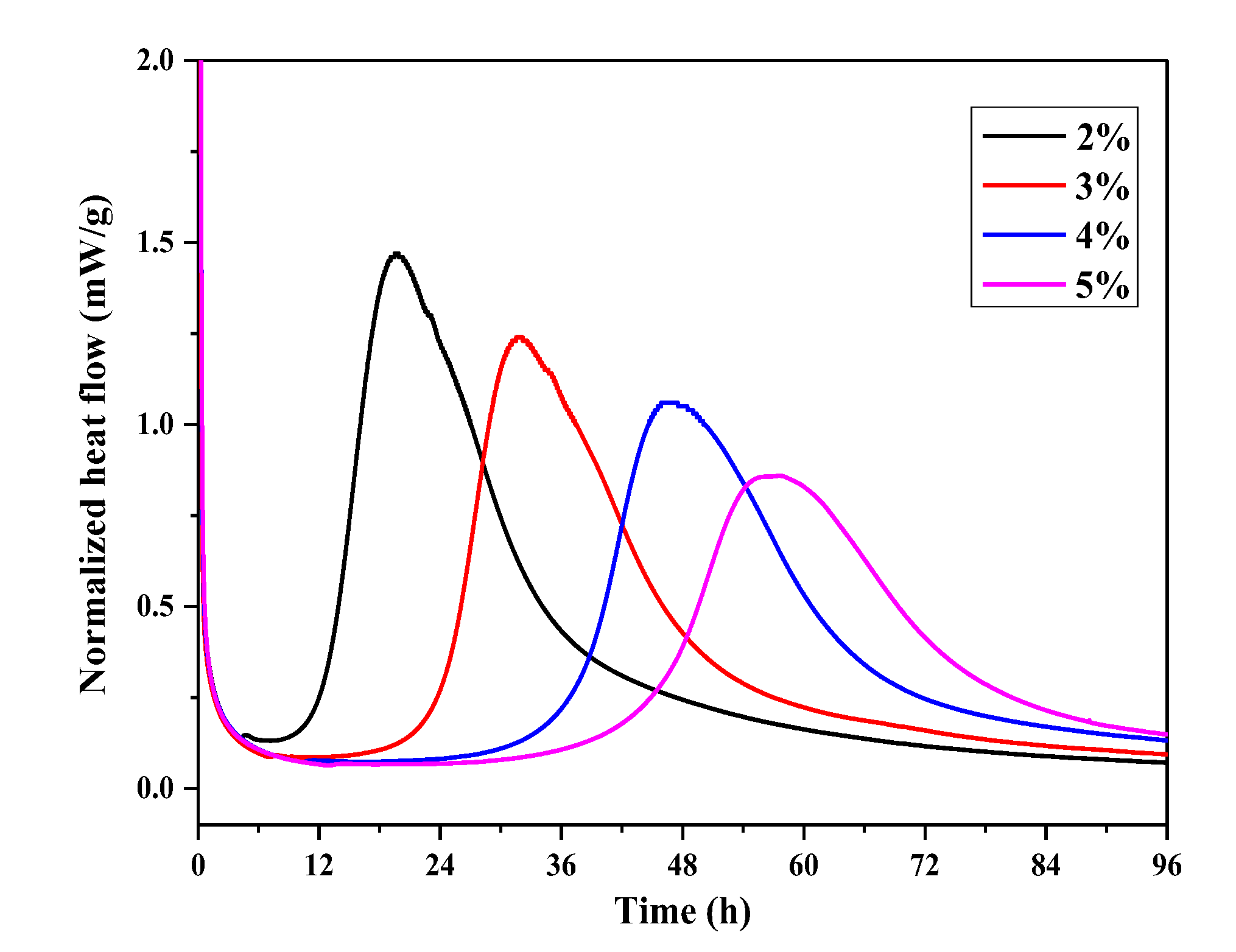
2.6.2. Heat Flow Calorimetry of UHPC Paste
2.7. Dispersibility of OIH Admixture
2.7.1. Flowability Test
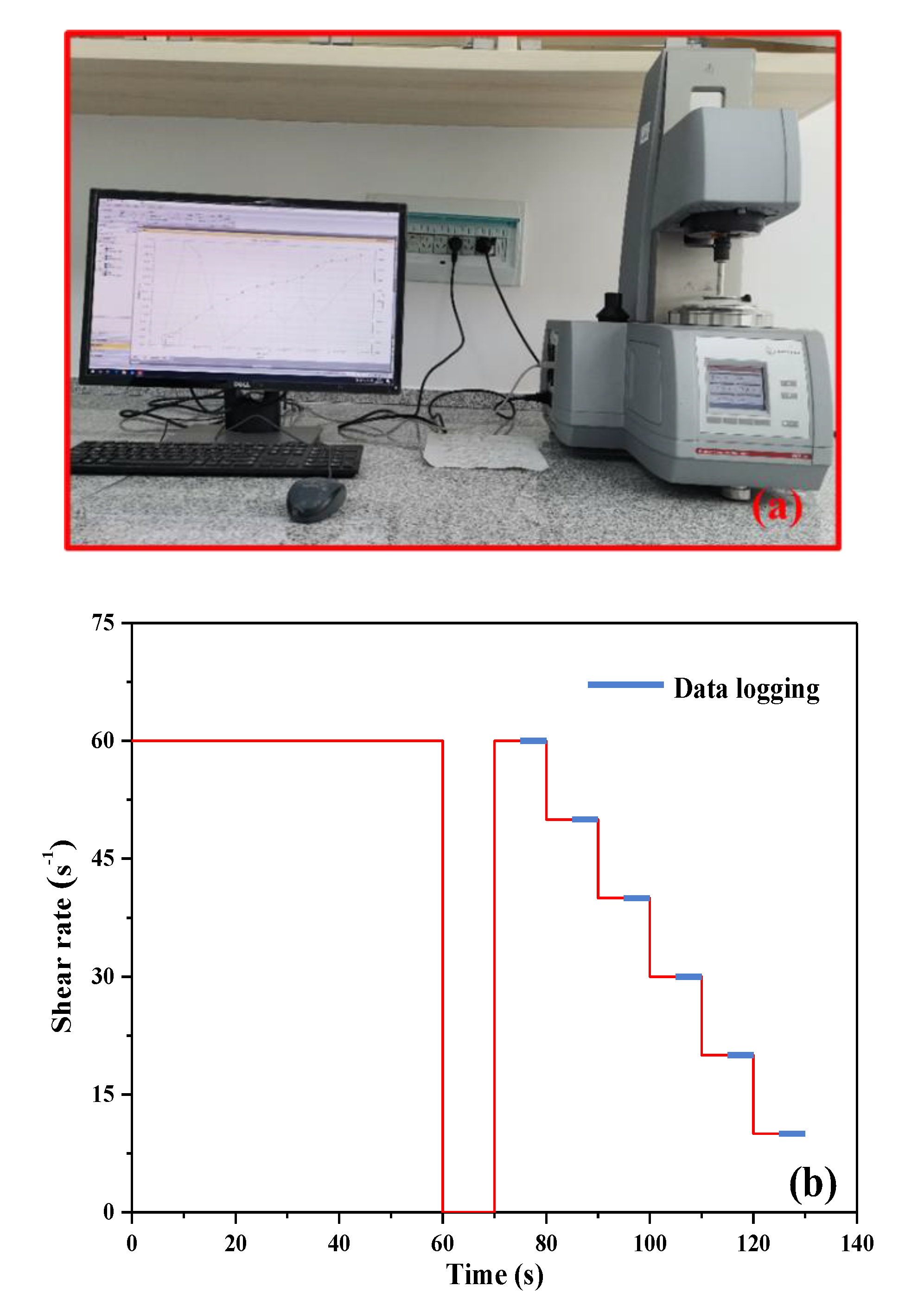
2.7.2. Rheological Behavior Measurement
2.7.3. Mechanical Properties Measurement
3. Results and Discussion
3.1. Characterization of OIH Admixture
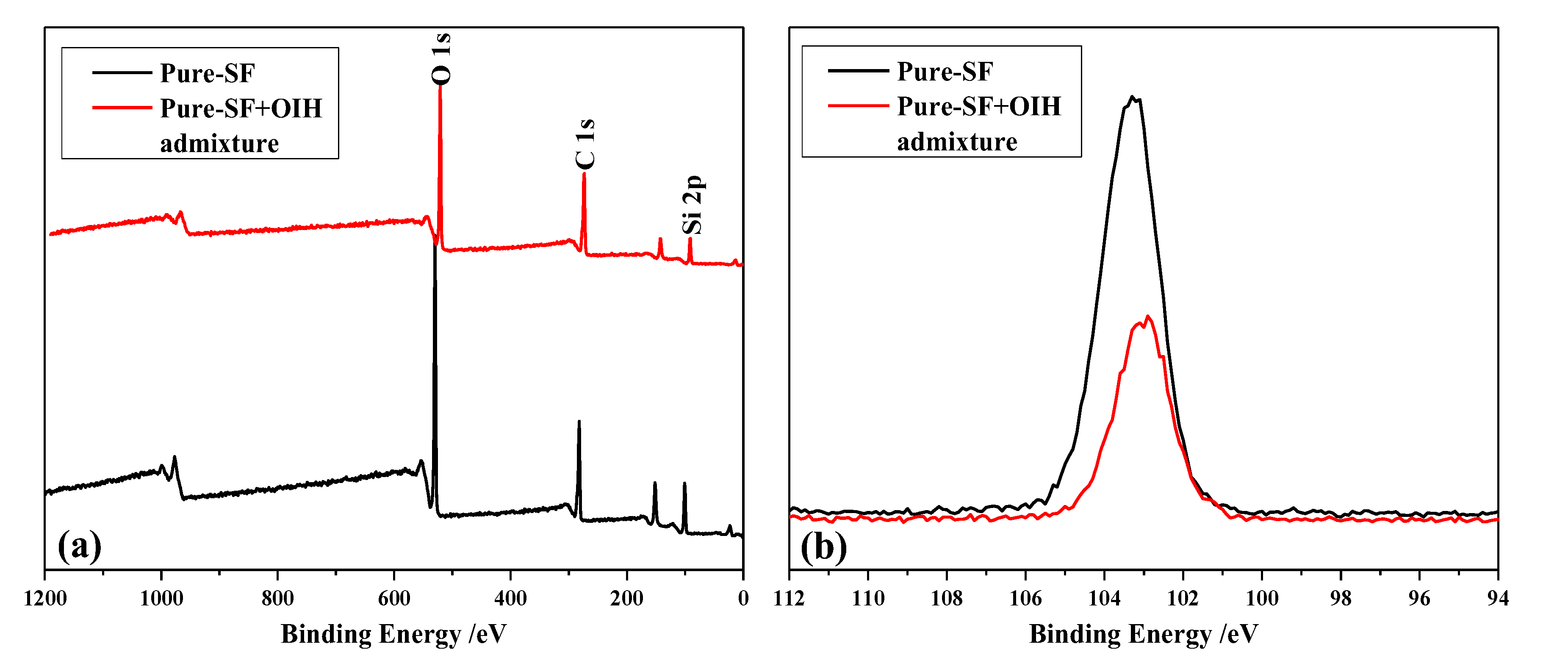
3.2. The Adsorption Behavior of OIH Admixture onto Cement and Pure-SF Particles
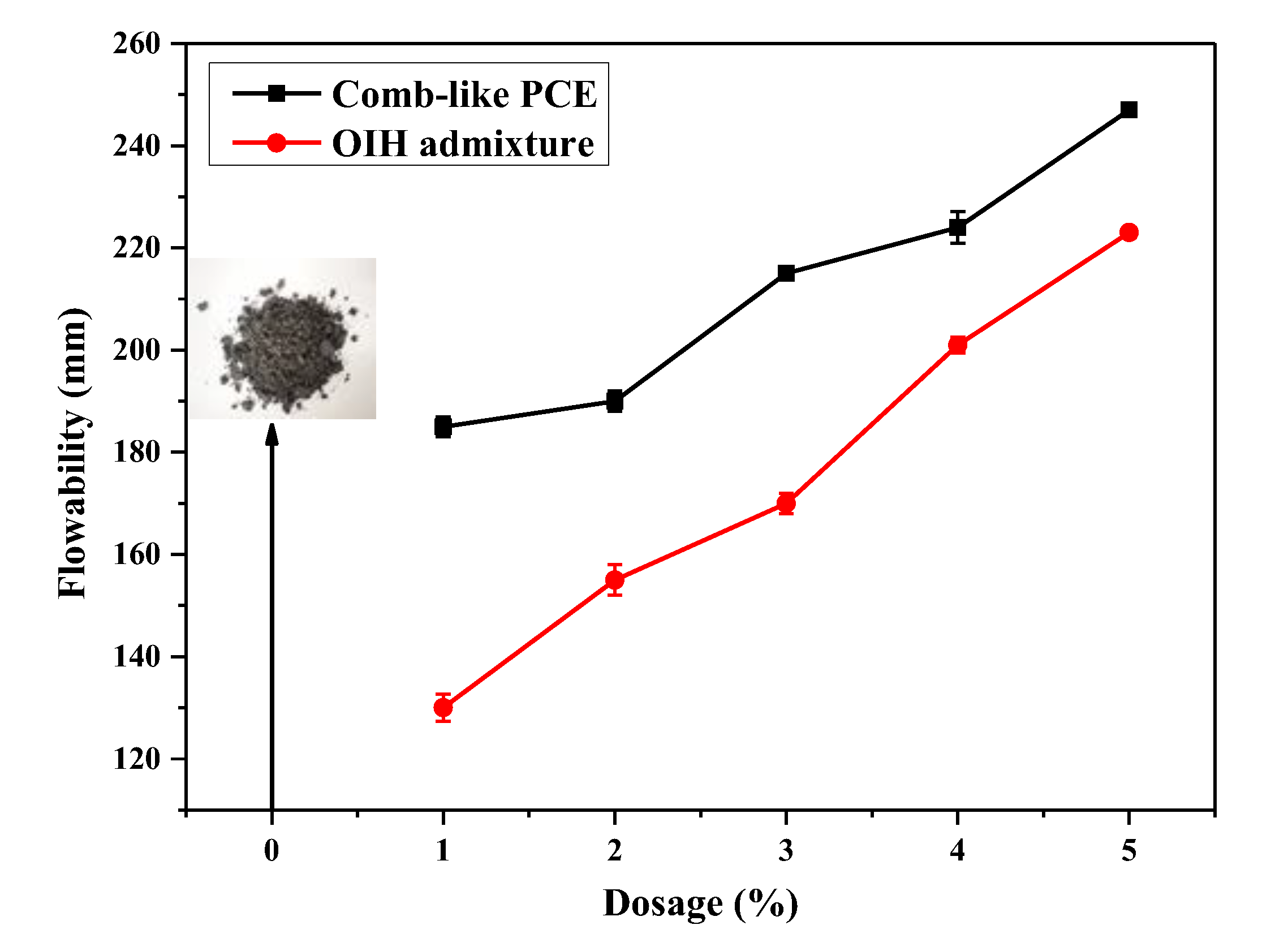
3.3. Flowability of UHPC Paste
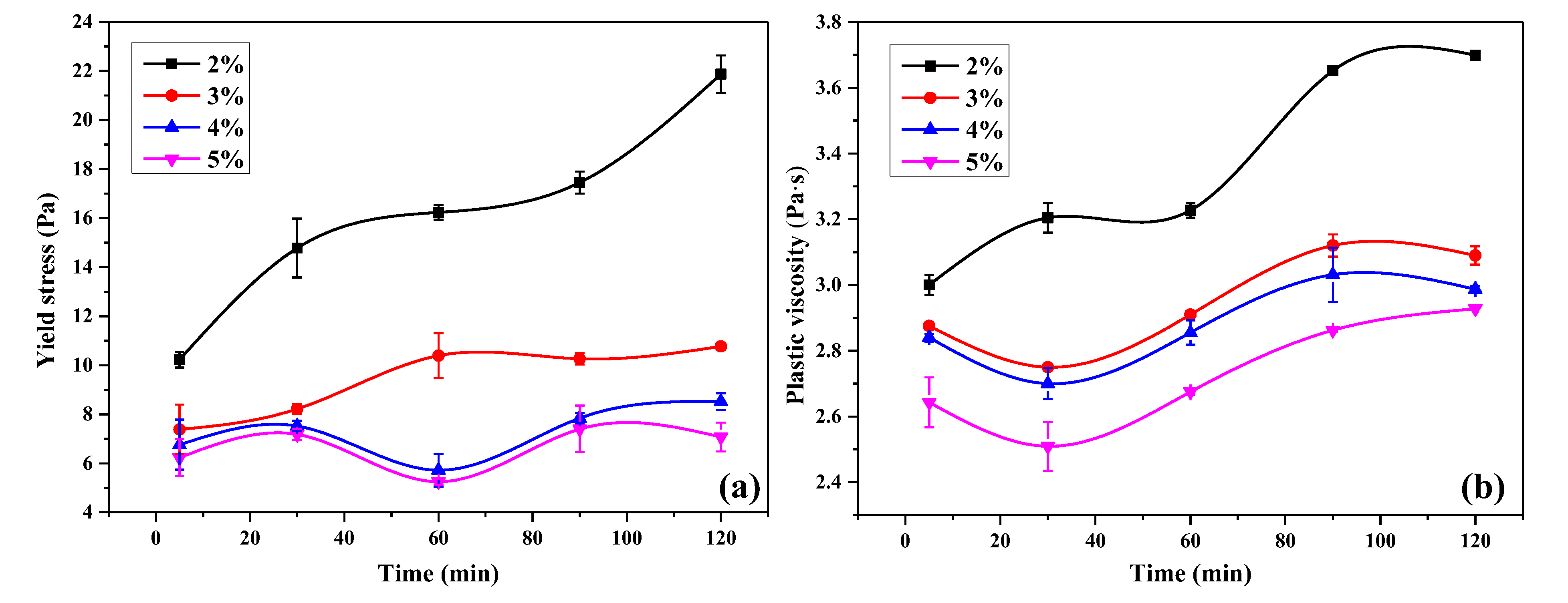
3.4. Flowability Retention Behavior of UHPC Paste
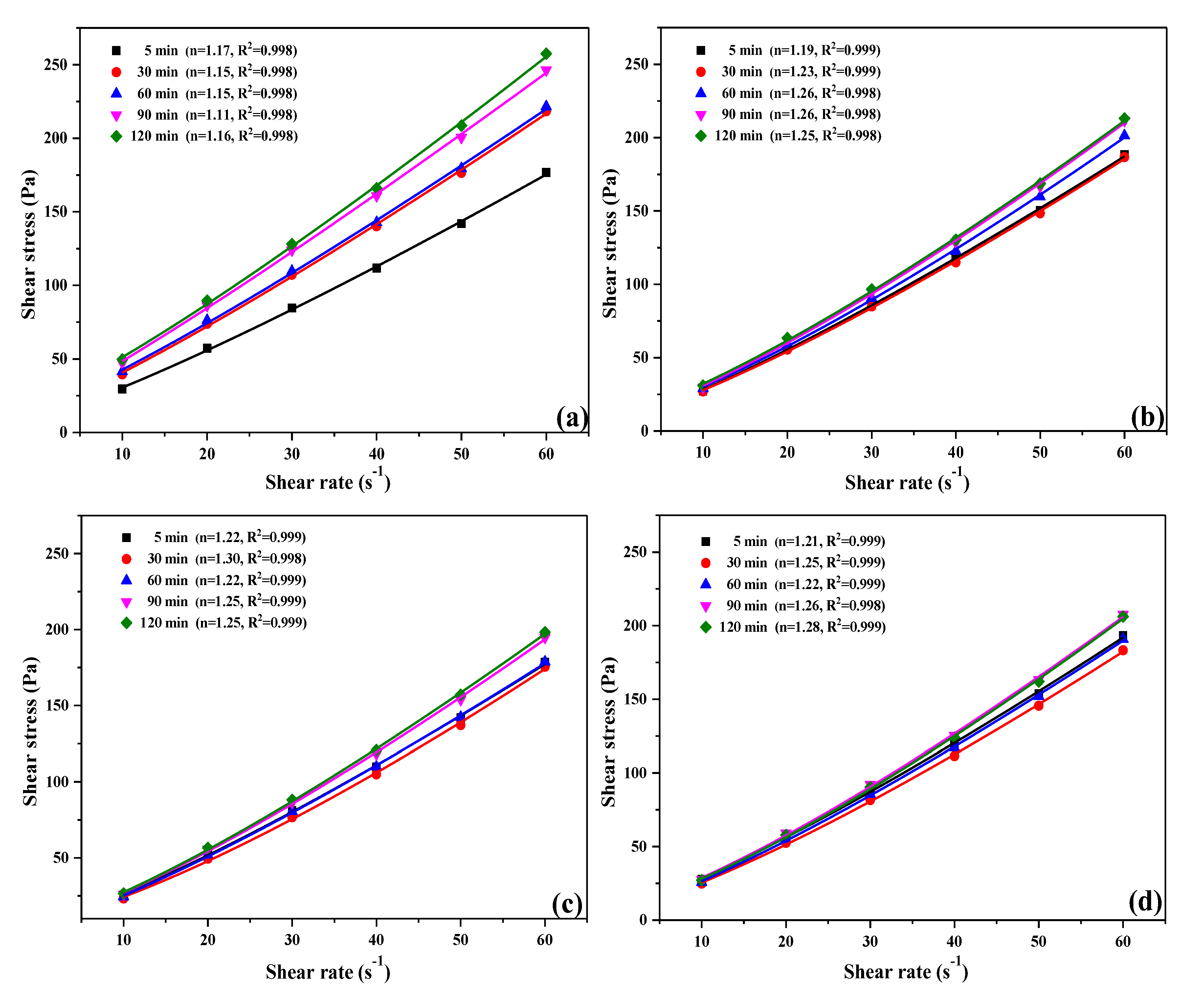
3.5. Rheological Properties of UHPC Paste
3.6. Mechanical Properties of UHPC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.-Y.; Zhang, J.; Liu, C.; Zheng, Z.-L.; Lambert, P. Effect of Graphene Oxide Nanosheets on Physical Properties of Ultra-High-Performance Concrete with High Volume Supplementary Cementitious Materials. Materials 2020, 13, 1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, D.-Y.; Banthia, N. Mechanical properties of ultra-high-performance fiber-reinforced concrete: A review. Cem. Concr. Compos. 2016, 73, 267–280. [Google Scholar] [CrossRef]
