Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells
Abstract
1. Introduction
1.1. Steel and Titanium Implants
1.2. Implant Removal
1.3. Laser Ablation Structuring
2. Materials and Methods
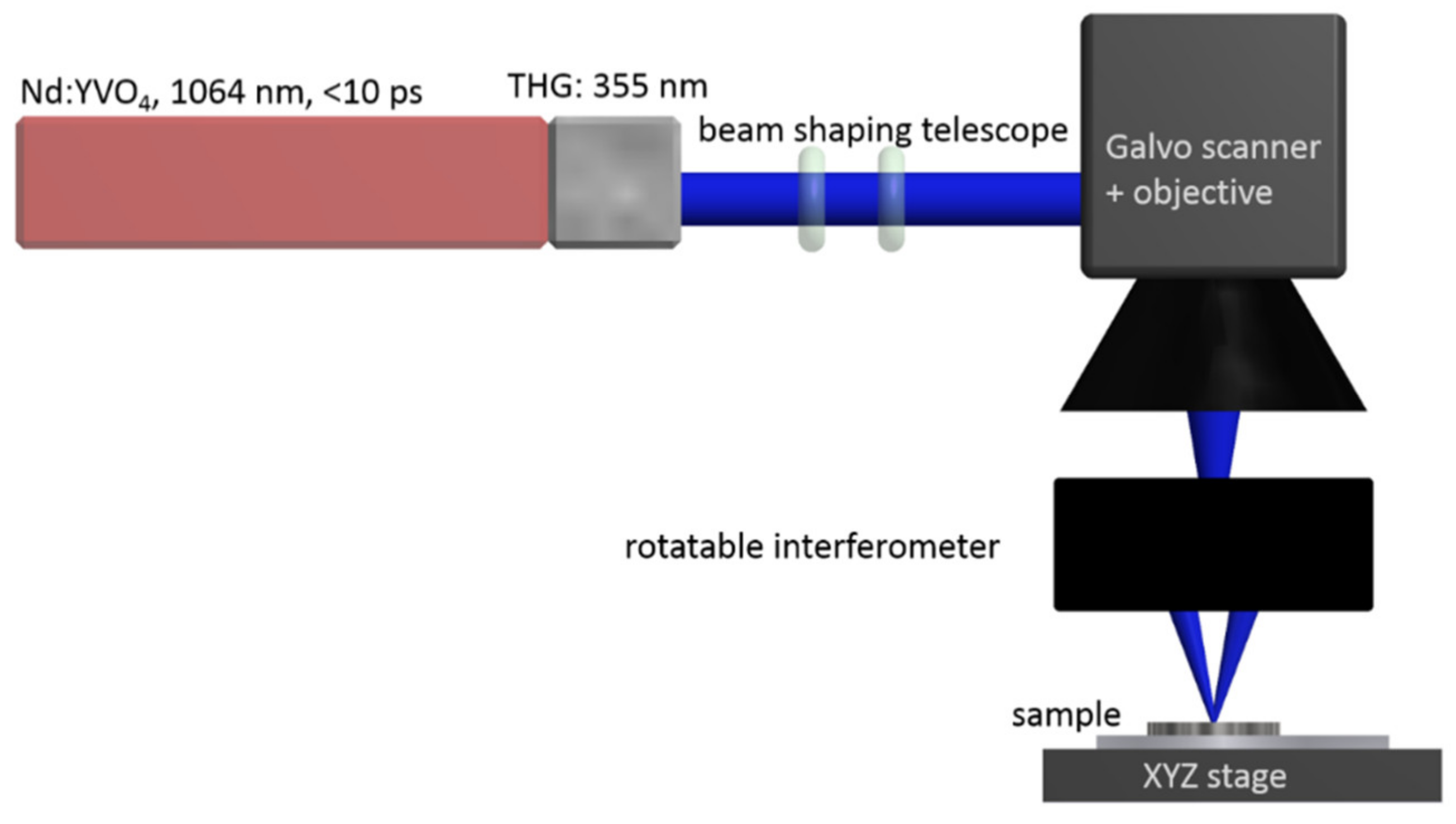
2.1. Surface Structuring of Implant Materials
2.2. Analysis of the Surface Topology
2.3. Cell Culture
2.4. Lentivirus Production
2.5. Generation of Stable SCP1-GFP Cell Lines
2.6. Cell Adhesion Determination
2.7. Co-Culture Implants with Mesenchymal Stem Cells (MSCs) and Osteogenic Differentiation
2.8. RNA Isolation, Reverse Transcription and Gene Expression Analysis
2.9. Statistical Analysis
2.10. Circularity Calculation
3. Results
3.1. Influence of Different Nano-Topographies on Cell Adhesion
3.2. Influence of Surface Structure on Cell Phenotype
3.3. Surface Modifications Lead to Osteogenic Inhibition of Human Mesenchymal Stem Cells (MSCs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Augat, P.; Simon, U.; Liedert, A.; Claes, L. Mechanics and mechano-biology of fracture healing in normal and osteoporotic bone. Osteoporos. Int. Suppl. 2005, 16, S36–S43. [Google Scholar] [CrossRef]
- Arora, R.; Gabl, M.; Erhart, S.; Schmidle, G.; Dallapozza, C.; Lutz, M. Aspects of current management of distal radius fractures in the elderly individuals. Geriatr. Orthop. Surg. Rehabil. 2011, 2, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, S.; Driessen, J.H.M.; Vestergaard, P.; van den Bergh, J.; Boonen, A.; de Vries, F.; Burden, A.M. Secular trends in major osteoporotic fractures among 50+ adults in Denmark between 1995 and 2010. Arch. Osteoporos. 2018, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, M.; Guler, O.; Mahirogullari, M.; Donmez, F.; Gereli, A.; Mutlu, S. Complications during removal of stainless steel versus titanium nails used for intramedullary nailing of diaphyseal fractures of the tibia. Ann. Med. Surg. (Lond.) 2018, 26, 38–42. [Google Scholar] [CrossRef]
- Duan, X.; Al-Qwbani, M.; Zeng, Y.; Zhang, W.; Xiang, Z. Intramedullary nailing for tibial shaft fractures in adults. Cochrane Database Syst. Rev. 2012, 1, CD008241. [Google Scholar] [CrossRef]
- Ürgüden, M.; Özdemir, H.; Söyüncü, Y.; Oruç, F. The treatment of tibial fractures with unreamed interlocking nails. Joint Dis. Rel. Surg. 2005, 16, 49–54. [Google Scholar]
- Mutschler, W.; Höntzsch, D. Stahl oder Titan in der Osteosynthese. Unfallchirurg 2017, 120, 94–95. [Google Scholar] [CrossRef][Green Version]
- Heyland, M.; Duda, G.N.; Märdian, S.; Schütz, M.; Windolf, M. Stahl oder Titan bei der Osteosynthese: Eine mechanobiologische Perspektive. Unfallchirurg 2017, 120, 103–109. [Google Scholar] [CrossRef]
