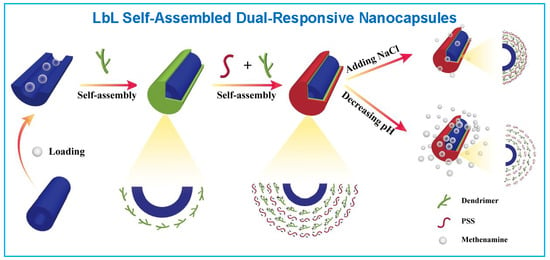
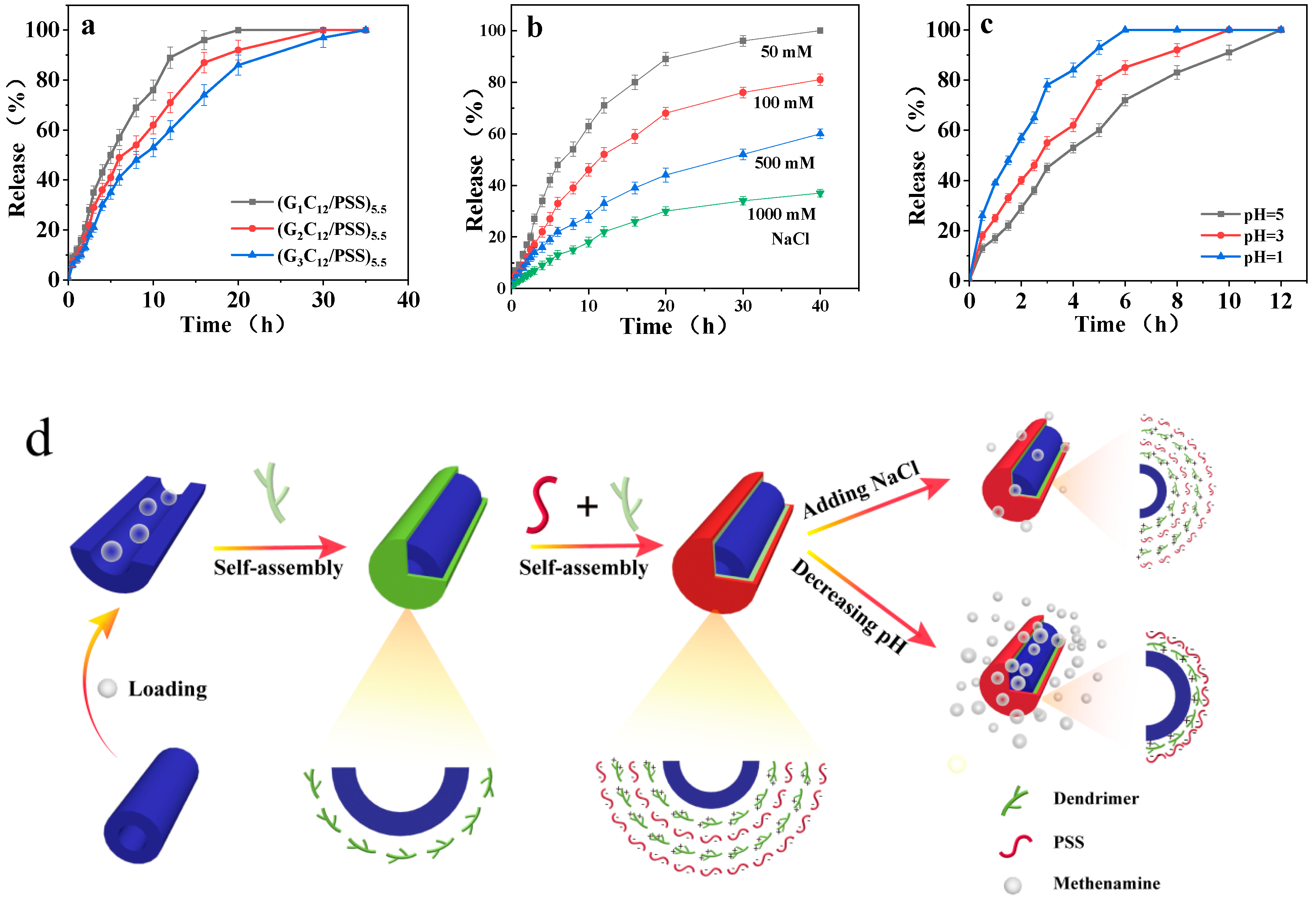
Dual-Responsive Nanotubes Assembled by Amphiphilic Dendrimers: Controlled Release and Crosslinking
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. Fabrication of Methenamine-Loaded HNTs
2.4. LbL Assembly of GnC12/PSS Multilayers, Modifying the Methenamine-Loaded HNTs
2.5. Controllable Release of Methenamine
2.6. Crosslinking Evaluation
3. Results and Discussion
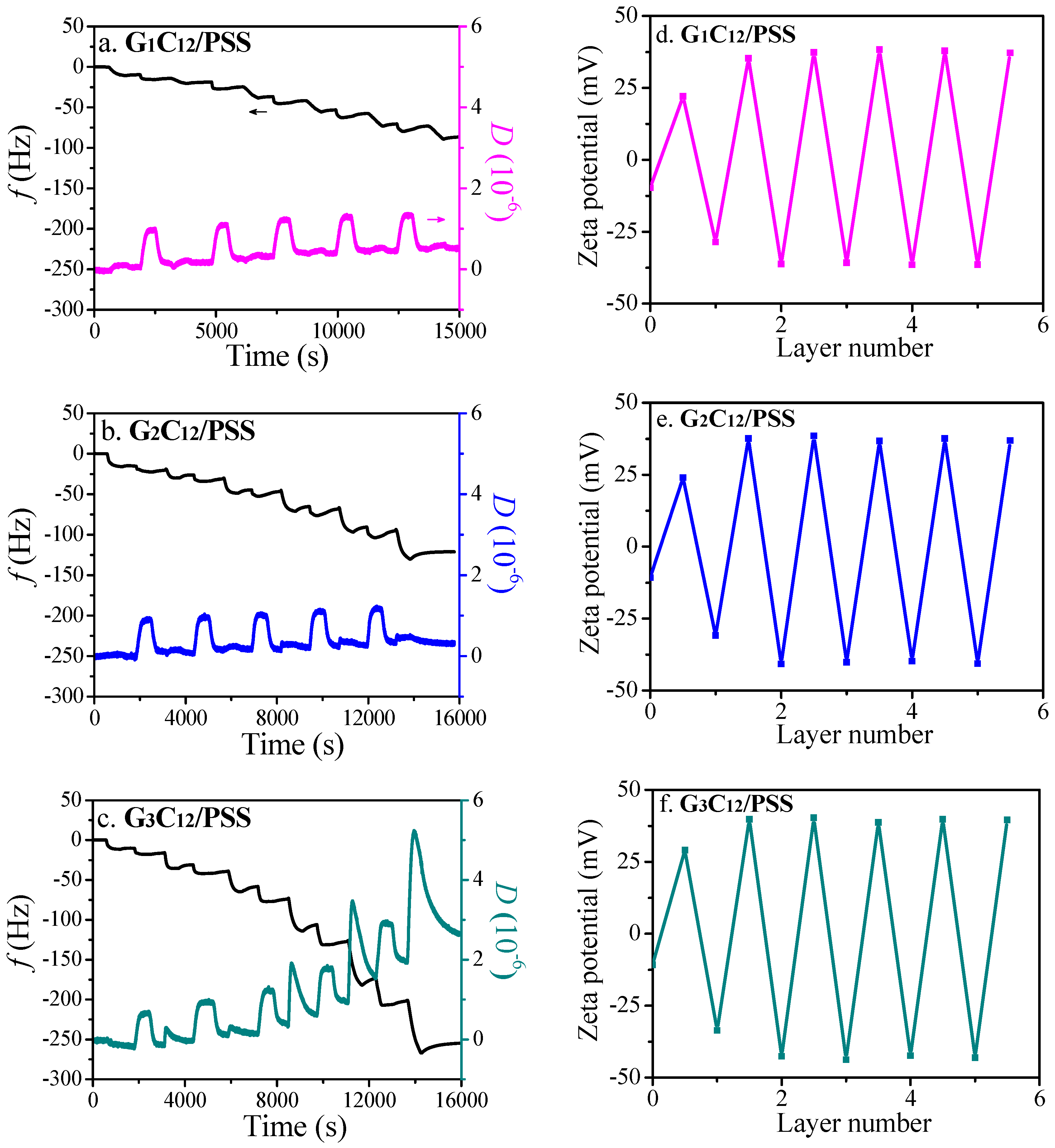
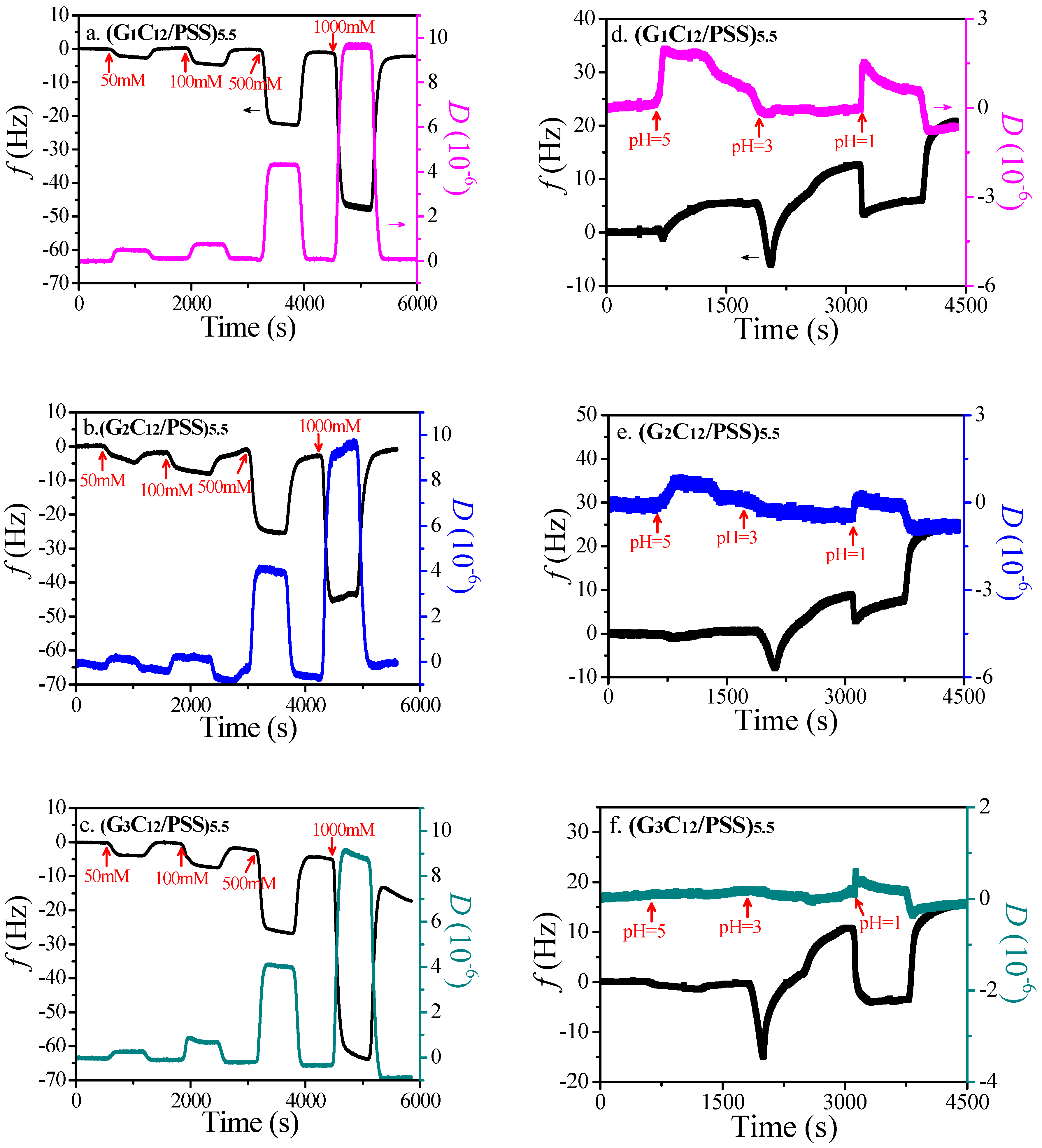
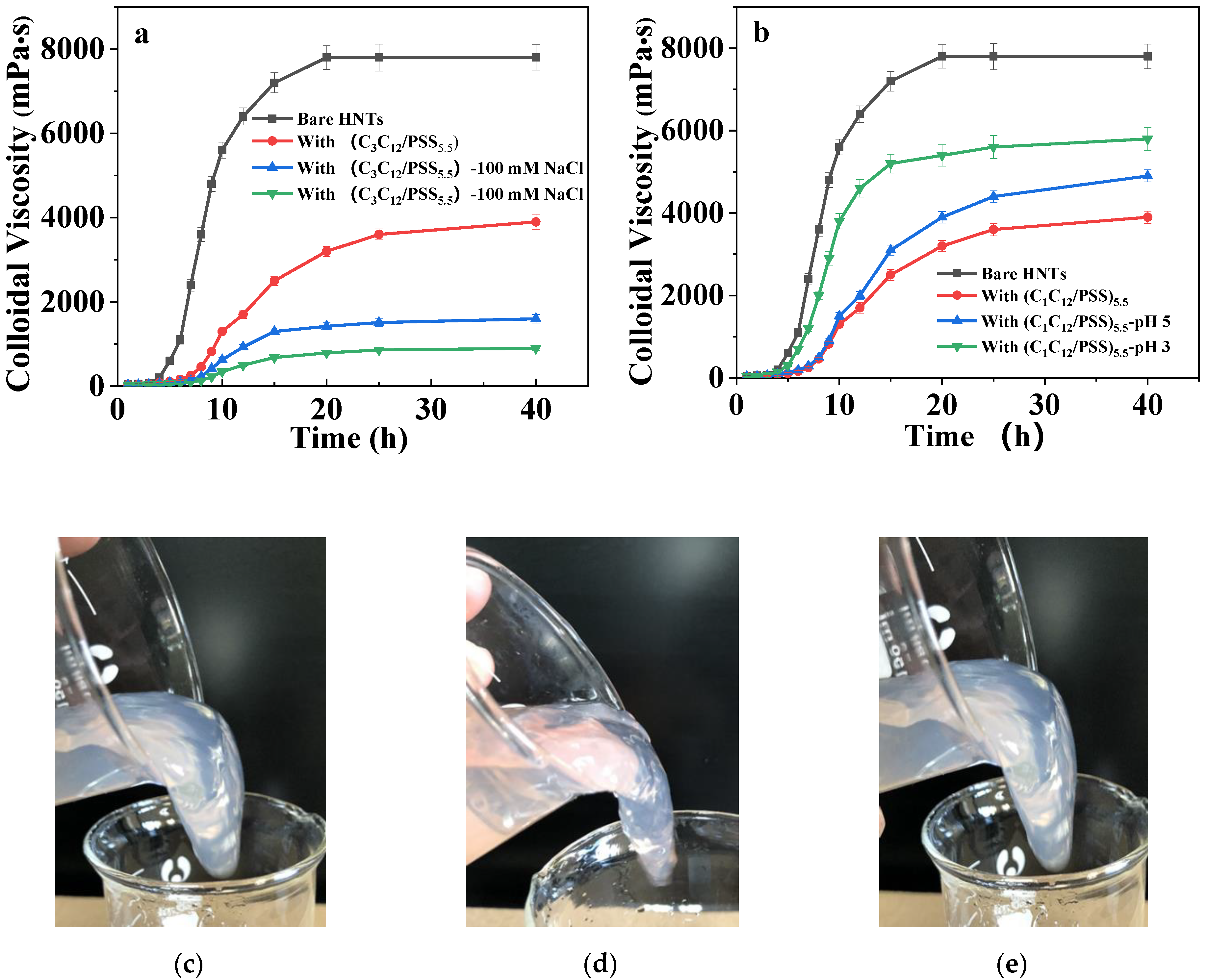
3.1. LbL Assembly and Responsiveness to Salt and pH
3.2. Release Performance of the Loaded Methenamine
3.3. Crosslinking Efficiency in PAM System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Chen, Q.; Zhou, S. Carbon-Based Hybrid Nanogels: A Synergistic Nanoplatform for Combined Biosensing, Bioimaging, and Responsive Drug Delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems Based on Magnetic Nanoparticles and Thermo- or pH-Responsive Polymers: An Update and Future Perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.; Yoon, J.; Chen, X. Cancer-Associated, Stimuli-Driven, Turn on Theranostics for Multimodality Imaging and Therapy. Adv. Mater. 2017, 29, 1606857. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, S.; Tao, Y.; Ma, H.; Wu, J. Research and Application of the HTHS Anti-H2S Gel Valve for Underbalanced Drilling. J. Pet. Sci. Technol. 2018, 164, 174–181. [Google Scholar] [CrossRef]