- Arora, A.; Aguayo, M.; Hansen, H.; Castro, C.; Federspiel, E.; Mobasher, B.; Neithalath, N. Microstructural packing- and rheology-based binder selection and characterization for Ultra-high Performance Concrete (UHPC). Cem. Concr. Res 2018, 103, 179–190. [Google Scholar] [CrossRef]
- Othman, H.; Marzouk, H.; Sherif, M. Effects of variations in compressive strength and fibre content on dynamic properties of ultra-high performance fibre-reinforced concrete. Constr. Build. Mater. 2019, 195, 547–556. [Google Scholar] [CrossRef]
- Yang, S.; Millard, S.; Soutsos, M.; Barnett, S.; Le, T. Influence of aggregate and curing regime on the mechanical properties of ultra-high performance fibre reinforced concrete (UHPFRC). Constr. Build. Mater. 2009, 23, 2291–2298. [Google Scholar] [CrossRef]
- Yajun, J.; Cahyadi, J.H. Effects of densified silica fume on microstructure and compressive strength of blended cement pastes. Cem. Concr. Res. 2003, 33, 1543–1548. [Google Scholar] [CrossRef]
- Lei, D.-Y.; Guo, L.; Sun, W.; Liu, J.; Shu, X.; Guo, X. A new dispersing method on silica fume and its influence on the performance of cement-based materials. Constr. Build. Mater. 2016, 115, 716–726. [Google Scholar] [CrossRef]
- Hommer, H. Interaction of polycarboxylate ether with silica fume. J. Eur. Ceram. Soc. 2009, 29, 1847–1853. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Wang, W.; Du, H. Surface modification of silica fume and adsorption property of Pb (II) ions. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 052022. [Google Scholar] [CrossRef]
- Khayat, K.H.; Meng, W.; Vallurupalli, K.; Teng, L. Rheological properties of ultra-high-performance concrete—An overview. Cem. Concr. Res. 2019, 124, 105828. [Google Scholar] [CrossRef]
- Meng, W.; Khayat, K.H. Improving flexural performance of ultra-high-performance concrete by rheology control of suspending mortar. Compos. Part B Eng. 2017, 117, 26–34. [Google Scholar] [CrossRef]
- Chu, S.; Kwan, A. Mixture design of self-levelling ultra-high performance FRC. Constr. Build. Mater. 2019, 228, 116761. [Google Scholar] [CrossRef]