- Ochs, B.G.; Gonser, C.E.; Baron, H.C.; Stöckle, U.; Badke, A.; Stuby, F.M. Refrakturen nach Entfernung von Osteosynthesematerialien. Eine vermeidbare Komplikation? Unfallchirurg 2012, 115, 323–329. [Google Scholar] [CrossRef]
- Evers, B.; Habelt, R.; Gerngross, H. O2109 indication, timing and complications of plate removal after forearm fractures: Results of a metanalysis including 635 cases. Orthop. Proc. 2004, 86, 289. [Google Scholar]
- Joeris, A.; Goldhahn, S.; Rometsch, E.; Höntzsch, D. Titan oder Stahl als Osteosynthesematerial: Systematische Literaturrecherche über die klinische Evidenz. Unfallchirurg 2017, 120, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.; Krieg, B.; Dengler, M.; Füchtmeier, B. Die Implantatentfernung-eine Risiko-Nutzen-Abwägung? OP J. 2016, 32, 80–88. [Google Scholar] [CrossRef][Green Version]
- Klein-Wiele, J.-H.; Blumenstein, A.; Simon, P.; Ihlemann, J. Laser interference ablation by ultrashort UV laser pulses via diffractive beam management. Adv. Opt. Technol. 2020, 9, 41–52. [Google Scholar] [CrossRef]
- Rung, S.; Bokan, K.; Kleinwort, F.; Schwarz, S.; Simon, P.; Klein-Wiele, J.-H.; Esen, C.; Hellmann, R. Possibilities of Dry and Lubricated Friction Modification Enabled by Different Ultrashort Laser-Based Surface Structuring Methods. Lubricants 2019, 7, 43. [Google Scholar] [CrossRef]
- Ahmmed, K.; Grambow, C.; Kietzig, A.-M. Fabrication of Micro/Nano Structures on Metals by Femtosecond Laser Micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- Klein-Wiele, J.-H.; Simon, P. Sub-100nm pattern generation by laser direct writing using a confinement layer. Opt. Express 2013, 21, 9017–9023. [Google Scholar] [CrossRef]
- Klein-Wiele, J.-H.; Simon, P. Sub-wavelength pattern generation by laser direct writing via repeated irradiation. Opt. Express 2013, 21, 626–630. [Google Scholar] [CrossRef]
- Bekesi, J.; Meinertz, J.; Ihlemann, J.; Simon, P. Fabrication of large-area grating structures through laser ablation. Appl. Phys. A 2008, 93, 27–31. [Google Scholar] [CrossRef]
- Bekesi, J.; Meinertz, J.; Simon, P.; Ihlemann, J. Sub-500-nm patterning of glass by nanosecond KrF excimer laser ablation. Appl. Phys. A 2013, 110, 17–21. [Google Scholar] [CrossRef]
- Bekesi, J.; Simon, P.; Ihlemann, J. Deterministic sub-micron 2D grating structures on steel by UV-fs-laser interference patterning. Appl. Phys. A 2014, 114, 69–73. [Google Scholar] [CrossRef]
- Böcker, W.; Yin, Z.; Drosse, I.; Haasters, F.; Rossmann, O.; Wierer, M.; Popov, C.; Locher, M.; Mutschler, W.; Docheva, D.; et al. Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J. Cell. Mol. Med. 2008, 12, 1347–1359. [Google Scholar] [CrossRef] [PubMed]