- Chen, H.; Tang, H.; Wu, X.; Liu, Y.; Bai, J.; Zhao, F. Synthesis, Characterization, and Property Evaluation of a Hydrophobically Modified Polyacrylamide as Enhanced Oil Recovery Chemical. J. Pet. Sci. Technol. 2015, 37, 486–495. [Google Scholar] [CrossRef]
- Alnazghah, M.; Kerans, C. Late Pennsylvanian Glaciation: Evidence of Icehouse Conditions from Canyon and Cisco Units, Midland Basin, Texas. Mar. Pet. Geol. 2018, 94, 198–211. [Google Scholar] [CrossRef]
- Mao, J.; Wang, D.; Yang, X.; Zhang, Y.; Zhao, J.; Li, Y.; Zhao, J. Application and Optimization: Non-Aqueous Fracturing Fluid from Phosphate Ester Synthesized with Single Alcohol. J. Pet. Sci. Technol. 2016, 147, 356–360. [Google Scholar] [CrossRef]
- Saghafi, H.R.; Naderifar, A.; Gerami, S.; Emadi, M.A. Improvement in Thermo-Chemical Stability of Nanocomposite Preformed Particle Gels for Conformance Control in Harsh Oil Reservoir Conditions. Can. J. Chem. Eng. 2016, 94, 1880–1890. [Google Scholar] [CrossRef]
- Li, G.; Zheng, Z.; Möhwald, H.; Shchukin, D.G. Silica/Polymer Double-Walled Hybrid Nanotubes: Synthesis and Application as Stimuli-Responsive Nanocontainers in Self-Healing Coatings. ACS Nano 2013, 7, 2470–2478. [Google Scholar] [CrossRef]