- Sadrmomtazi, A.; Tajasosi, S.; Tahmouresi, B. Effect of materials proportion on rheology and mechanical strength and microstructure of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2018, 187, 1103–1112. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.-J.; Ryu, G.-S.; Koh, K.T. Tensile behavior of Ultra High Performance Hybrid Fiber Reinforced Concrete. Cem. Concr. Compos. 2012, 34, 172–184. [Google Scholar] [CrossRef]
- Song, Q.; Yu, R.; Wang, X.; Rao, S.; Shui, Z. A novel Self-Compacting Ultra-High Performance Fibre Reinforced Concrete (SCUHPFRC) derived from compounded high-active powders. Constr. Build. Mater. 2018, 158, 883–893. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Brouwers, H. Effect of PCE-type superplasticizer on early-age behaviour of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2017, 153, 740–750. [Google Scholar] [CrossRef]
- Lesti, M.; Ng, S.; Plank, J. Ca2+-Mediated Interaction Between Microsilica and Polycarboxylate Comb Polymers in a Model Cement Pore Solution. J. Am. Ceram. Soc. 2010, 93, 3493–3498. [Google Scholar] [CrossRef]
- Schröfl, C.; Gruber, M.; Plank, J. Preferential adsorption of polycarboxylate superplasticizers on cement and silica fume in ultra-high performance concrete (UHPC). Cem. Concr. Res. 2012, 42, 1401–1408. [Google Scholar] [CrossRef]
- Puertas, F.; Varga, C.; Alonso, M. Rheology of alkali-activated slag pastes. Effect of the nature and concentration of the activating solution. Cem. Concr. Compos. 2014, 53, 279–288. [Google Scholar] [CrossRef]
- Palacios, M.; Alonso, M.M.; Varga, C.; Puertas, F. Influence of the alkaline solution and temperature on the rheology and reactivity of alkali-activated fly ash pastes. Cem. Concr. Compos. 2019, 95, 277–284. [Google Scholar] [CrossRef]
- De Larrard, F.; Ferraris, C.F.; Sedran, T. Fresh concrete: A Herschel-Bulkley material. Mater. Struct. 1998, 31, 494–498. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Wang, B.; Shi, Q.; Gao, J.; Cui, Z.; Wan, Y. Dopamine functionalization for improving crystallization behaviour of polyethylene glycol in shape-stable phase change material with silica fume as the matrix. J. Clean. Prod. 2019, 208, 951–959. [Google Scholar] [CrossRef]
