NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Parametric Model Initial Predictions
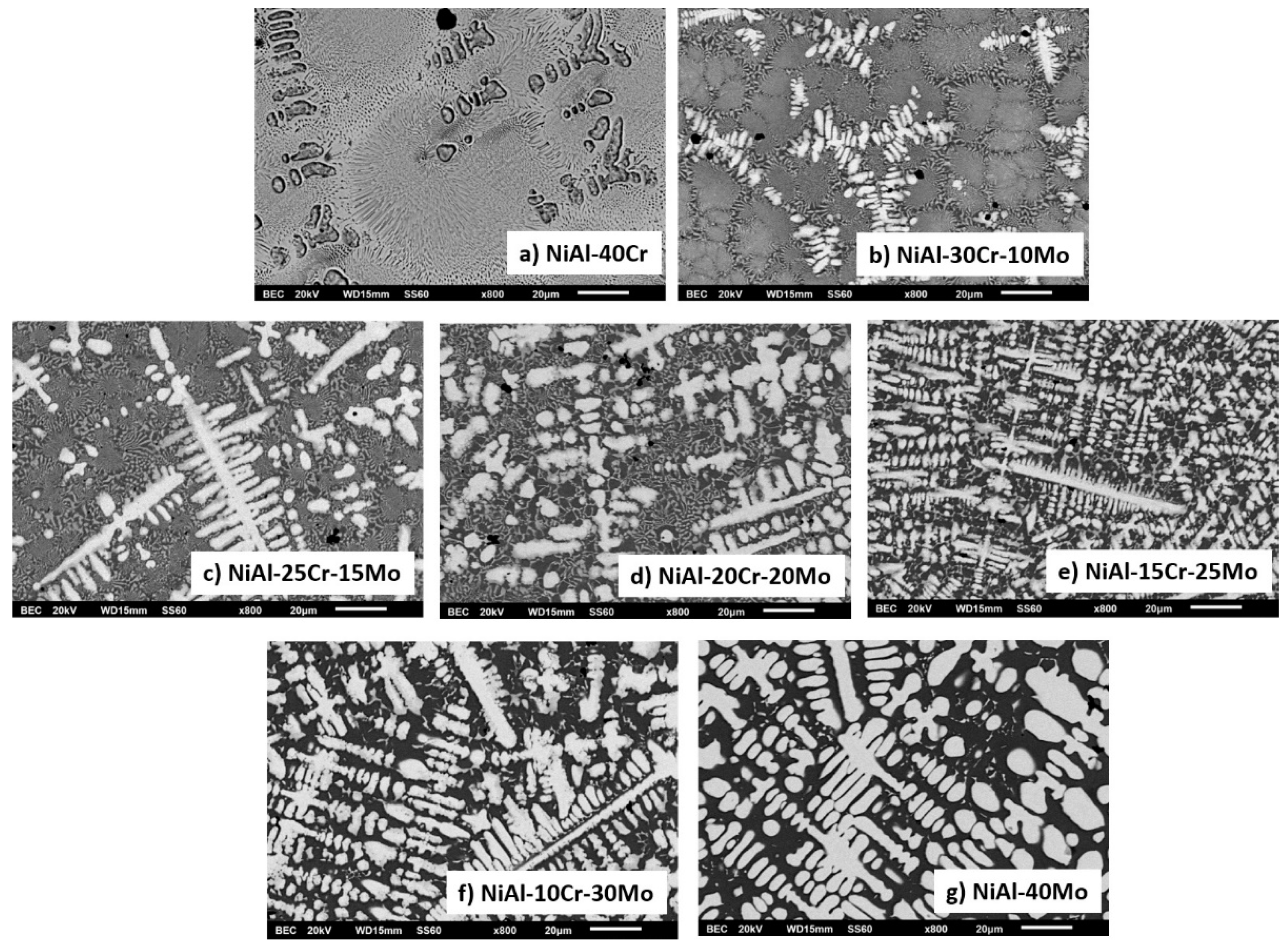
3.2. Microstructural Features
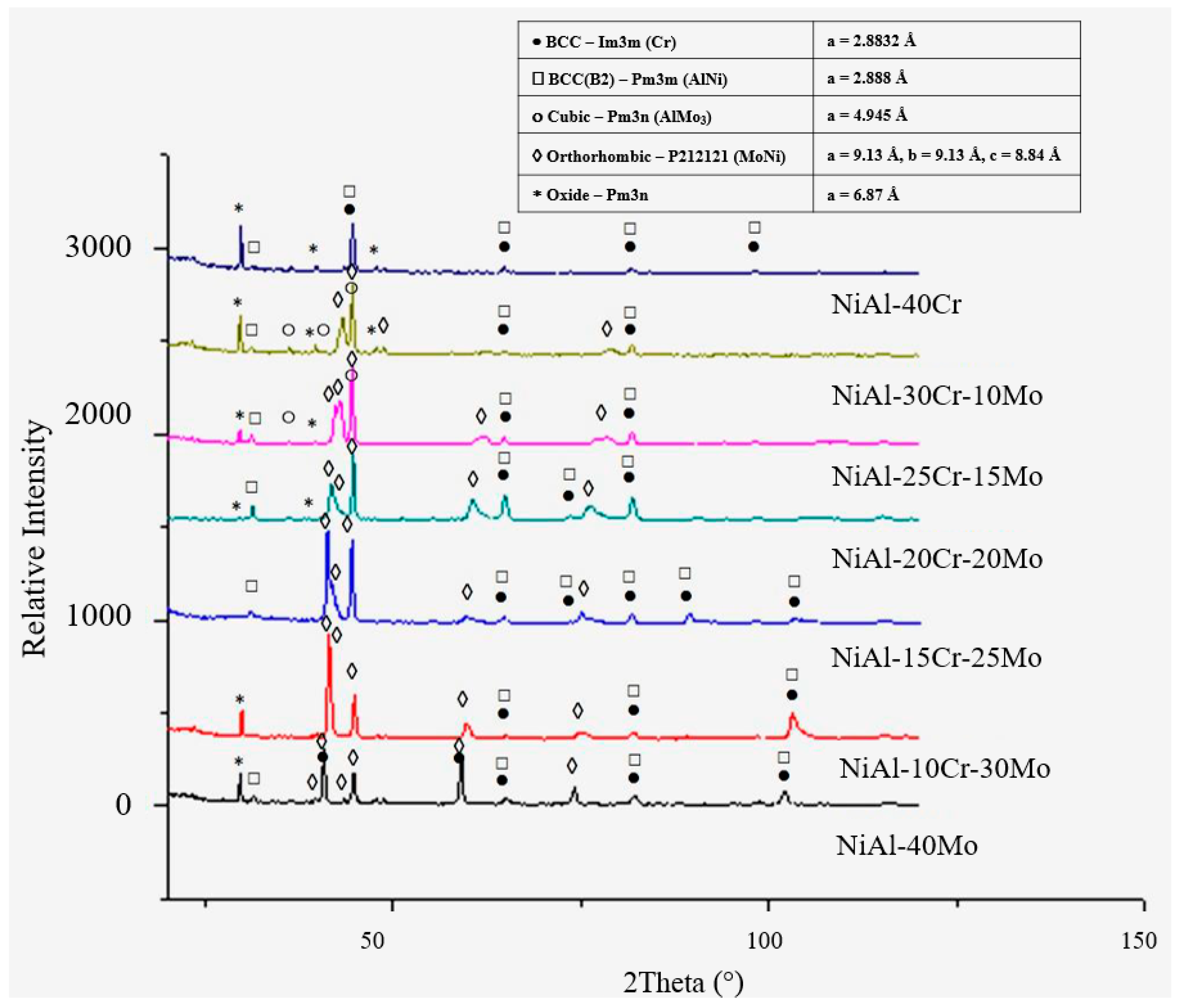
3.3. Solidification and Phase Formation Considerations
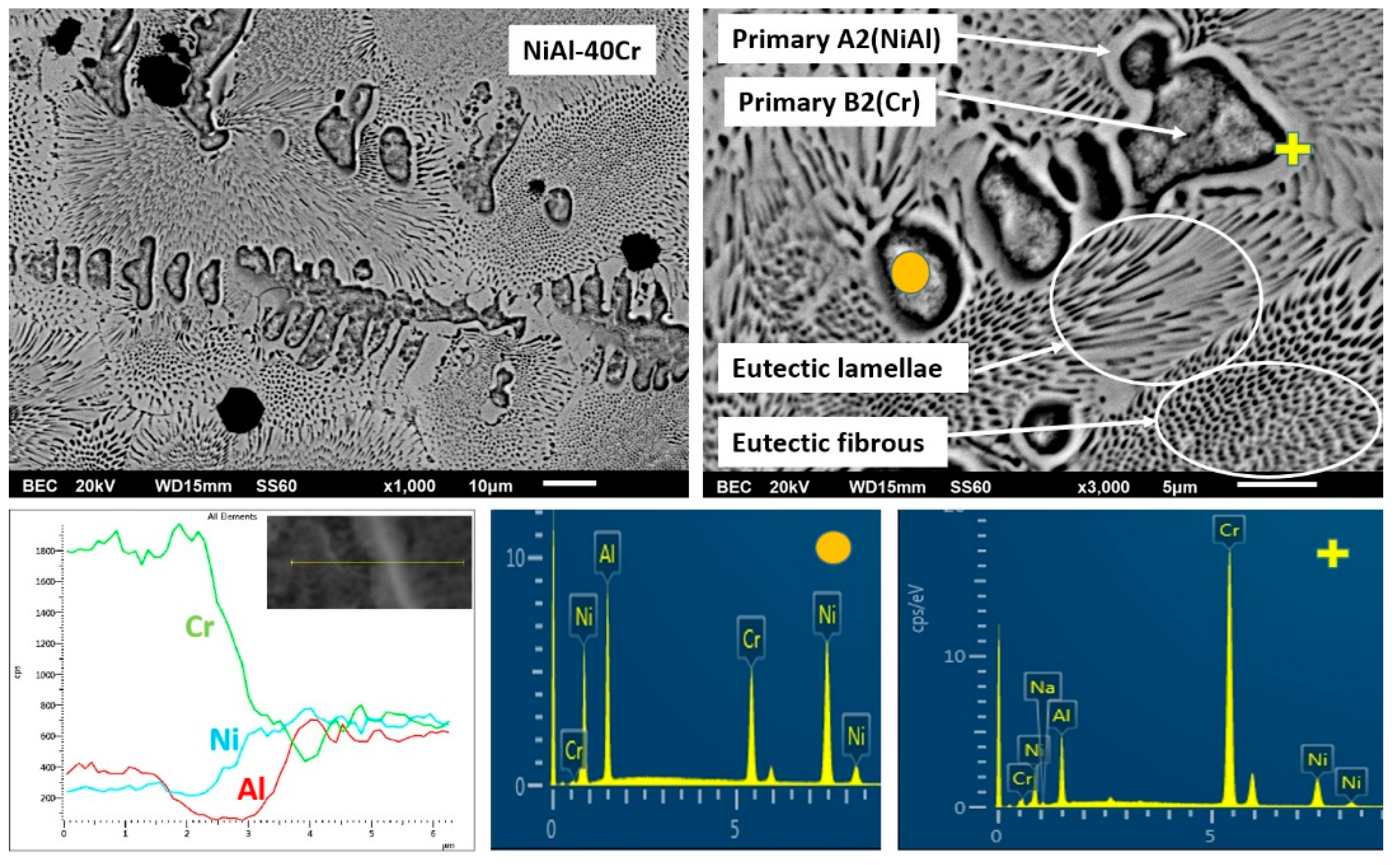
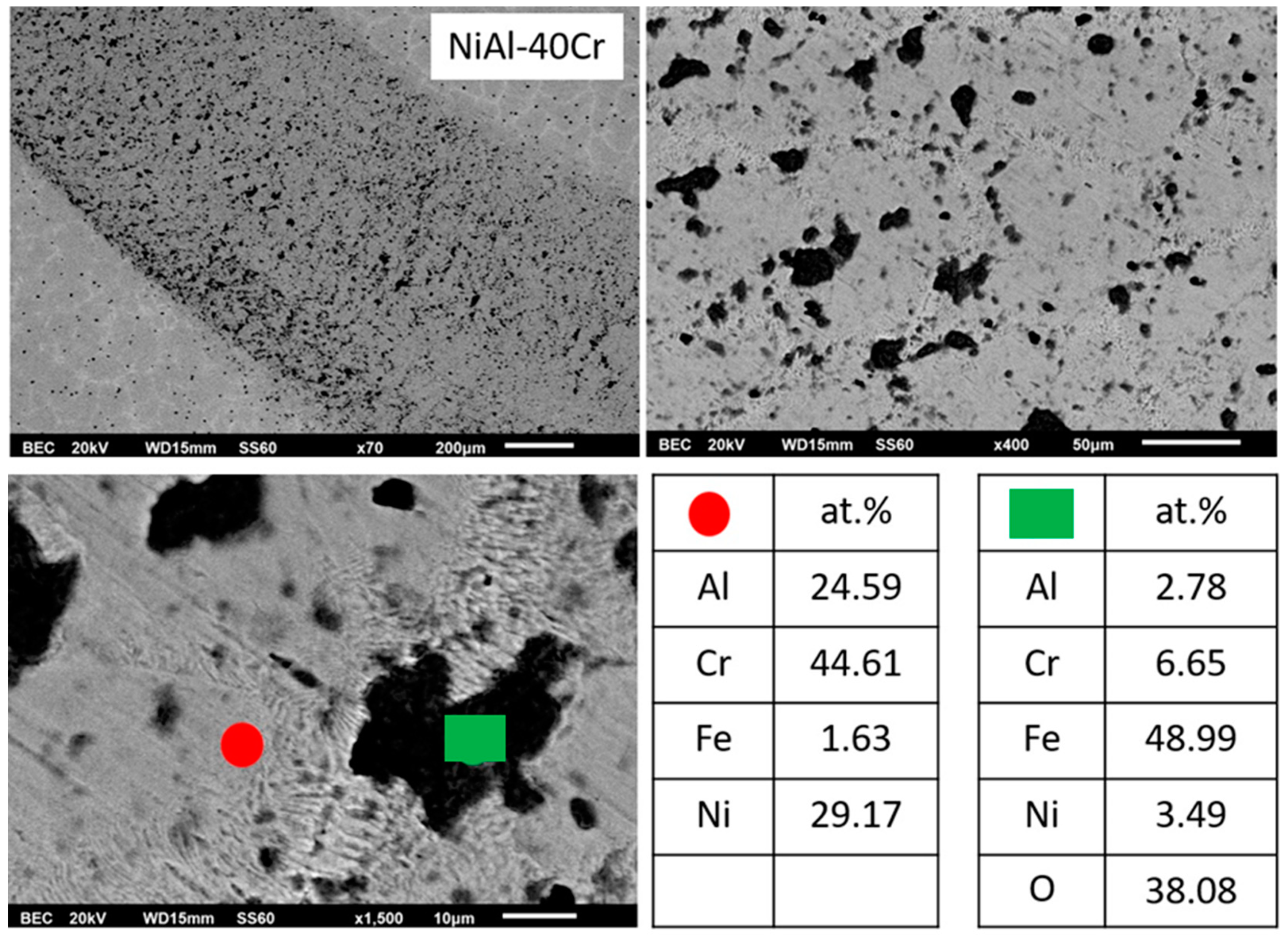
3.3.1. Alloy A:NiAl-40Cr
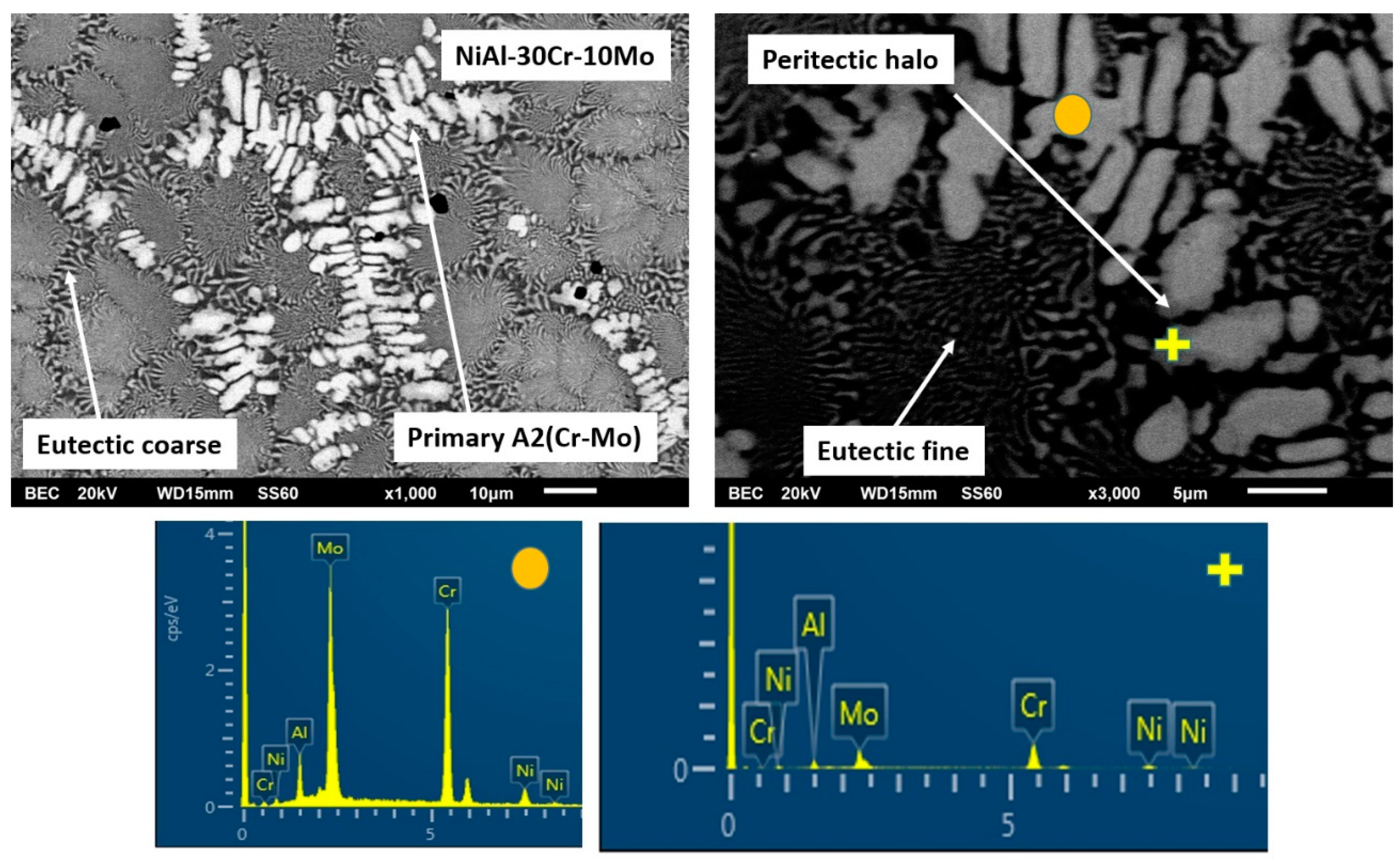
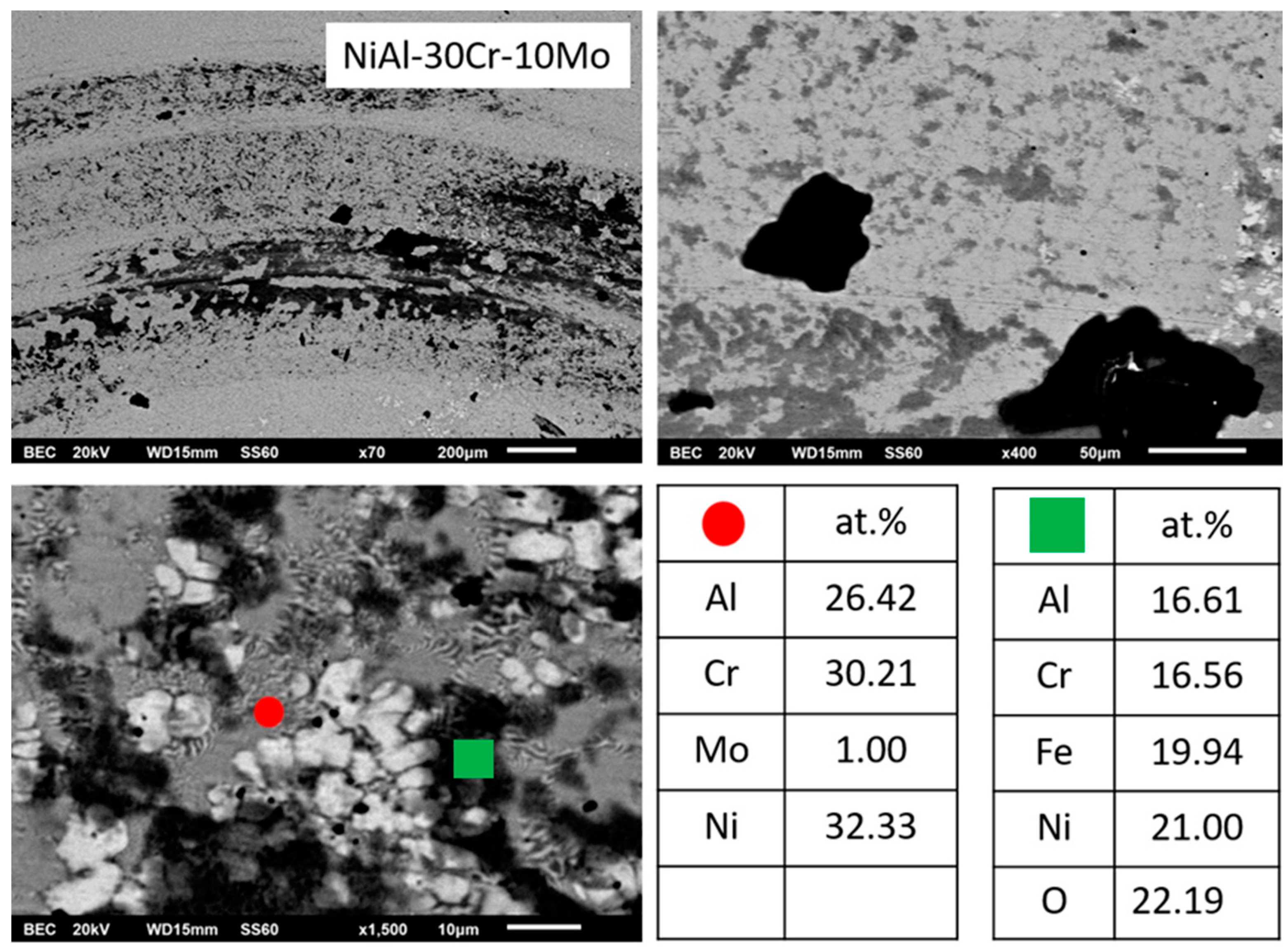
3.3.2. Alloy B: NiAl-30Cr-10Mo
- (1)
- Based on EDS analysis (Table 1), the primary A2 phase contains Cr and Mo in relative ratio of Cr/Mo ≈ 70/30. According to the Cr-Mo phase diagram [48], this ratio leads to an increase of the liquidus temperature by several degrees compared to the melting point of Cr. This observation, in conjunction with the fact that Cr-Mo show complete solubility, leads to the fact that the presence of Mo controls the initiation of the solidification process, binds Cr form the liquid phase, and forms a primary modified A2 Cr phase rich in Mo. It is worth noticing, as well, that if the value of the melting temperature of the primary phase, as calculated by the parametric models (~2200 K), is taken into consideration, the tendency for the primary Cr(Mo) formation becomes even stronger.