- Su, A.; Tan, S.; Thapa, F.P.B.N.; Ford, W.T. Highly Ordered Langmuir-Blodgett Films of Amphiphilic Poly (propylene imine) Dendrimers. J. Phys. Chem. C 2007, 111, 4695–4701. [Google Scholar] [CrossRef]
- Ito, M.; Imae, T.; Aoi, K.; Tsutsumiuchi, K.; Noda, H.; Okada, M. In Situ Investigation of Adlayer Formation and Adsorption Kinetics of Amphiphilic Surface-Block Dendrimers on Solid Substrates. Langmuir 2002, 18, 9757–9764. [Google Scholar] [CrossRef]
- Schenning, A.P.H.J.; Elissen-Roman, C.; Weener, J.-W.; Baars, M.W.P.L.; van der Gaast, S.J.; Meijer, E.W. Amphiphilic Dendrimers as Building Blocks in Supramolecular Assemblies. J. Am. Chem. Soc. 1998, 120, 8199–8208. [Google Scholar] [CrossRef]
- Tan, S.; Su, A.; Ford, W.T. Aggregation of a Hydrophobically Modified Poly (propylene imine) Dendrimer. Eur. Phys. J. E 2008, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Nummelin, S.; Selin, M.; Legrand, S.; Ropponen, J.; Seitsonen, J.; Nykanen, A.; Koivisto, J.; Hirvonen, J.; Kostiainen, M.A.; Bimbo, L.M. Modular Synthesis of Self-Assembling Janus-Dendrimers and Facile Preparation of Drug-Loaded Dendrimersomes. Nanoscale 2017, 9, 7189–7198. [Google Scholar] [CrossRef] [PubMed]
- Avila-Salas, F.; Pereira, A.; Rojas, M.A.; Saavedra-Torres, M.; Montecinos, R.; Bonardd, S.; Quezada, C.; Saldías, S.; Díaz, D.D.; Leiva, A.; et al. An Experimental and Theoretical Comparative Study of the Entrapment and Release of Dexamethasone from Micellar and Vesicular Aggregates of PAMAM-PCL Dendrimers. Eur. Polym. J. 2017, 93, 507–520. [Google Scholar] [CrossRef]
