Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects
Abstract
1. Introduction
2. Results
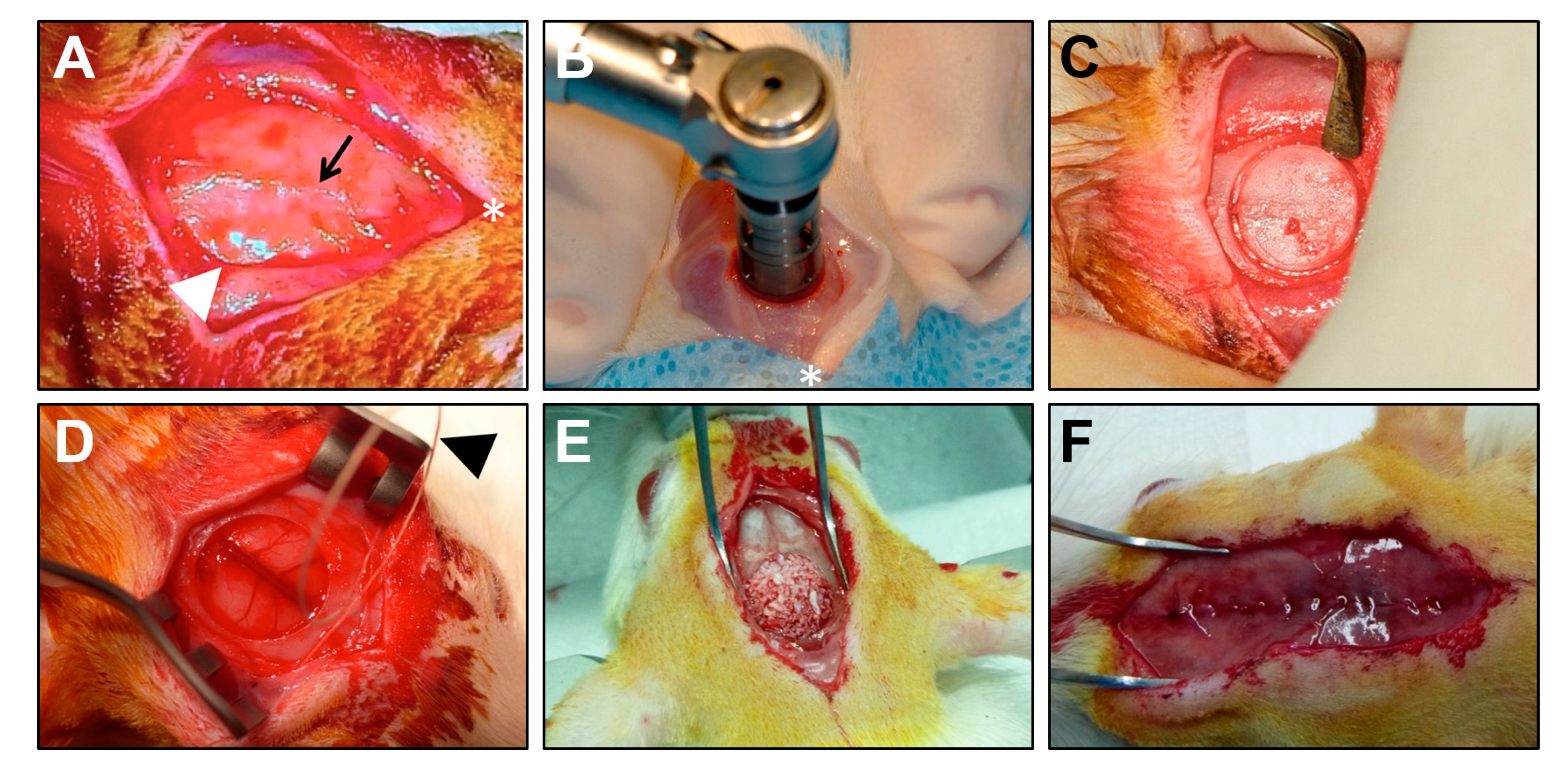
2.1. Filling of Critical-Sized Calvarial Defect with Bovine HA, DBM, and COLL Is Safe for Experimental Animals
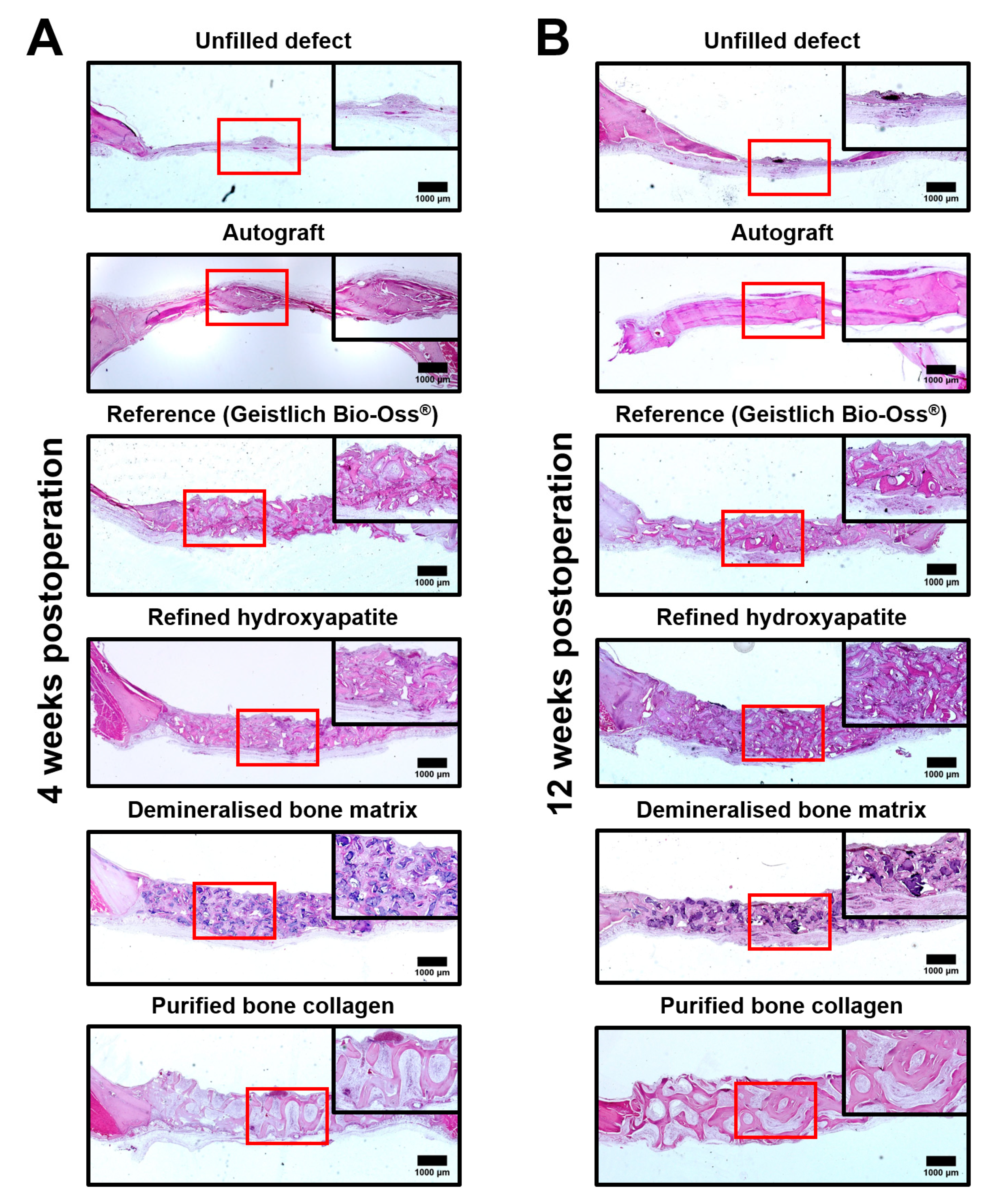
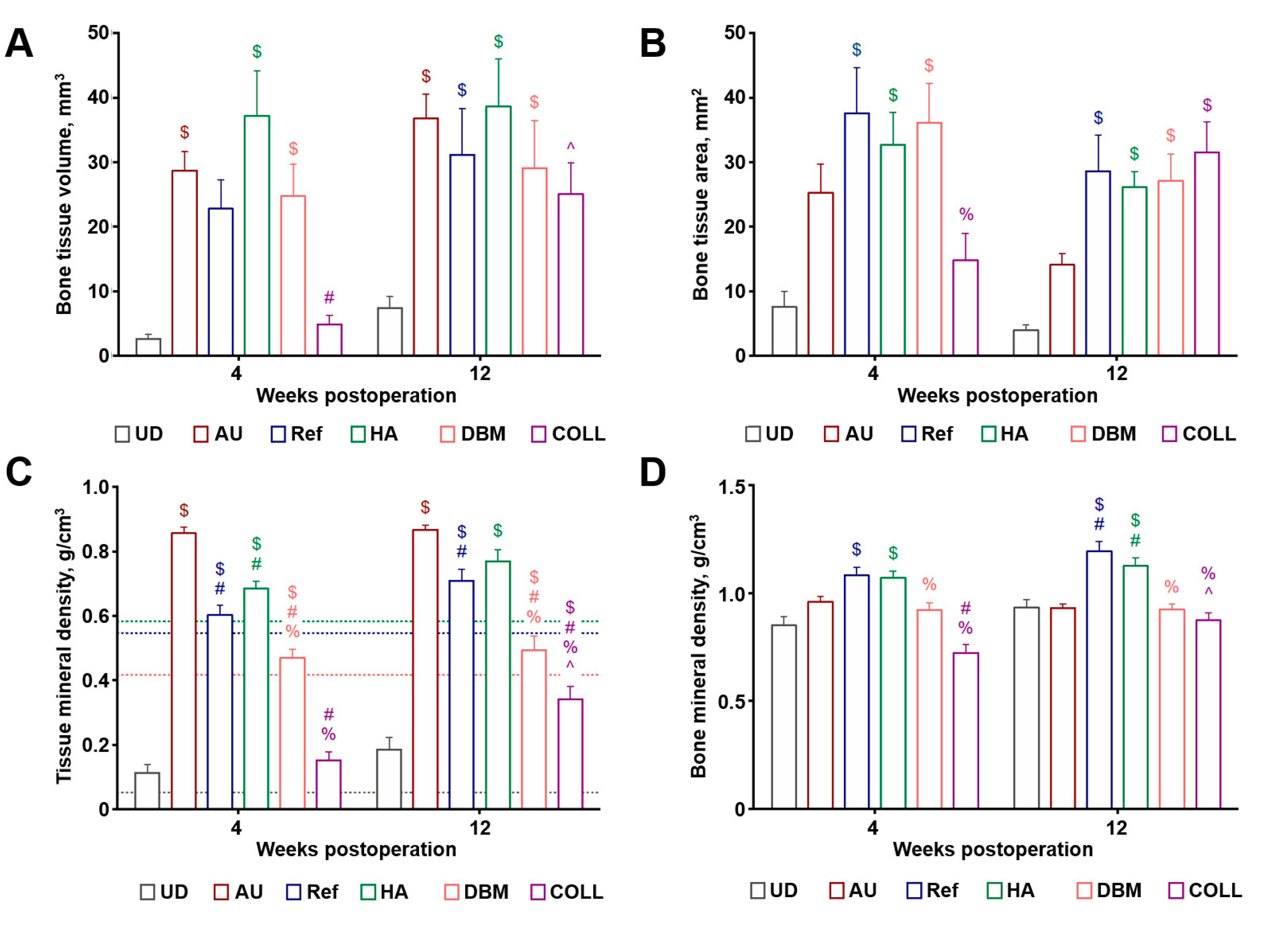
2.2. Bovine HA and DBM Are Highly and Equally Efficient for the Repair of Critical-Sized Rat Calvarial Defect While COLL Demonstrates the Highest Bone Repair Rate
3. Discussion
4. Materials and Methods
4.1. Implant Preparation
4.2. Animal Model
4.3. Complete Bblood Count and Biochemical Analysis
4.4. Microcomputed Tomography
4.5. Histological Examination
4.6. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Courtney, P.M. Advances in Orthopedics-2; Jaypee Brothers Medical Publishers: New Delhi, India, 2018; pp. 1–220. [Google Scholar]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Haagsma, J.; Graetz, N.; Bolliger, I.; Naghavi, M.; Higashi, H.; Mullany, E.C.; Abera, S.F.; Abraham, J.P.; Adofo, K.; Alsharif, U.; et al. The global burden of injury: Incidence, mortality, disability-adjusted life years and time trends from the Global Burden of Disease study 2013. Inj. Prev. 2015, 22, 3–18. [Google Scholar] [CrossRef]
- Global Burden of Disease Child and Adolescent Health Collaboration; Kassebaum, N.; Kyu, H.H.; Zoeckler, L.; Olsen, H.E.; Thomas, K.; Pinho, C.; Bhutta, Z.A.; Dandona, L.; Ferrari, A.; et al. Child and Adolescent Health From 1990 to 2015: Findings From the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study. JAMA Pediatr. 2017, 171, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Mokdad, A.H.; Forouzanfar, M.H.; Daoud, F.; A Mokdad, A.; El Bcheraoui, C.; Moradi-Lakeh, M.; Kyu, H.H.; Barber, R.M.; Wagner, J.; Cercy, K.; et al. Global burden of diseases, injuries, and risk factors for young people’s health during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2016, 387, 2383–2401. [Google Scholar] [CrossRef]