- (2)
- The presence of Mo most likely affects the T0-A2 temperature presented by Tang et al. [15]. The presence of Mo, as stated previously, raises considerably the liquidus temperature. Having taken into consideration the fact that during arc melting, the temperature of heating and melting of the various systems was kept constant (arc parameters were not modified or changed), it is logical to assume that the increase of the liquidus temperature corresponds to a lower degree of liquid phase overheating, compared with the case of the NiAl-40Cr alloy. A lower degree of liquid phase overheating, as Mei and Li [49] and Yang et al. [50] postulate, leads to a reduction of the necessary for nucleation undercooling. In the case of the present work, this practically implies that the presence of Mo shifts the T0-A2 temperatures towards higher values, i.e., the necessary undercooling for primary Cr(Mo) A2 phase nucleation is reduced. It is strongly likely, that the established upon the initial stages of solidification, undercooling conditions were dropped below the affected T0-A2 Cr(Mo) values, and as such, the formation of A2 Cr(Mo) phase became dominant.
- (3)
- Synergistically to these potential mechanisms, another parameter that may also enhance the stability of the primary A2 Cr(Mo) phase is the lattice distortion value δ. As shown in Table 1, the calculated lattice distortion δ in the case of the primary NiAl B2 in alloy NiAl-40Cr is roughly 6.8, whereas in the case of the primary A2 Cr(Mo) in the present system, δ is roughly 4.8. This difference makes the A2 Cr(Mo) phase more favorable both in stability and kinetics of formation.
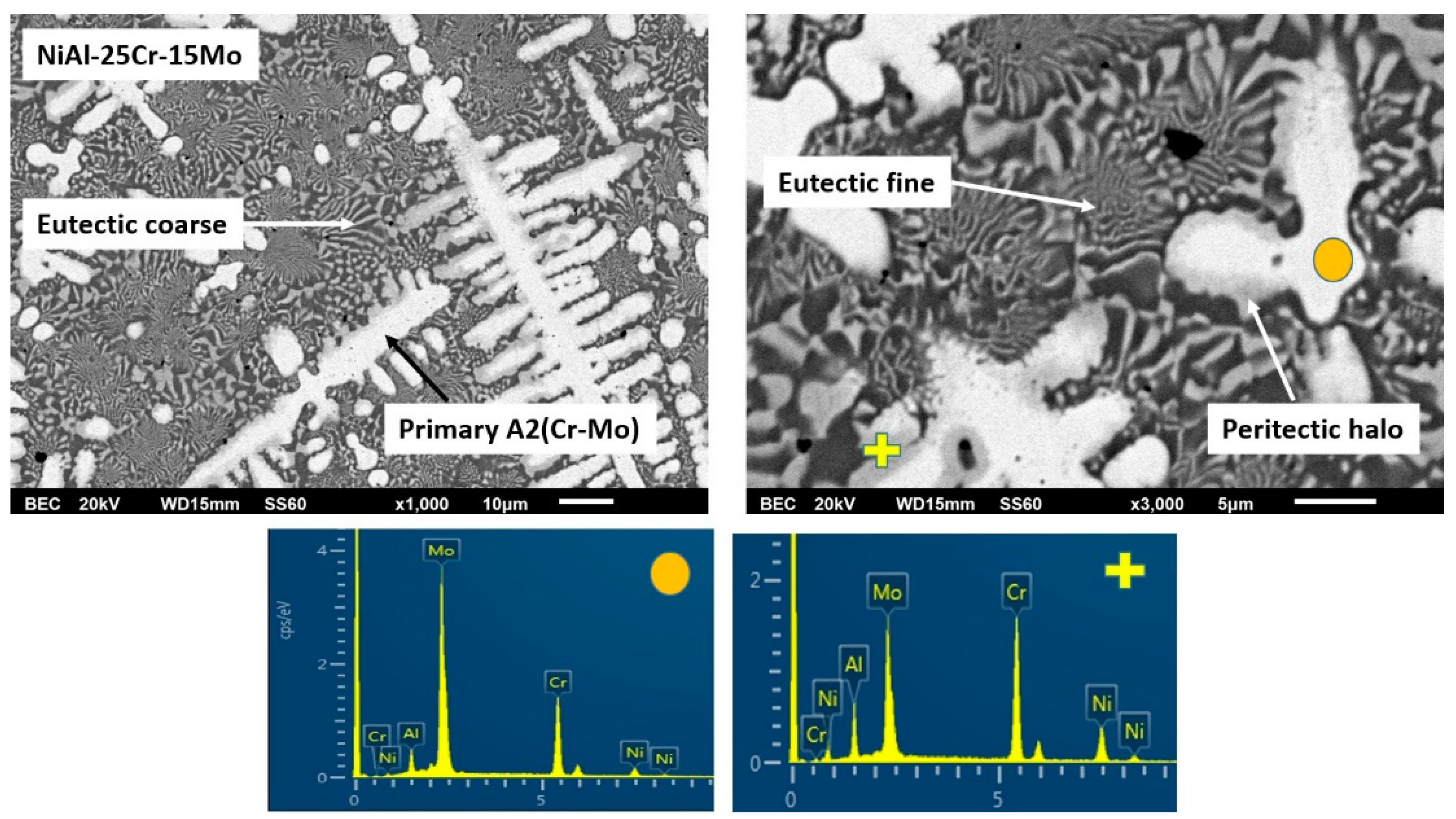
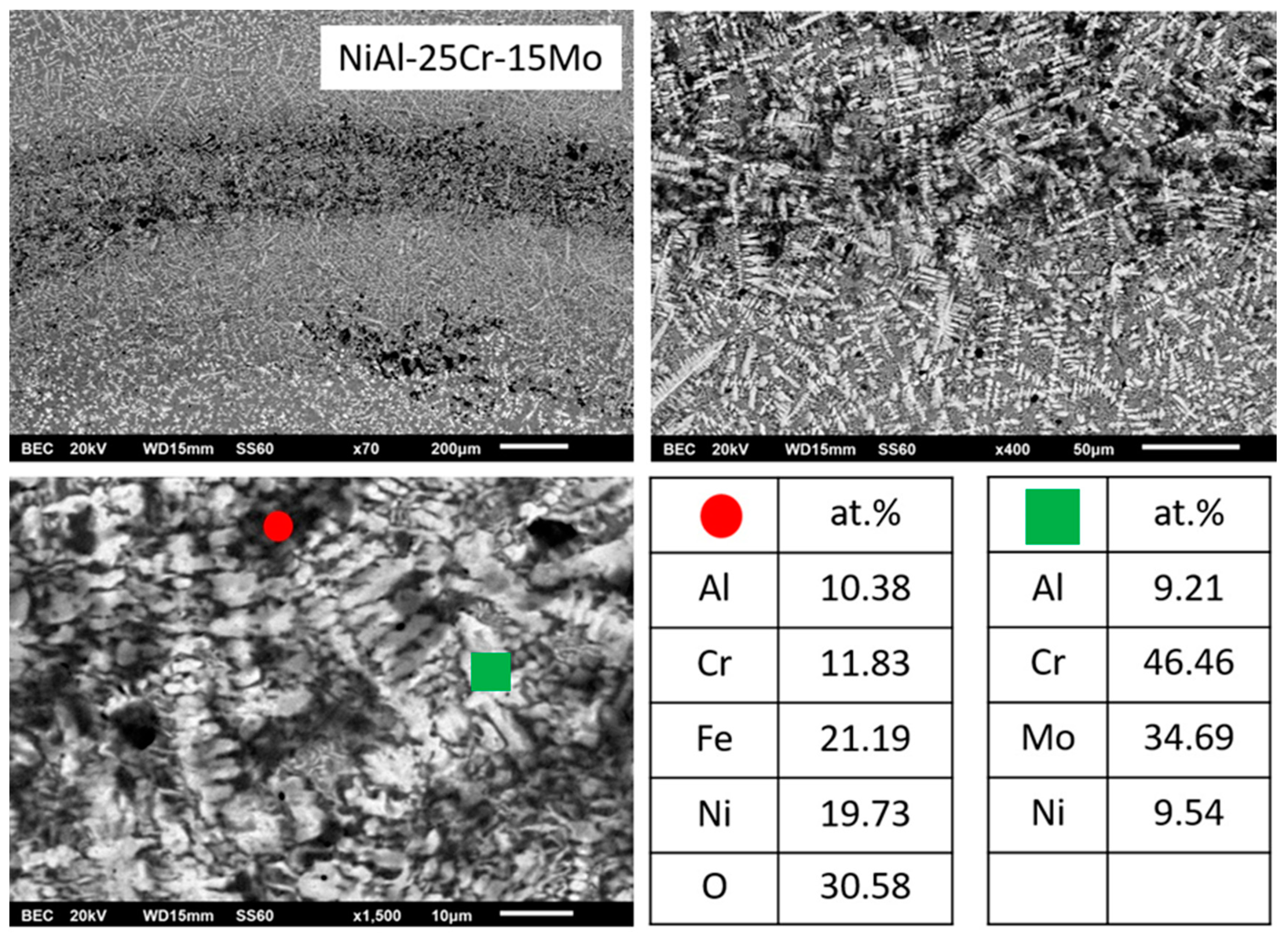
3.3.3. Alloy C: NiAl-25Cr-15Mo
3.3.4. Alloy D:NiAl-20Cr-20Mo
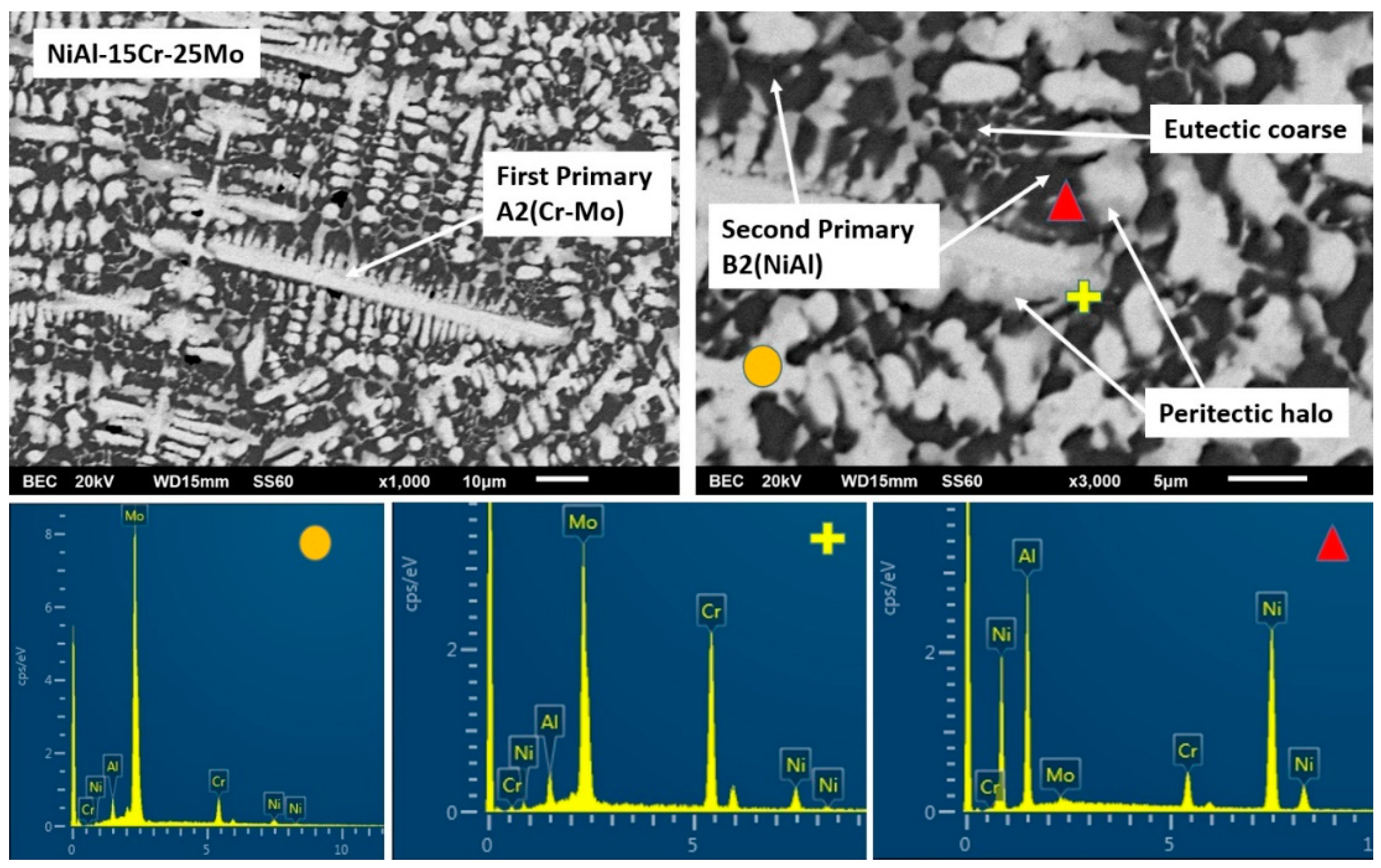
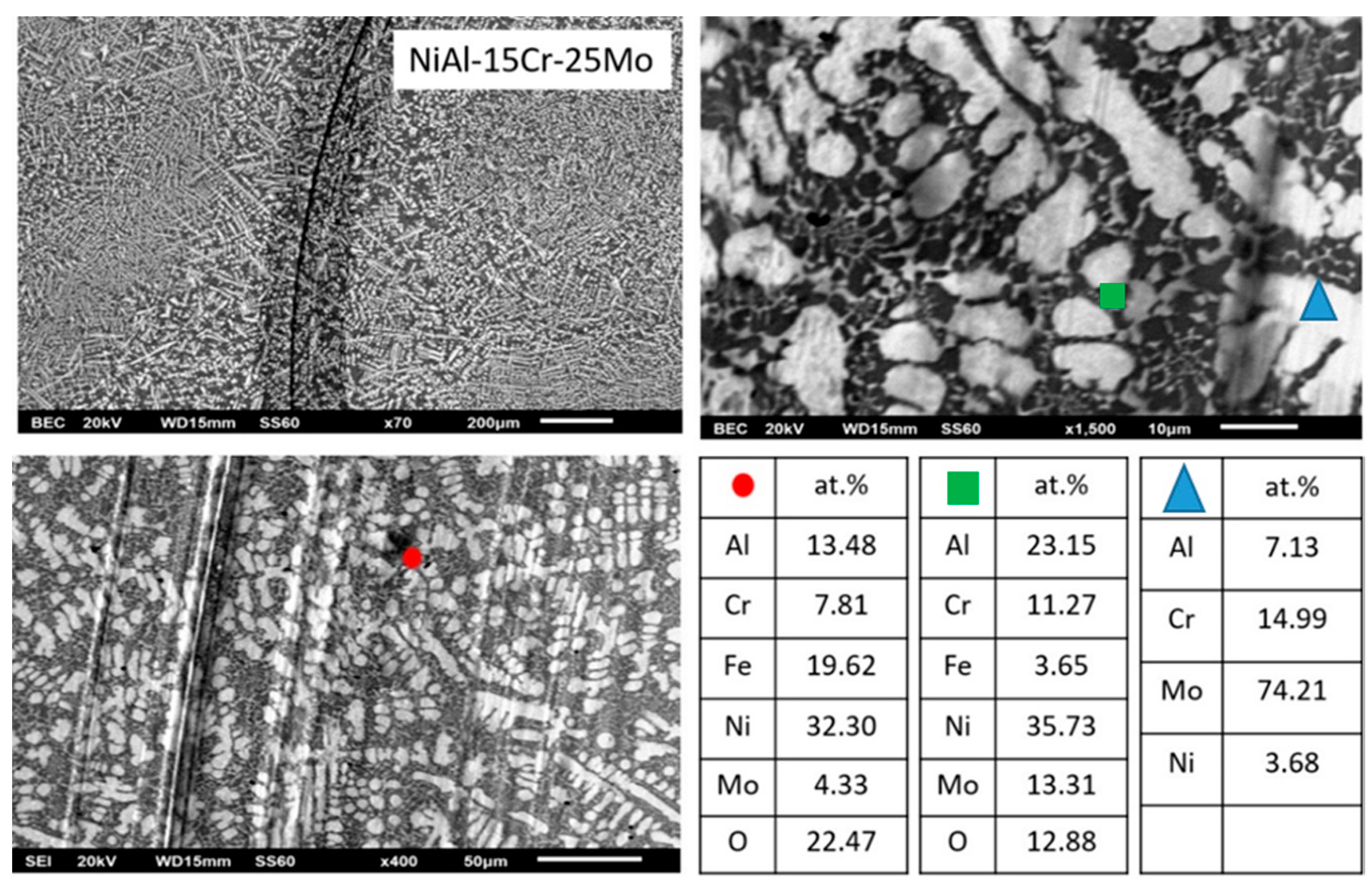
3.3.5. Alloy E: NiAl-15 Cr-25 Mo
3.3.6. Alloy F: NiAl-10Cr-30 Mo
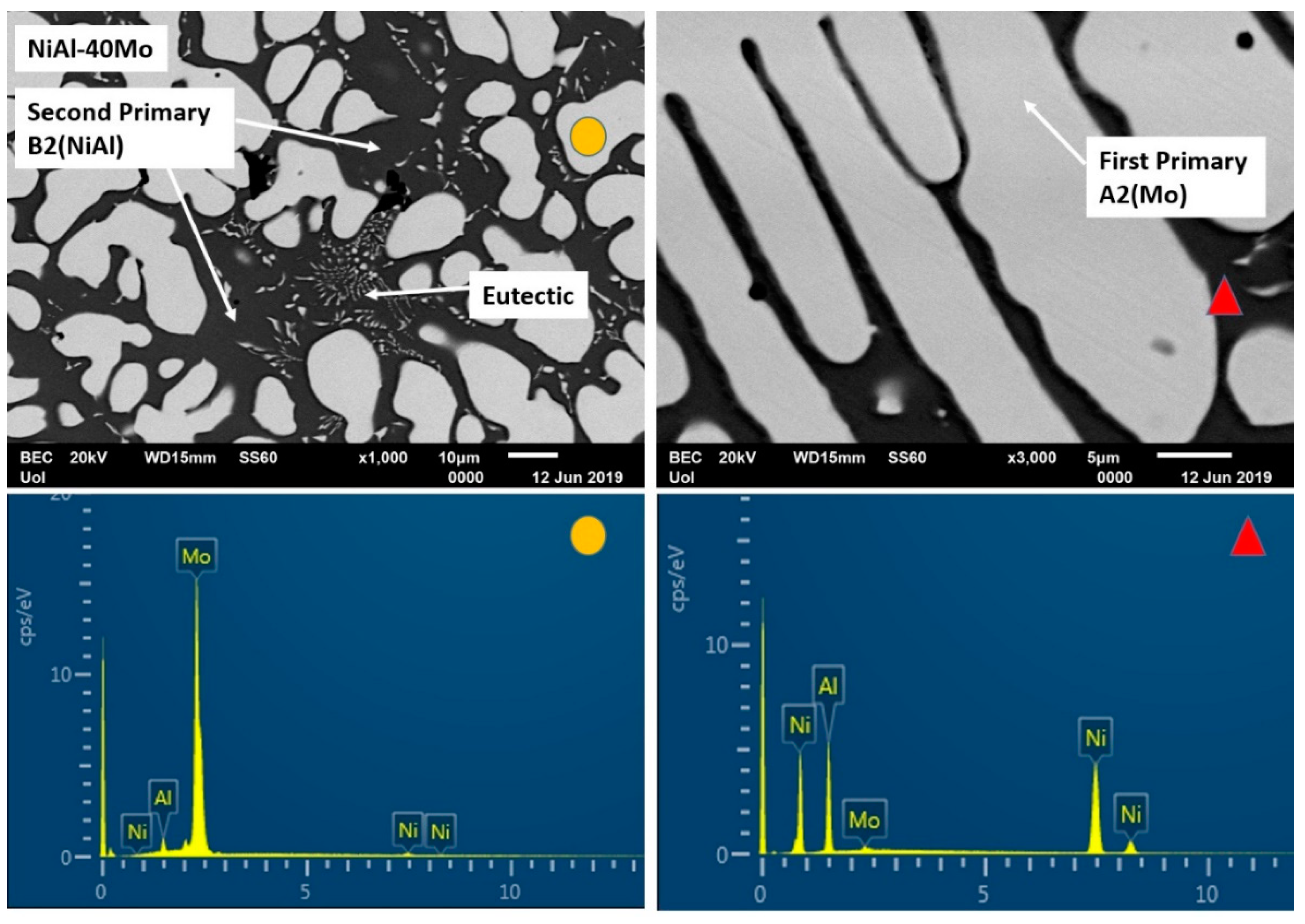
3.3.7. Alloy G: NiAl-40Mo
- (1)
- Upon solidification initiation, undercooling falls below the T0-A2 (Mo) and T0-B2 (NiAl) temperatures establishing such conditions for decoupled, partitionless growth of Mo and NiAl phases, with Mo being the first one to form due to its higher melting point.
- (2)
- As soon as recalescence is expressed, temperature is raised so that Mo continues to grow in a partitioning to the liquid mode, whereas NiAl proceeds in a partitionless way.
- (3)
- Further increase of temperature in the remaining liquid establishes conditions for partitioning growth of both NiAl and Mo establishing conditions for the development of the eutectic mixture growth, as the final stage of the solidification process.