- Campeau, E.; Ruhl, V.E.; Rodier, F.; Smith, C.L.; Rahmberg, B.L.; Fuss, J.O.; Campisi, J.; Yaswen, P.; Cooper, P.K.; Kaufman, P.D. A Versatile Viral System for Expression and Depletion of Proteins in Mammalian Cells. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.Y.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Heitz, J.; Plamadeala, C.; Muck, M.; Armbruster, O.; Baumgartner, W.; Weth, A.; Steinwender, C.; Blessberger, H.; Kellermair, J.; Kirner, S.V.; et al. Femtosecond laser-induced microstructures on Ti substrates for reduced cell adhesion. Appl. Phys. A 2017, 123, S78. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Ogita, H.; Hirota, T.; Kawakatsu, T.; Fukuyama, T.; Yasumi, M.; Kanzaki, N.; Ozaki, M.; Takai, Y. Interaction of integrin alpha(v)beta3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J. Biol. Chem. 2006, 281, 19631–19644. [Google Scholar] [CrossRef]
- Turner, N.; Armitage, M.; Butler, R.; Ireland, G. An in vitro model to evaluate cell adhesion to metals used in implantation shows significant differences between palladium and gold or platinum. Cell Biol. Int. 2004, 28, 541–547. [Google Scholar] [CrossRef]
- Zaidel-Bar, R.; Cohen, M.; Addadi, L.; Geiger, B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004, 32, 416–420. [Google Scholar] [CrossRef]
- Richert, L.; Variola, F.; Rosei, F.; Wuest, J.D.; Nanci, A. Adsorption of proteins on nanoporous Ti surfaces. Surf. Sci. 2010, 604, 1445–1451. [Google Scholar] [CrossRef]
- Schwarz, R.I. Collagen I and the fibroblast: High protein expression requires a new paradigm of post-transcriptional, feedback regulation. Biochem. Biophys. Rep. 2015, 3, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, B.; Green, H. An analysis of collagen secretion by stablished mouse fibroblast lines. J. Cell Biol. 1964, 22, 227–258. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Sandhage, K.H.; Schwartz, Z.; Tannenbaum, R.; Boyan, B.D. Surface Modification under Controlled Oxidative Environment. U.S. Patent Application No. 61/299,433, 29 January 2009. [Google Scholar]
- Zhao, G.; Zinger, O.; Schwartz, Z.; Wieland, M.; Landolt, D.; Boyan, B.D. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin. Oral Implants Res. 2006, 17, 258–264. [Google Scholar] [CrossRef]
- Webster, T. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Dedhar, S.; Ruoslahti, E.; Argraves, S.; Suzuki, S. An adhesion variant of the MG-63 osteosarcoma cell line displays an osteoblast-like phenotype. Ciba Found. Symp. 1988, 136, 131–141. [Google Scholar] [CrossRef]
- Saldaña, L.; Bensiamar, F.; Boré, A.; Vilaboa, N. In search of representative models of human bone-forming cells for cytocompatibility studies. Acta Biomater. 2011, 7, 4210–4221. [Google Scholar] [CrossRef]