- Pericet-Camara, R.; Cahill, B.P.; Papastavrou, G.; Borkovec, M. Nano-Patterning of Substrates by Adsorbed Dendrimers. Chem. Commun. 2007, 3, 266–268. [Google Scholar] [CrossRef]
- Cahill, B.P.; Papastavrou, G.; Koper, G.J.M.; Borkovec, M. Adsorption of Poly (Amido Amine) (PAMAM) Dendrimers on Silica: Importance of Electrostatic Three-Body Attraction. Langmuir 2008, 24, 465–473. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Zhou, L.J.; Wang, J.; Yan, H. Dilational Properties of Novel Amphiphilic Dendrimers at Water-Air and Water-Heptane Interfaces. J. Phys. Chem. B 2012, 116, 12760–12768. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Zhang, P. Controllable Self-Assembly of Amphiphilic Dendrimers on a Silica Surface: The Effect of Molecular Topological Structure and Salinity. J. Phys. Chem. B 2016, 120, 10990–10999. [Google Scholar] [CrossRef]
- Hanif, M.; Jabbar, F.; Sharif, S.; Abbas, G.; Farooq, A.; Aziz, M. Halloysite Nanotubes as a New Drug-Delivery System: A Review. Clay Miner. 2018, 51, 469–477. [Google Scholar] [CrossRef]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; D’Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite Nanotubes for Efficient Loading, Stabilization and Controlled Release of Insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, J.; Pan, J.; Luo, J.; Niu, X.; Zhang, T.; Qiu, F. Two Are Better than One: Halloysite Nanotubes-Supported Surface Imprinted Nanoparticles Using Synergy of Metal Chelating and Low pKa Boronic Acid Monomers for Highly Specific Luteolin Binding under Neutral Condition. ACS Appl. Mater. Interfaces 2017, 38, 33191–33202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhong, B.; Jia, Z.; Zhang, Q.; Chen, Y.; Jia, D. Halloysite Nanotubes as Nanocarriers for Plant Herbicide and its Controlled Release in Biodegradable Polymers Composite Film. Appl. Clay Sci. 2019, 171, 20–28. [Google Scholar] [CrossRef]
- Pan, Q.; Li, N.; Hong, Y.; Tang, H.; Zheng, Z.; Weng, S.; Zheng, Y.; Huang, L. Halloysite Clay Nanotubes as Effective Nanocarriers for the Adsorption and Loading of Vancomycin for Sustained Release. RSC Adv. 2017, 7, 21352–21359. [Google Scholar] [CrossRef]
- O’Neal, J.T.; Dai, E.Y.; Zhang, Y.; Clark, K.B.; Wilcox, K.G.; George, I.M.; Ramasamy, N.E.; Enriquez, D.; Batys, P.; Sammalkorpi, M.; et al. QCM-D Investigation of Swelling Behavior of Layer-by-Layer Thin Films upon Exposure to Monovalent Ions. Langmuir 2018, 34, 999–1009. [Google Scholar] [CrossRef]
- Benselfelt, T.; Pettersson, T.; Wågberg, L. Influence of Surface Charge Density and Morphology on the Formation of Polyelectrolyte Multilayers on Smooth Charged Cellulose Surfaces. Langmuir 2017, 33, 968–979. [Google Scholar] [CrossRef]