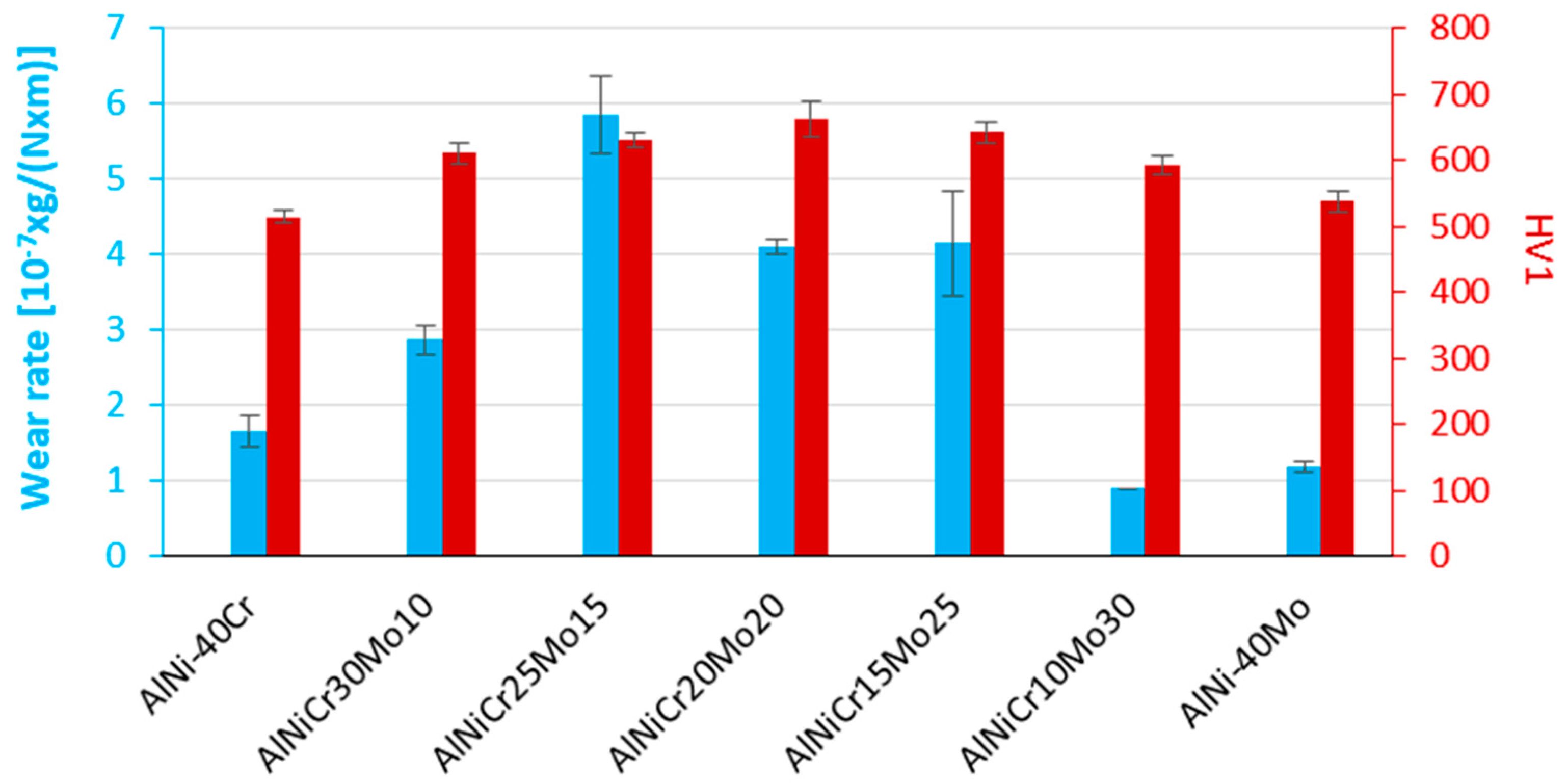
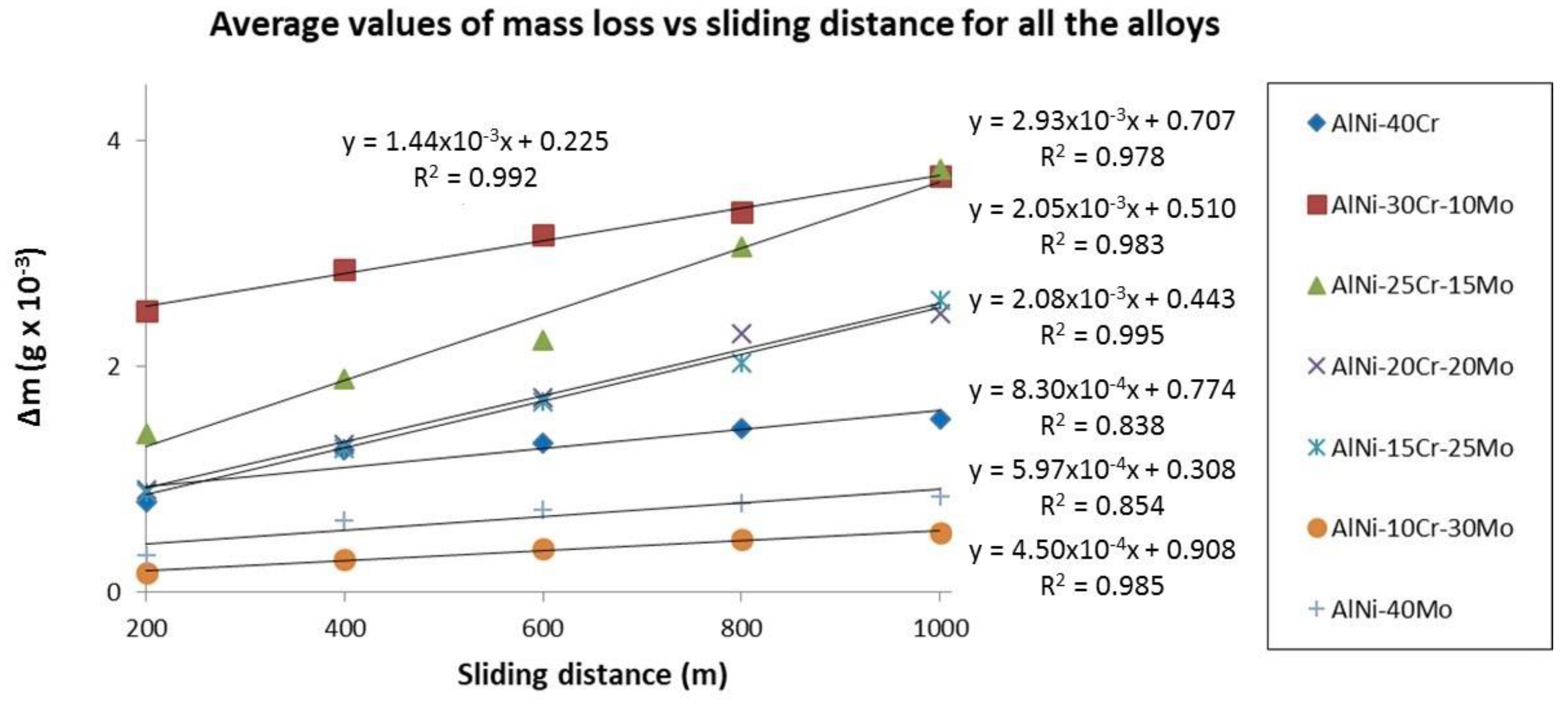
3.4. Microhardness and Wear Response
3.4.1. Case 1: the NiAl-Cr alloy
3.4.2. Case 2: alloys with progressively increasing Mo content with Cr being present (alloys B to F)
- (1)
- A peak value of the wear rate is observed for alloy B, which is gradually reduced until the case of alloy F. This practically means that the introduction of small amount of Mo bursts the wear rate at very high values, yet the progressive increase of the Mo content leads to a gradual reduction of the wear rates.
- (2)
- All wear tracks (Figure 14, Figure 15, Figure 16, Figure 17 and Figure 18) show an extensive material loss at the areas of the lastly solidified liquid, irrespectively of this area’s morphological feature (being a eutectic microconstituent or not). This observation is very crucial as it suggests that the interfacial areas between the lastly to solidify liquid and the primary phases are weak and their rigidity is highly questionable.
- (3)
- The oxide phases being present within the cavities of the removed materials are also of great importance. EDS analysis (Figure 14, Figure 15, Figure 16, Figure 17 and Figure 18) on these selective areas shows that in the case of alloy B, the oxides phases (debris formed upon testing) are a mixture of Fe and Cr oxides. As the Mo content increases, the composition of these oxides is altered. In alloy C, the oxide phases are rich in Fe rather in Cr, and in the case of alloy D, the Mo content of the oxide phases is taking the lead. Further increase of Mo, alloy E, shows that the Ni is the dominant element followed by Fe. The Cr content is significantly reduced. Finally, in the case of alloy F, the Ni content is further increased and the Cr content is further reduced. As it will be discussed in a following paragraph, these elemental variations play a vital role on the lubricating action of the oxide phases being formed.
- (4)
- Even among these alloys, a classification of their wear rates can be conducted: alloys B and C forming the first group of high wear rates, alloys D and E forming a second group with reduced wear rates, and finally alloy F with considerably reduced wear rate. There must be a reason for this kind of transition which will be addressed later.
- (5)
- Another observation that will help in the explanation of the wear behavior of the different systems is the morphology of the primary phases and, especially, the morphology of the contour of the primary phases. By recalling the involved microstructures (Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9), it can be observed that the periphery of the primary phases in the cases of alloys B to E is characterized by an intensive jigsaw like morphology with acicular protrusions towards the lastly to solidify liquid. Alloy G shows a more planar, yet smoother perimetric contour of the primary phase.
- (1)
- The first crucial factor, as already mentioned, is the nature of the oxides formed. The observed oxides in these systems are Fe-based, Ni-based, Mo-based, and Cr-based oxides. According to the extensive reviews of Glascott et al. [58] and Stott and Wood [59], among these oxides, the most effective in performing lubricating action are the Fe-, Ni-, and Mo-based oxides which belong to the category of the so-called ductile oxides. Glascott et al. [58] postulates that these oxides under conditions of hydrostatic pressure show significant ductile behavior and can deform plastically. Glascott et al. [58] also mention that under sliding wear conditions, a high portion of hydrostatic pressure field (even as high as 60% of the overall stress field) especially at the areas of oxide asperity junctions, exists. Under this frame, the ductile oxides deform plastically and absorb a great amount of the shear stresses involved energy and provide to the system an effective lubrication action. Cr-based oxides on the other hand, despite the fact that they are harder, they are also very brittle and do not deform plastically as the other oxides, and as such, their lubrication action is limited. Stott et al. [59] also mentioned that another problem with the Cr-based oxides is their poor adhesion with the substrate especially in the case of Ni-Cr containing alloys. If we recall not the present experimental findings, it can be seen, in reverse order, in the alloys of high Mo content (alloys G, E, and D) that the oxides being formed are mainly Mo, Ni, and Fe-based, whereas the presence of Cr-based is very restricted. It is expected, thus, that in these systems, the oxides been present will perform a strong lubrication action. In the case of alloys C and B, it can be observed that the relative amounts of Cr are increased and as such, the lubricating action is reduced.