- Hasan, A.; Byambaa, B.; Morshed, M.; Cheikh, M.I.; Shakoor, R.A.; Mustafy, T.; Marei, H.E. Advances in osteobiologic materials for bone substitutes. J. Tissue Eng. Regen. Med. 2018, 12, 1448–1468. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Manivasagam, G.; Sen, D. Progress of Regenerative Therapy in Orthopedics. Curr. Osteoporos. Rep. 2018, 16, 169–181. [Google Scholar] [CrossRef]
- Smith, W.R.; Hudson, P.W.; Ponce, B.A.; Manoharan, S.R.R. Nanotechnology in orthopedics: A clinically oriented review. BMC Musculoskelet. Disord. 2018, 19, 67. [Google Scholar] [CrossRef]
- Azi, M.L.; Aprato, A.; Santi, I.; Kfuri, M.; Masse, A.; Joeris, A.; Junior, M.K. Autologous bone graft in the treatment of post-traumatic bone defects: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2016, 17, 465. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J.J.; Resident, O.S. Orthopedic Surgery Professor and Department Chair Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef]
- Bhatt, R.A.; Rozental, T.D. Bone Graft Substitutes. Hand Clin. 2012, 28, 457–468. [Google Scholar] [CrossRef]
- Mansour, A.; Mezour, M.A.; Badran, Z.; Tamimi, F. Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Eng. Part A 2017, 23, 1436–1451. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, Z. A comparative study of the effect of Bio-Oss ® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting. J. Oral Pathol. Med. 2016, 46, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Khojasteh, A.; Fahimipour, F.; Jafarian, M.; Sharifi, D.; Jahangir, S.; Khayyatan, F.; Eslaminejad, M.B. Bone engineering in dog mandible: Coculturing mesenchymal stem cells with endothelial progenitor cells in a composite scaffold containing vascular endothelial growth factor. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Murata, K.; Omokawa, S.; Akahane, M.; Shimizu, T.; Kawamura, K.; Kawate, K.; Tanaka, Y. Promotion of Osteogenesis and Angiogenesis in Vascularized Tissue-Engineered Bone Using Osteogenic Matrix Cell Sheets. Plast. Reconstr. Surg. 2016, 137, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.L.; Da Rosa, W.L.O.; Cuevas-Suárez, C.E.; Carreño, N.L.; Da Silva, A.F.; Guim, T.N.; Dellagostini, O.A.; Piva, E. Histological Evaluation of Bone Repair with Hydroxyapatite: A Systematic Review. Calcif. Tissue Int. 2017, 101, 341–354. [Google Scholar] [CrossRef]
- Boskey, A.L. Bone composition: Relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep. 2013, 2, 447. [Google Scholar] [CrossRef]
- Clarke, B.L. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3, S131–S139. [Google Scholar] [CrossRef]
- Sobczak-Kupiec, A.; Kowalski, Z.; Wzorek, Z. Preparation of hydroxyapatite from animal bones. Acta Bioeng. Biomech. 2009, 11, 23–28. [Google Scholar]
- Bagher, Z.; Rajaei, F.; Shokrgozar, M.A. Comparative study of bone repair using porous hydroxyapatite/β-tricalcium phosphate and xenograft scaffold in rabbits with tibia defect. Iran. Biomed. J. 2012, 16, 18–24. [Google Scholar]
- Li, Q.; Zhou, G.; Yu, X.; Wang, T.; Xi, Y.; Tang, Z. Porous deproteinized bovine bone scaffold with three-dimensional localized drug delivery system using chitosan microspheres. Biomed. Eng. Online 2015, 14, 33. [Google Scholar] [CrossRef]