- Wu, Y.-N.; Law, J.B.K.; He, A.Y.; Low, H.Y.; Hui, J.H.P.; Lim, C.T.; Yang, Z.; Lee, E.H. Substrate topography determines the fate of chondrogenesis from human mesenchymal stem cells resulting in specific cartilage phenotype formation. Nanomedicine 2014, 10, 1507–1516. [Google Scholar] [CrossRef]
- Mustafa, H.; Mezera, M.; Matthews, D.T.A.; Römer, G.R.B.E. Effect of surface roughness on the ultrashort pulsed laser ablation fluence threshold of zinc and steel. Appl. Surf. Sci. 2019, 488, 10–21. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.D.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. The combination of micron and nanotopography by H(2)SO(4)/H(2)O(2) treatment and its effects on osteoblast-specific gene expression of hMSCs. J. Biomed. Mater. Res. A 2010, 94, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.-A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef] [PubMed]
- Hori, N.; Iwasa, F.; Ueno, T.; Takeuchi, K.; Tsukimura, N.; Yamada, M.; Hattori, M.; Yamamoto, A.; Ogawa, T. Selective cell affinity of biomimetic micro-nano-hybrid structured TiO2 overcomes the biological dilemma of osteoblasts. Dent. Mater. 2010, 26, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef]
- Folwaczny, E.F.; Stürmer, K.M. Unusual Complications during Removal of Intramedullary Nails after Lower Leg Fractures Tibial Shaft Fissure and Inability to Remove the Nail. Eur. J. Trauma 2001, 27, 184–190. [Google Scholar] [CrossRef]
- Mehraj, M.; Shah, I. Effects of intramedullary nail removal after tibial fracture repair: A prospective study. Int. J. Orthop. Sci. 2018, 4, 886–888. [Google Scholar] [CrossRef]
- Busam, M.L.; Esther, R.J.; Obremskey, W.T. Hardware removal: Indications and expectations. J. Am. Acad. Orthop. Surg. 2006, 14, 113–120. [Google Scholar] [CrossRef]
- Bonifacio, M.A.; Cometa, S.; Dicarlo, M.; Baruzzi, F.; de Candia, S.; Gloria, A.; Giangregorio, M.M.; Mattioli-Belmonte, M.; de Giglio, E. Gallium-modified chitosan/poly(acrylic acid) bilayer coatings for improved titanium implant performances. Carbohydr. Polym. 2017, 166, 348–357. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.-M.; Limmer, A.; Giersig, M. Long-term release of antibiotics by carbon nanotube-coated titanium alloy surfaces diminish biofilm formation by Staphylococcus epidermidis. Nanomedicine 2017, 13, 1587–1593. [Google Scholar] [CrossRef]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cell Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, A.A.; Kanlic, E. Removal of a broken cannulated intramedullary nail: Review of the literature and a case report of a new technique. Case Rep. Orthop. 2013, 2013, 461703. [Google Scholar] [CrossRef] [PubMed]