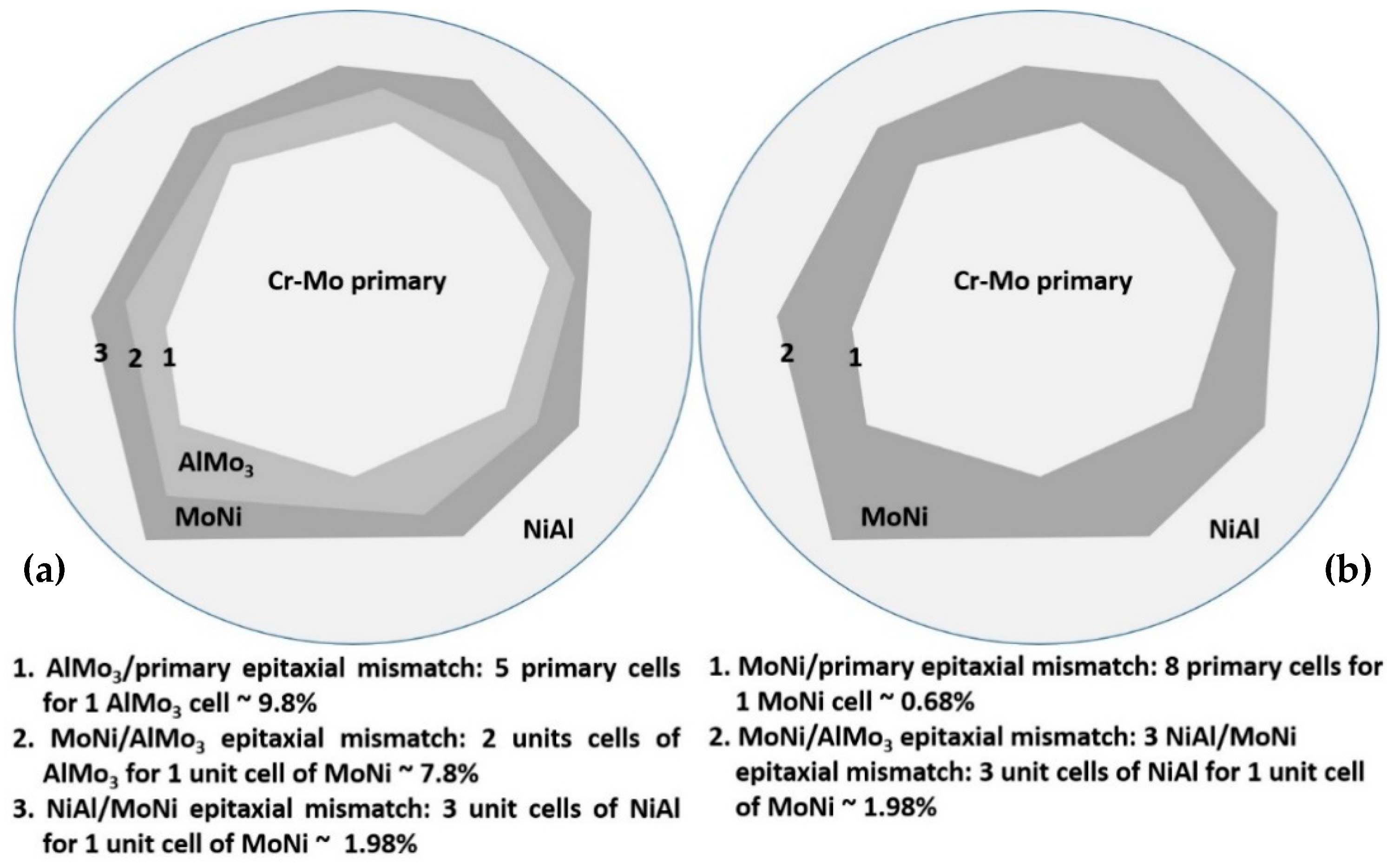
- (2)
- The severe mass loss, as already mentioned, is related to the extensive detachment of the last to solidify liquid areas. It should, hence, be a gradual weakening of the interfacial area between the primary phases and these lastly solidified regions. The difference between alloy A and alloys B to F, concerning these interfacial areas, is the presence of peritectic halos around the primary phases. As mentioned previously, the result of these peritectic reactions is the formation of strong and brittle intermetallic phases. Taking into consideration that as the Mo content increases from alloys B to C, the second primary phase to be formed is the NiAl intermetallic phase; Figure 19 shows a graphical representation of the possible epitaxial mismatches between the phases gradually formed from the core of the primary phases, through the formation of the peritectic halo intermetallics, to the last formation of the secondary primary NiAl. Alloys B and C belong to the first category of phase formation (Figure 19a) where both the peritectic intermetallics are present and the epitaxial mismatch starts from ~10%, reduced to ~8%, and finally, to ~2% at the outer layers. On the contrary, alloys D and E did not show the formation of the first peritectic halo reaction zone, and as such, the epitaxial mismatch (Figure 19b) starts with values as low as ~1% moving to ~2% at the outer layer. Intensive epitaxial mismatch can be a reason for intensive residual stresses being developed, and as such, the integrity and the stability of the interfacial areas are strongly and negatively affected [60]. This postulate is in agreement with the experimental findings of this effort. The interfacial rigidity in the case of alloys B and C is more affected due to the higher values of epitaxial mismatches, and as such, the danger of a detachment event is highly increased. On the contrary, the progression from the primary phase to the last solidified areas is smother in the case of alloys D and E, in terms of interfacial stability, and as such, the possibility of a forthcoming detachment event is reduced. Indeed, this is what is actually observed in the present case where alloys B and C showed significant material loss events in the last solidified regions compared to the same phenomenon in the case of alloys D and E. Furthermore, in the case of alloy F where the formation of peritectic halo is limited, the wear rate is further decreased.
3.4.3. Case 3: the NiAl-40Mo Alloy
4. Concluding Remarks
4.1. Microstructural Features
- (1)
- The microstructure of the NiAl-40Cr alloy is a result of solidification sequence according to which primary A2(Cr) and B2(NiAl) phases are developed partitionless due to undercooling phenomenon. Recalescence and its involved heat release lead to the development of a final eutectic structure.
- (2)
- The substitution of Cr by 10% Mo, in the case of alloy B, stabilizes the A2 phase as a primary phase as both Mo and undercooling do not favor a parallel B2 growth in a partitionless way. Phase diagram predictions verified in practice and multiple AlMo3 and MoNi intermetallic phases are developed at the periphery of the primary grains due to peritectic reactions. Recalescence leads to the final stage of eutectic phase formation.
- (3)
- By increasing the Mo content in all the preceding alloys, the A2 phase is further established as the primary phase, AlMo3 is eventually vanished, and the eutectic phase is continuously restricted. B2 (NiAl) is gradually increased as a secondary (following A2) “primary” phase. All these microstructural sequences are related to partitioning/partitionless growth, recalescence, and undercooling phenomenon.
- (4)
- The NiAl-40Mo alloy consists of primary Mo, NiAl, and NiAl-Mo eutectic constituents. The same phenomena, as in the previous cases, are responsible for this solidification sequence.
- (5)
- The increase of the Mo content leads to progressive elimination of the eutectic microconstituent and establishes significantly simpler microstructures of greater controlling potential.
4.2. Sliding Wear Response
- (1)
- The sliding wear behavior of alloy NiAl-40Cr showed extensive material loss due to localized delamination of the eutectic areas. Oxides of Fe and Cr are the main oxides being present.
- (2)
- An increased wear rate was observed in the case of alloy B (NiAl-30Cr-10Mo) by extensive material loss at the eutectic areas, due to the presence of intermetallic peritectic halos and the nonlubricating action of the formed oxides. The increase of the Mo content leads to the reduction of the wear rate (from alloy B to alloy F), due to the gradual restriction of the intermetallic phases and the gradual formation of oxides phases with intensive lubrication action.
- (3)
- The NiAl-40Mo is characterized by a relatively low wear rate due to the absence of intermetallic phases and the presence of lubricating oxides.
Author Contributions
Funding
Conflicts of Interest
Appendix A. Parametric Models
References
- Miracle, D.B. The physical and mechanical properties of NiAl. Acta Mater. 1993, 41, 649. [Google Scholar] [CrossRef]
- Ferrandini, P.; Batista, W.W.; Caram, R. Influence of Growth Rate on the Microstructure and Mechanical Behaviour of a NiAl-Mo Eutectic Alloy. J. Alloy. Compd. 2004, 381, 91–98. [Google Scholar] [CrossRef]
- Walter, J.L.; Cline, H.E. The Effect of Solidification Rate on Structure and High-Temperature Strength of the Eutectic NiAl-Cr. Metall. Mater. Trans. 1970, 1, 1221–1229. [Google Scholar] [CrossRef]
- Bogner, S.; Hu, L.; Hollad, S.; Hu, W.; Gottstein, G.; Bührig-Polaczek, A. Microstructure of a Eutectic NiAl-Mo Alloy Directionally Solidified using an Industrial Scale and a Laboratory Scale Bridgman Furnace. Int. J. Mater. Res. 2012, 103, 17–23. [Google Scholar]
- Hu, L.; Hu, W.; Gottstein, G.; Bogner, S.; Hollad, S.; Bührig-Polaczek, A. Investigation into Microstructure and Mechanical Properties of NiAl-Mo Composites Produced by Directional Solidification. Mater. Sci. Eng. A 2012, 539, 211–222. [Google Scholar] [CrossRef]
- Johnson, D.R.; Chen, X.F.; Oliver, B.F.; Noebe, R.D.; Whittenberger, J.D. Directional Solidification and Mechanical Properties of NiAl Single Bond NiAlTa Alloys. Intermetallics 1995, 3, 141–152. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Guo, J.T.; Ye, H.Q. Microstructure and mechanical properties of NiAl–Cr(Mo)/Nb eutectic alloy prepared by injection-casting. Mater. Des. 2009, 30, 964–969. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lill, J.V.; Chen, S.L.; Simmons, J.P. A ternary phase-field model incorporating commercial CALPHAD software and its application to precipitation in superalloys. Acta Mater. 2010, 58, 875–885. [Google Scholar] [CrossRef]
- Chen, S.T.; Tang, W.Y.; Kuo, Y.F.; Chen, S.Y.; Tsau, C.H.; Shun, T.T.; Yeh, J.W. Microstructure and properties of age-hardenable AlxCrFe1.5MnNi0.5 alloys. Mater. Sci. Eng. A 2010, 527, 5818–5825. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Juan, C.C.; Sheu, T.S.; Chen, S.K.; Yeh, J.W. Effect of aluminum content on microstructure and mechanical properties of AlxCoCrFeMo0.5Ni high- entropy alloys. J. Met. 2013, 65, 1840–1847. [Google Scholar]
- Hsu, C.Y.; Sheu, T.S.; Yeh, J.W.; Chen, S.K. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Wu, J.M.; Lin, S.J.; Yeh, J.W.; Chen, S.K.; Huang, Y.S.; Chen, H.C. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Munitz, A.; Meshi, L.; Kaufman, M.J. Heat treatments’ effects on the microstructure and mechanical properties of an equiatomic Al-Cr-Fe-Mn-Ni high entropy alloy. Mater. Sci. Eng. A 2017, 689, 384–394. [Google Scholar] [CrossRef]