- Kostoglou, M.; Bikiaris, D. Kinetic Analysis of Nanocomposites Prepared in situ Consisting of an Aliphatic Biodegradable Polyester and Fumed Silica Nanoparticles. Macromol. React. Eng. 2011, 5, 178–189. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Zheng, S.; Farhan, S.; Yao, H.; Jiang, H. Research on the chemical mechanism in the polyacrylate latex modified cement system. Cem. Concr. Res. 2015, 76, 62–69. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S.; Wang, Z.; Du, C.; Jiang, H. Research on the mechanism of polymer latex modified cement. Constr. Build. Mater. 2016, 111, 710–718. [Google Scholar] [CrossRef]
- Arend, J.; Wetzel, A.; Middendorf, B. Fluorescence Microscopic Investigations of the Retarding Effect of Superplasticizers in Cementitious Systems of UHPC. Materials 2020, 13, 1057. [Google Scholar] [CrossRef] [Green Version]
- Jansen, D.; Neubauer, J.; Goetz-Neunhoeffer, F.; Haerzschel, R.; Hergeth, W.-D. Change in reaction kinetics of a Portland cement caused by a superplasticizer — Calculation of heat flow curves from XRD data. Cem. Concr. Res. 2012, 42, 327–332. [Google Scholar] [CrossRef]