- Kürkçü, M.; Benlidayi, M.E.; Cam, B.; Sertdemir, Y. Anorganic bovine-derived Hydroxyapatite vs β-tricalcium phosphate in sinus augmentation: A comparative histomorphometric study. J. Oral Implantol. 2012, 38, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.A.; Monteiro, F. Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration. J. Biomed. Mater. Res. Part A 2015, 104, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-K.; Lee, J.-S.; Kim, M.-S.; Choi, S.-H.; Cho, K.-S.; Jung, U.-W. Sinus augmentation using BMP-2 in a bovine hydroxyapatite/collagen carrier in dogs. J. Clin. Periodontol. 2013, 41, 86–93. [Google Scholar] [CrossRef]
- Lewis, C.S.; Supronowicz, P.R.; Zhukauskas, R.M.; Gill, E.; Cobb, R.R. Local antibiotic delivery with demineralized bone matrix. Cell Tissue Bank. 2011, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Z.; Chen, B.; Yang, M.; Zhao, Y.; Sun, W.; Xiao, Z.; Zhang, J.; Dai, J. Loading of VEGF to the heparin cross-linked demineralized bone matrix improves vascularization of the scaffold. J. Mater. Sci. Mater. Electron. 2009, 21, 309–317. [Google Scholar] [CrossRef]
- Chen, B.; Lin, H.; Wang, J.; Zhao, Y.; Wang, B.; Zhao, W.; Sun, W.; Dai, J. Homogeneous osteogenesis and bone regeneration by demineralized bone matrix loading with collagen-targeting bone morphogenetic protein-2. Biomaterials 2007, 28, 1027–1035. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Glattauer, V.; Ramshaw, J.A.M.; Werkmeister, J.A. Evaluation of the immunogenicity and cell compatibility of avian collagen for biomedical applications. J. Biomed. Mater. Res. Part A 2009, 9999, 1235–1244. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef]