- Tang, B.; Cogswell, D.A.; Hu, G.; Milenkovic, S.; Cui, Y. The formation mechanism of eutectic microstructures in NiAl-Cr composites. Phys. Chem. Chem. Phys. 2016, 18, 19773–19786. [Google Scholar] [CrossRef]
- Duprin, N.; Ansara, I.; Sundman, B. Thermodynamic Re-Assessment of the Ternary System Al-Cr-Ni. Calphad 2001, 25, 279–298. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P. Microstructures and Mechanical Properties of a Directionally Solidified NiAl-Mo Eutectic Alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Zhang, J.F.; Shen, J.; Shang, Z.; Feng, Z.R.; Wang, L.S.; Fu, H.Z. Microstructure and Room Temperature Fracture Toughness of Directionally Solidified NiAl-Mo Eutectic in situ Composites. Intermetallics 2012, 21, 18–25. [Google Scholar] [CrossRef]
- Chen, X.F.; Johnson, D.R.; Noebe, R.D.; Oliver, B.F. Deformation and Fracture of a Directionally Solidified NiAl-28Cr-6Mo Eutectic Alloy. J. Mater. Res. 1995, 10, 1159–1170. [Google Scholar] [CrossRef]
- Whittenberger, J.D.; Raj, S.V.; Locci, I.E.; Salem, J.A. Effect of Growth Rate on Elevated Temperature Plastic Flow and Room Temperature Fracture Toughness of Directionally Solidified NiAl-31Cr-3Mo. Intermetallics 1999, 7, 1159–1168. [Google Scholar] [CrossRef]
- Raj, S.V.; Locci, I.E. Microstructural Characterization of a Directionally-Solidified Ni-33 (at.%) Al-31Cr-3Mo Eutectic Alloy as a Function of Withdrawal Rate. Intermetallics 2001, 9, 217–227. [Google Scholar] [CrossRef][Green Version]
- Raj, S.V.; Locci, I.E.; Salem, J.A.; Pawlik, R.J. Effect of Directionally Solidified Microstructures on the Room-Temperature Fracture-toughness Properties of Ni-33(at.%)Al-33Cr-1Mo and Ni-33(at.%)Al-31Cr-3Mo Eutectic Alloys Grown at Different Solidification Rates. Metall. Mater. Trans. A 2002, 33, 597–612. [Google Scholar] [CrossRef]
- Shang, Z.; Shen, J.; Wang, L.; Du, Y.; Xiong, Y.; Fu, H. Investigations on the Microstructure and Room Temperature Fracture Toughness of Directionally Solidified NiAl-Cr(Mo) Eutectic Alloy. Intermetallics 2015, 57, 25–33. [Google Scholar] [CrossRef]
- Peng, J.; Franke, P.; Seifert, H.J. Experimental Investigation and CALPHAD Assessment of the Eutectic Trough in the System NiAl-Cr-Mo. J. Phase Equilib. Diffus. 2016, 37, 592–600. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Sheng, L.; Zhou, L.; Yuan, C.; Chen, Z.; Song, L. Wear properties of NiAl based materials. Prog. Nat. Sci. Mater. Int. 2012, 22, 414–425. [Google Scholar] [CrossRef]
- Mathiou, C.; Giorspyros, K.; Georgatis, E.; Karantzalis, A.E. Microstructural verification of the theoretically predicted morphologies of the NiAl–Cr pseudo-binary alloy systems and NiAl–Cr eutectic structure modification by Mo addition. SN Appl. Sci. 2019, 1, 1292. [Google Scholar] [CrossRef]
- Sheng, L.; Zhang, W.; Guo, J.; Ye, H. Microstructure and mechanical properties of Hf and Ho doped NiAl-Cr (Mo) near eutectic alloy prepared by suction casting. Mater. Charact. 2009, 60, 1311–1316. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L.; Xi, T.; Zheng, Y.; Ye, H. Microstructure, precipitates and compressive properties of various holmium doped NiAl/Cr (Mo, Hf) eutectic alloys. Mater. Des. 2011, 32, 4810–4817. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar]
- Wang, Z.; Huang, Y.; Yang, Y.; Wang, J.; Liu, C.T. Atomic-size effect and solid solubility of multicomponent alloys. Scr. Mater. 2015, 94, 28–31. [Google Scholar] [CrossRef]
- King, D.J.M.; Middleburgh, S.C.; McGregor, A.G.; Cortie, M.B. Predicting the formation and stability of single phase high-entropy alloys. Acta Mater. 2016, 104, 172–179. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Troparevsky, M.C.; Morris, J.R.; Kent, P.R.C.; Lupini, A.R.; Stocks, G.G. Criteria for predicting the formation of single-phase high-entropy alloys. Phys. Rev. X 2015, 5, 011041-1–011041-6. [Google Scholar]
- Senkov, O.N.; Miracle, D.B. A new thermodynamic parameter to predict formation of solid solution or intermetallic phases in high entropy alloys. J. Alloys Compd. 2016, 658, 603–607. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Poulia, A.; Georgatis, E.; Mathiou, C.; Karantzalis, A.E. Phase segregation discussion in a Hf25Zr30Ti20Nb15V10 high entropy alloy: The effect of the high melting point element. Mater. Chem. Phys. Mater. 2018, 210, 251–258. [Google Scholar] [CrossRef]
- Mathiou, C.; Poulia, A.; Georgatis, E.; Karantzalis, A.E. Microstructural features and dry—Sliding wear response of MoTaNbZrTi high entropy alloy. Mater. Chem. Phys. Mater. 2018, 210, 126–135. [Google Scholar] [CrossRef]
- Karantzalis, A.E.; Poulia, A.; Georgatis, E.; Petroglou, D.; Mathiou, C. New MoWHfZrTi refractory high entropy alloy system: A microstructural verification of phase formation criteria approach. Res. Rep. Met. 2017, 1, 1–6. [Google Scholar]
- Boettinger, W.J.; Banerjee, D.K. Physical Metallourgy, 5th ed.; Laughlin, D.E., Hono, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, p. 668. ISBN 978-0-444-59598-0. [Google Scholar]
- Mathiou1, C.; Ganara1, D.; E. Georgatis1, E.; Poulia1, A.; Lentzaris1, K.; Karantzalis1, A.E. Adjustment of hardness of (CrFeMn)x(NiyAl)1−x, (y = 1,3 and x = 0.6,0.72,0.80) High Entropy Alloys by Deliberate Control of Intermetallic Phase Formation: Microstructural Evolution, Hardness and Dry-Sliding Wear Response. Met. Mater. Int. 2020, 1–18. [Google Scholar] [CrossRef]
- Shang, Z.; Shen, J.; Zhang, J.; Wang, L.; Wang, L.; Fu, H. Effect of microstructures on the room temperature fracture toughness of NiAl–32Cr–6Mo hypereutectic alloy directionally solidified at different withdrawal rates. Mater. Sci. Eng. A 2014, 611, 306–312. [Google Scholar] [CrossRef]
- Yang, J.-M.; Jeng, S.M.; Bain, K.; Amato, R.A. Microstructure and mechanical behavior of in-situ directional solidified NiAl/Cr(Mo) eutectic composite. Acta Mater. 1997, 45, 295–305. [Google Scholar] [CrossRef]
- Tang, L.-Z.; Zhang, Z.-G.; Li, S.S.; Gong, S.-K. Mechanical behaviors of NiAl-Cr(Mo)-based near eutectic alloy with Ti, Hf, Nb and W additions. Trans. Nonferrous Met. Soc. China 2010, 20, 212–216. [Google Scholar] [CrossRef]
- Wang, L.; Shen, J.; Zhang, Y.; Xu, H.; Fu, H. Microstructure and mechanical properties of NiAl-based hypereutectic alloy obtained by liquid metal cooling and zone melted liquid metal cooling directional solidification techniques. J. Mater. Res. 2016, 31, 646–654. [Google Scholar] [CrossRef]
- Guo, S.; Chun, N.; Liu, C.T. Sunflower-like Solidification Microstructure in a Near-eutectic High-entropy alloy. Mater. Res. Lett. 2013, 1, 228–232. [Google Scholar] [CrossRef]
- Goetzinger, R.; Barth, M.; Herlach, D.M. Growth of lamellar eutectic dendrites in undercooled melts. J. Appl. Phys. 1998, 84, 1643–1649. [Google Scholar] [CrossRef]
- Li, M.; Kuribayashi, K. Free Solidification of Undercooled Eutectics. Mater. Trans. 2006, 47, 2889–2897. [Google Scholar] [CrossRef][Green Version]
- Available online: http://resource.npl.co.uk/mtdata/phdiagrams/crmo.htm (accessed on 28 May 2020).
- Mei, Q.; Li, J. Dependence of Liquid Supercooling on Liquid Overheating Levels of Al Small Particles. Materials 2016, 9, 7. [Google Scholar] [CrossRef]
- Yang, B.; Perepezko, J.H.; Schmelzer, J.W.P.; Gao, Y.; Schick, C. Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 2014, 140, 104513-1–104513-7. [Google Scholar] [CrossRef]
- Available online: http://resource.npl.co.uk/mtdata/phdiagrams/moni.htm (accessed on 28 May 2020).
- Available online: http://resource.npl.co.uk/mtdata/phdiagrams/almo.htm (accessed on 28 May 2020).