- Lothenbach, B.; Le Saoût, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Yahia, A. Shear-thickening behavior of high-performance cement grouts — Influencing mix-design parameters. Cem. Concr. Res. 2011, 41, 230–235. [Google Scholar] [CrossRef]



| Material | Mass Fraction/wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | Loss | |
| Cement | 22.87 | 4.47 | 3.48 | 64.05 | 2.46 | 2.44 | 0.52 | 0.9 | 1.21 |
| Silica fume | 95.38 | - | 0.61 | 1.84 | 0.26 | - | 0.16 | 0.85 | 2.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yao, H. A Novel Organic–Inorganic Hybrid Admixture for Increasing Flowability and Reducing Viscosity of Ultra-High Performance Paste. Materials 2020, 13, 3385. https://doi.org/10.3390/ma13153385
Wang M, Yao H. A Novel Organic–Inorganic Hybrid Admixture for Increasing Flowability and Reducing Viscosity of Ultra-High Performance Paste. Materials. 2020; 13(15):3385. https://doi.org/10.3390/ma13153385
Chicago/Turabian StyleWang, Min, and Hao Yao. 2020. "A Novel Organic–Inorganic Hybrid Admixture for Increasing Flowability and Reducing Viscosity of Ultra-High Performance Paste" Materials 13, no. 15: 3385. https://doi.org/10.3390/ma13153385