- Lynn, A.K.; Yannas, I.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Schaller, B.; Saulacic, N.; Zhang, Y.; Miron, R.J. Growth factor delivery of BMP9 using a novel natural bovine bone graft with integrated atelo-collagen type I: Biosynthesis, characterization, and cell behavior. J. Biomed. Mater. Res. Part A 2016, 105, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-H.; Lee, Y.-J.; Rhie, J.-W.; Suh, D.-S.; Oh, D.Y.; Lee, J.-H.; Kim, Y.-J.; Kim, S.-M.; Jun, Y.-J. Comparative study of the effectiveness and safety of porcine and bovine atelocollagen in Asian nasolabial fold correction. J. Plast. Surg. Hand Surg. 2014, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Brydone, A.S.; Meek, D.; MacLaine, S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1329–1343. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, R.J.; Mao, J.J. Bone tissue engineering and regeneration: From discovery to the clinic—An overview. Tissue Eng. Part B Rev. 2011, 17, 389–392. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Li, B.; Zhang, Z.; Zhao, L. Gene expression profile study on osteoinductive effect of natural hydroxyapatite. J. Biomed. Mater. Res. Part A 2013, 102, 2833–2841. [Google Scholar] [CrossRef]
- Dodds, R.A.; York-Ely, A.M.; Zhukauskas, R.; Arola, T.; Howell, J.; Hartill, C.; Cobb, R.R.; Fox, C. Biomechanical and radiographic comparison of demineralized bone matrix, and a coralline hydroxyapatite in a rabbit spinal fusion model. J. Biomater. Appl. 2009, 25, 195–215. [Google Scholar] [CrossRef]
- Alidadi, S.; Oryan, A.; Bigham-Sadegh, A.; Moshiri, A. Comparative study on the healing potential of chitosan, polymethylmethacrylate, and demineralized bone matrix in radial bone defects of rat. Carbohydr. Polym. 2017, 166, 236–248. [Google Scholar] [CrossRef]