- Demirtas, H.; Gungor, A. Effect of alloying elements on the microstructure and mechanical properties of NiAl-Cr(Mo) eutectic alloy. Sci. Proc. XII Int. Congr. Macnines Technol. Mater. 2015, 2, 4–8. [Google Scholar]
- Tsai, M.; Yeh, J.W. High-Entropy Alloys: A Critical Review. J. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.-P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef]
- Booser, E.R. Tribology Data Handbook; CRC Press: New York, NY, USA, 1997. [Google Scholar]
- Glascott, J.; Stott, F.H.; Wood, G.C. The Effectiveness of Oxides in Reducing Sliding Wear of Alloys. Oxid. Met. 1985, 24, 99–114. [Google Scholar] [CrossRef]
- Stott, F.H.; Wood, G.C. The influence of Oxides on the Friction and Wear of Alloys. Tribol. Int. 1978, 11, 211–218. [Google Scholar] [CrossRef]
- Moridi, A.; Ruan, H.; Zhang, L.C.; Liu, M. Residual stresses in thin film systems: Effects of lattice mismatch, thermal mismatch and interface dislocations. Int. J. Solids Struct. 2013, 50, 3562–3569. [Google Scholar] [CrossRef]
- Kirkpatrick, R.J. Crystal growth from the melt—A review. Am. Miner. 1975, 60, 798–814. [Google Scholar]
- Sheng, L.Y.; Yang, F.; Xi, T.F.; Guo, J.T. Investigation on microstructure and wear behavior of the NiAl-TiC-Al2O3 composite fabricated by self-propagation high-temperature synthesis with extrusion. J. Alloy. Compd. 2013, 554, 182–188. [Google Scholar] [CrossRef]
- Sheng, L.Y. Microstructure, mechanical and tribological properties of the rapidly solidified NiAl/Cr (Mo, Dy) hypoeutectic alloy. Mater. Sci. Forum 2016, 849, 590–596. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505-1–103505-5. [Google Scholar] [CrossRef]
- Guo, S.; Hu, Q.; Ng, C.; Liu, C.T. More than entropy in high entropy alloys: Forming solid solutions or amorphous phase. Intermetallics 2013, 41, 96–103. [Google Scholar] [CrossRef]






| System | Composition (at.%) | Al | Ni | Cr | Mo | Mo/Cr + Mo | Mo/Ni + Mo | Mo/Al + Mo | Cr/NiAl + Cr | δ |
|---|---|---|---|---|---|---|---|---|---|---|
| Alloy A: AlNi-40Cr | Nominal | 30 | 30 | 40 | 0 | 0 | 0 | 0 | 57/100 | 4.96 |
| Actual | 26.21 | 33 | 40.76 | 0 | 0 | 0 | 0 | 0 | ||
| Primary Dark | 11.88 | 10.70 | 77.42 | 0 | 0 | 0 | 0 | 87.5/100 | ||
| Primary Light | 35.02 | 46.34 | 18.65 | 0 | 0 | 0 | 0 | 32/100 | ||
| Eutectic Overall | 36.92 | 43.76 | 19.32 | 0 | 0 | 0 | 0 | 32/100 | ||
| Alloy B: AlNi-30Cr-10Mo | Nominal | 30 | 30 | 30 | 10 | 25/100 | 25/100 | 25/100 | 50/100 | 4.83 |
| Actual | 25.7 | 33.2 | 30.99 | 10.11 | 25.5/100 | 23/100 | 28/100 | 52/100 | ||
| Primary Light | 8.09 | 7.01 | 58.98 | 25.91 | 30.5/100 | 78/100 | 76/100 | 88/100 | ||
| Primary Halo | 14.77 | 12.9 | 53.26 | 19.07 | 24/100 | 60/100 | 56/100 | 80/100 | ||
| Eutectic Overall | 39.47 | 47.83 | 10.77 | 1.92 | 15/100 | 4/100 | 4.5/100 | 20/100 | ||
| Alloy C: AlNi-25Cr-15Mo | Nominal | 30 | 30 | 25 | 15 | 37.5/100 | 33/100 | 33/100 | 45/100 | 4.87 |
| Actual | 23.74 | 33.96 | 25.99 | 16.31 | 39/100 | 32/100 | 41/100 | 47/100 | ||
| Primary Light | 9.54 | 6.47 | 39.48 | 44.52 | 53/100 | 87/100 | 82/100 | 83/100 | ||
| Primary Halo | 14.72 | 19.38 | 45.05 | 20.85 | 32/100 | 52/100 | 58/100 | 73/100 | ||
| Eutectic Overall | 29.26 | 39.06 | 21.66 | 10.01 | 32/100 | 20/100 | 26/100 | 39/100 | ||
| Dark Phase | 37.01 | 52.16 | 9.40 | 1.42 | 13/100 | 3/100 | 4/100 | 18/100 | ||
| Alloy D: AlNi-20Cr-20Mo | Nominal | 30 | 30 | 20 | 20 | 50/100 | 40/100 | 40/100 | 40/100 | 4.33 |
| Actual | 23.88 | 35.10 | 19.43 | 21.59 | 53/100 | 38/100 | 47.5/100 | 40/100 | ||
| Primary Light | 8.04 | 4.83 | 24.09 | 63.04 | 72/100 | 93/100 | 89/100 | 75/100 | ||
| Primary Halo | 6.13 | 7.93 | 54.28 | 31.65 | 37/100 | 20/100 | 16/100 | 88/100 | ||
| Eutectic Overall | 29.03 | 41.11 | 17.36 | 12.50 | 42/100 | 23/100 | 30/100 | 33/100 | ||
| Dark Phase | 37.51 | 54.84 | 6.98 | 0.67 | 9/100 | 1/100 | 2/100 | 13/100 | ||
| Alloy E: AlNi-15Cr-25Mo | Nominal | 30 | 30 | 15 | 25 | 62.5/100 | 46/100 | 46/100 | 33/100 | 4.04 |
| Actual | 23.44 | 34.70 | 14.82 | 27.03 | 65/100 | 44/100 | 54/100 | 34/100 | ||
| Primary Light | 7.3 | 3.58 | 21.91 | 67.11 | 75/100 | 95/100 | 90/100 | 80/100 | ||
| Primary Halo | 5.20 | 7.92 | 53.40 | 33.48 | 39/100 | 81/100 | 87/100 | 86/100 | ||
| Eutectic Overall | 28.77 | 41.58 | 15.82 | 13.83 | 47/100 | 25/100 | 33/100 | 31/100 | ||
| Dark Phase | 39.53 | 53.70 | 6.07 | 0.70 | 10/100 | 1/100 | 2/100 | 12/100 | ||
| Alloy F: AlNi-10Cr-30Mo | Nominal | 30 | 30 | 10 | 30 | 75/100 | 50/100 | 50/100 | 25/100 | 3.63 |
| Actual | 23.71 | 35.51 | 10.06 | 30.73 | 75/100 | 46/100 | 56/100 | 25/100 | ||
| Primary Light | 6.32 | 3.45 | 15.45 | 74.79 | 83/100 | 94/100 | 92/100 | 76/100 | ||
| Peritectic Halo | Difficult to Ascertain | |||||||||
| Eutectic Overall | 33.99 | 47.46 | 7.33 | 11.82 | 62/100 | 20/100 | 26/100 | 15/100 | ||
| Dark Phase | 41.59 | 53.94 | 3.86 | 0.62 | 14/100 | 1/100 | 1.5/100 | 7.5/100 | ||
| Alloy G: AlNi-40Mo | Nominal | 30 | 30 | 0 | 40 | 100 | 57/100 | 57/100 | 0 | 1.96 |
| Actual | 23.06 | 37.30 | 0 | 39.65 | 100 | 52/100 | 63/100 | - | ||
| Primary Light | 6.91 | 3.11 | 0 | 89.98 | 100 | 97/100 | 93/100 | - | ||
| Eutectic Overall | 35.62 | 55.51 | 0 | 8.87 | - | 14/100 | 20/100 | 16/100 | ||
| Dark Phase | 41.13 | 57.94 | 0 | 0.96 | 100 | 1.5/100 | 2/100 | 2/100 | ||
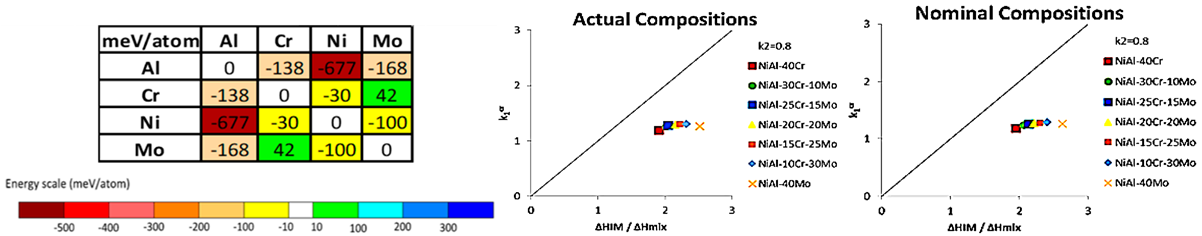
| System–Composition (at.%) | Atomic Percentage [at.%] | Zhang/Guo Criteria | King Criteria | Senkov Criteria | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ni | Cr | Mo | δ | ΔSmix [J/K·mol] | ΔHmix [kJ/mol] | ΔHf [kJ/mol] | ΔG [kJ/mol] | VEC | Ω | γ | Φ | Τm [K] | ΔHIM/ΔHmix | k1cr | ||
| NiAl-40Cr | Nominal | 30 | 30 | 40 | 0 | 6.33 | 9.05 | −16.08 | 16.57 | −33.11 | 6.30 | 0.93 | 1.158 | 0.70 | 1650 | 1.95 | 1.19 |
| Actual | 26.21 | 33 | 40.79 | 0 | 6.10 | 9.00 | −15.66 | 16.84 | −32.46 | 6.53 | 0.97 | 1.158 | 0.68 | 1683 | 1.91 | 1.19 | |
| NiAl-30Cr-10Mo | Nominal | 30 | 30 | 30 | 10 | 6.23 | 10.92 | −15.48 | 18.12 | −34.49 | 6.30 | 1.22 | 1.157 | 0.35 | 1726 | 2.07 | 1.24 |
| Actual | 25.7 | 33.2 | 30.99 | 10.11 | 6.05 | 10.89 | −15.03 | 18.45 | −33.84 | 6.56 | 1.28 | 1.158 | 0.35 | 1766 | 2.01 | 1.26 | |
| NiAl-25Cr-15Mo | Nominal | 30 | 30 | 25 | 15 | 6.14 | 11.25 | −15.18 | 18.90 | −33.73 | 6.30 | 1.31 | 1.157 | 0.35 | 1765 | 2.14 | 1.26 |
| Actual | 23.74 | 33.96 | 25.99 | 16.31 | 5.90 | 11.25 | −14.36 | 19.58 | −33.07 | 6.65 | 1.44 | 1.157 | 0.35 | 1834 | 2.04 | 1.29 | |
| NiAl-20Cr-20Mo | Nominal | 30 | 30 | 20 | 20 | 6.02 | 11.35 | −14.88 | 19.67 | −33.58 | 6.30 | 1.37 | 1.157 | 0.35 | 1803 | 2.22 | 1.27 |
| Actual | 23.88 | 35.1 | 19.43 | 21.59 | 5.83 | 11.29 | −14.29 | 20.35 | −32.94 | 6.69 | 1.47 | 1.157 | 0.35 | 1868 | 2.15 | 1.29 | |
| NiAl-15Cr-25Mo | Nominal | 30 | 30 | 15 | 25 | 5.87 | 11.25 | −14.58 | 20.45 | −33.68 | 6.30 | 1.19 | 1.156 | 0.35 | 1841 | 2.30 | 1.28 |
| Actual | 23.44 | 34.7 | 14.82 | 27.03 | 5.71 | 11.17 | −13.88 | 21.25 | −32.91 | 6.68 | 1.54 | 1.157 | 0.35 | 1916 | 2.22 | 1.31 | |
| NiAl-10Cr-30Mo | Nominal | 30 | 30 | 10 | 30 | 5.69 | 10.92 | −14.28 | 21.22 | −34.40 | 6.30 | 1.44 | 1.156 | 0.35 | 1880 | 2.40 | 1.29 |
| Actual | 23.71 | 35.51 | 10.06 | 30.73 | 5.62 | 10.82 | −13.88 | 21.77 | −33.30 | 6.71 | 1.51 | 1.157 | 0.35 | 1938 | 2.32 | 1.30 | |
| NiAl-40Mo | Nominal | 30 | 30 | 0 | 40 | 5.22 | 9.05 | −13.68 | 22.77 | −32.44 | 6.30 | 1.29 | 1.55 | 0.68 | 1956 | 2.63 | 1.26 |
| Actual | 23.06 | 37.3 | 0 | 39.65 | 5.31 | 8.92 | −13.54 | 23.16 | −30.92 | 6.80 | 1.32 | 1.156 | 0.66 | 2007 | 2.52 | 1.26 | |
 | |||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathiou, C.; Giorspyros, K.; Georgatis, E.; Poulia, A.; Karantzalis, A.E. NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response. Materials 2020, 13, 3445. https://doi.org/10.3390/ma13163445
Mathiou C, Giorspyros K, Georgatis E, Poulia A, Karantzalis AE. NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response. Materials. 2020; 13(16):3445. https://doi.org/10.3390/ma13163445
Chicago/Turabian StyleMathiou, Christina, Konstantinos Giorspyros, Emmanuel Georgatis, Anthoula Poulia, and Alexander E. Karantzalis. 2020. "NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response" Materials 13, no. 16: 3445. https://doi.org/10.3390/ma13163445
APA StyleMathiou, C., Giorspyros, K., Georgatis, E., Poulia, A., & Karantzalis, A. E. (2020). NiAl-Cr-Mo Medium Entropy Alloys: Microstructural Verification, Solidification Considerations, and Sliding Wear Response. Materials, 13(16), 3445. https://doi.org/10.3390/ma13163445