- Liodakis, E.; Krettek, C.; Kenawey, M.; Hankemeier, S. A new technique for removal of an incarcerated expandable femoral nail. Clin. Orthop. Relat. Res. 2010, 468, 1405–1409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, S.; Ni, J.; Liu, X.; Lu, H.; Yin, S.; Rong, M.; Guo, Z.; Zhou, L. Surface Characteristic of Pure Titanium Sandblasted with Irregular Zirconia Particles and Acid-Etched. Mater. Trans. 2012, 53, 913–919. [Google Scholar] [CrossRef]







| Plasmid | Depositor | Addgene Number |
|---|---|---|
| psPAX2 | Didier Trono | 12,260 |
| pLenti CMV GFP Neo | Eric Campeau [22] | 17,447 |
| pCMV-VSVG | Bob Weinberg [23] | 8454 |
| Component | Volume/Concentration | Company |
|---|---|---|
| DMEM (low glucose) | 500 mL | Sigma Aldrich, Germany |
| Fetal calf serum (FCS) | 50 mL (10%) | PAN Biotech, Germany |
| Penicillin/Streptavidin | 5 mL (1%) | PAN Biotech, Germany |
| Ascorbic acid-2 phosphate | 200 µM | Cayman chemical company, MI, USA |
| ß-glycerophosphate | 10 mM | Carl Roth, Germany |
| Gene | Forward Primer (5´→3´) | Reverse Primer (5´→3´) |
|---|---|---|
| β-2-microglobulin | TGTGCTCGCGCTACTCTCTCT | CGGATGGATGAAACCCAGACA |
| Osteocalcin | GGCGCTACCTGTATCAATGG | GTGGTCAGCCAACTCGTCA |
| Runx2 | TGGTTACTGTCATGGCGGGTA | TCTCAGATCGTTGAACCTTGCTA |
| Collagen 1 | GTGCGATGACGTGATCTGTGA | CGGTGGTTTCTTGGTCGGT |
| Collagen 2 | TGG ACG CCA TGA AGG TTT TCT | TGG GAG CCA GAT TGT CAT CTC |
| Aggrecan | GTGCCTATCAGGACAAGGTCT | GATGCCTTTCACCACGACTTC |
| Sox9 | AGCGAACGCACATCAAGAC | CTGTAGGCGATCTGTTGGGG |
| Material | Structure 1-5 | Structure 2-5 | Structure 5-5 |
|---|---|---|---|
| Stainless Steel | 880 nm | 750 and 990 nm | 1050, 1300 and 1400 nm |
| Titanium | 700 nm | 400 and 600 nm | 900, 1200 and 1300 nm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Böker, K.O.; Kleinwort, F.; Klein-Wiele, J.-H.; Simon, P.; Jäckle, K.; Taheri, S.; Lehmann, W.; Schilling, A.F. Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells. Materials 2020, 13, 3526. https://doi.org/10.3390/ma13163526
Böker KO, Kleinwort F, Klein-Wiele J-H, Simon P, Jäckle K, Taheri S, Lehmann W, Schilling AF. Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells. Materials. 2020; 13(16):3526. https://doi.org/10.3390/ma13163526
Chicago/Turabian StyleBöker, Kai Oliver, Frederick Kleinwort, Jan-Hendrick Klein-Wiele, Peter Simon, Katharina Jäckle, Shahed Taheri, Wolfgang Lehmann, and Arndt F. Schilling. 2020. "Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells" Materials 13, no. 16: 3526. https://doi.org/10.3390/ma13163526
APA StyleBöker, K. O., Kleinwort, F., Klein-Wiele, J.-H., Simon, P., Jäckle, K., Taheri, S., Lehmann, W., & Schilling, A. F. (2020). Laser Ablated Periodic Nanostructures on Titanium and Steel Implants Influence Adhesion and Osteogenic Differentiation of Mesenchymal Stem Cells. Materials, 13(16), 3526. https://doi.org/10.3390/ma13163526