- Bigham-Sadegh, A.; Karimi, I.; Alebouye, M.; Shafie-Sarvestani, Z.; Oryan, A. Evaluation of bone healing in canine tibial defects filled with cortical autograft, commercial-DBM, calf fetal DBM, omentum and omentum-calf fetal DBM. J. Vet. Sci. 2013, 14, 337–343. [Google Scholar] [CrossRef]
- Salamanca, E.; Hsu, C.-C.; Huang, H.-M.; Teng, N.-C.; Lin, C.-T.; Pan, Y.-H.; Chang, W.-J. Bone regeneration using a porcine bone substitute collagen composite in vitro and in vivo. Sci. Rep. 2018, 8, 984. [Google Scholar] [CrossRef]

| Experimental Group (n = 12 Per Each) | Sample Biomaterial |
|---|---|
| HA | Refined bovine hydroxyapatite powder |
| DBM | Demineralised bovine bone matrix powder |
| COLL | Purified bovine bone collagen membranes |
| Ref | Geistlich Bio-Oss®, powder |
| AU | Autograft (excised rat calvarial bones) |
| UD | None (unfilled defect) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veremeev, A.; Bolgarin, R.; Nesterenko, V.; Andreev-Andrievskiy, A.; Kutikhin, A. Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects. Materials 2020, 13, 3393. https://doi.org/10.3390/ma13153393
Veremeev A, Bolgarin R, Nesterenko V, Andreev-Andrievskiy A, Kutikhin A. Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects. Materials. 2020; 13(15):3393. https://doi.org/10.3390/ma13153393
Chicago/Turabian StyleVeremeev, Alexey, Roman Bolgarin, Vladimir Nesterenko, Alexander Andreev-Andrievskiy, and Anton Kutikhin. 2020. "Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects" Materials 13, no. 15: 3393. https://doi.org/10.3390/ma13153393
APA StyleVeremeev, A., Bolgarin, R., Nesterenko, V., Andreev-Andrievskiy, A., & Kutikhin, A. (2020). Native Bovine Hydroxyapatite Powder, Demineralised Bone Matrix Powder, and Purified Bone Collagen Membranes Are Efficient in Repair of Critical-Sized Rat Calvarial Defects. Materials, 13(15), 3393. https://doi.org/10.3390/ma13153393