- Zhang, Y.; Arugula, M.A.; Kirsch, J.S.; Yang, X.; Olsen, E.; Simonian, A.L. Layer-by-Layer Assembled Carbon Nanotube-Acetylcholinesterase/Biopolymer Renewable Interfaces: SPR and Electrochemical Characterization. Langmuir 2015, 31, 1462–1468. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Yang, H.; Wu, J.; Yang, S.; Yu, D.; Wu, X.; Ma, A.; Sun, K.; Wang, J. Dual-Responsive Nanotubes Assembled by Amphiphilic Dendrimers: Controlled Release and Crosslinking. Materials 2020, 13, 3479. https://doi.org/10.3390/ma13163479
Zhang M, Yang H, Wu J, Yang S, Yu D, Wu X, Ma A, Sun K, Wang J. Dual-Responsive Nanotubes Assembled by Amphiphilic Dendrimers: Controlled Release and Crosslinking. Materials. 2020; 13(16):3479. https://doi.org/10.3390/ma13163479
Chicago/Turabian StyleZhang, Minghui, Hui Yang, Jiazhong Wu, Siyu Yang, Danfeng Yu, Xu Wu, Aiqing Ma, Keji Sun, and Jinben Wang. 2020. "Dual-Responsive Nanotubes Assembled by Amphiphilic Dendrimers: Controlled Release and Crosslinking" Materials 13, no. 16: 3479. https://doi.org/10.3390/ma13163479
APA StyleZhang, M., Yang, H., Wu, J., Yang, S., Yu, D., Wu, X., Ma, A., Sun, K., & Wang, J. (2020). Dual-Responsive Nanotubes Assembled by Amphiphilic Dendrimers: Controlled Release and Crosslinking. Materials, 13(16), 3479. https://doi.org/10.3390/ma13163479