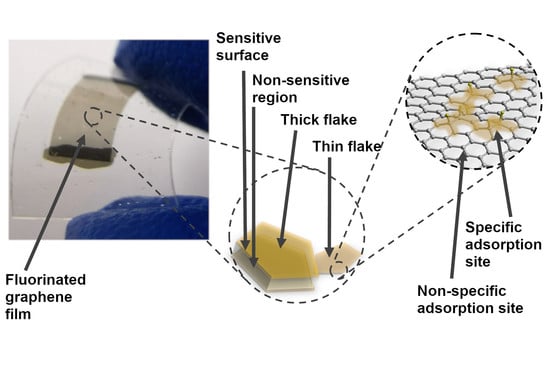
Chemiresistive Properties of Imprinted Fluorinated Graphene Films
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Materials Characterization
3.2. Sensor Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.H.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Yang, Z.; Wang, Y.; Zhang, L.; Wang, Y.; Huang, X.; Wei, H.; Wei, L.; Zhang, Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 2013, 25, 25502. [Google Scholar] [CrossRef] [PubMed]
- Fattah, A.; Khatami, S.M.A. Selective H2S gas sensing with a graphene/n-Si schottky diode. IEEE Sens. J. 2014, 14, 4104–4108. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.; Yang, S.S.; Cheng, M.M.-C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuators B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, D.-S.; Choi, H.K.; Lee, D.H.; Kim, J.-E.; Lee, J.Y.; Lee, W.J.; Kim, S.O.; Choi, S. Flexible room-temperature NO2 gas sensors based on carbon nanotubes/reduced graphene hybrid films. Appl. Phys. Lett. 2010, 96, 213105. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, A.; Huang, L.; Li, C.; Shi, G. High-performance NO2 sensors based on chemically modified graphene. Adv. Mater. 2012, 25, 766–771. [Google Scholar] [CrossRef]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef]
- Moos, R.; Müller, R.; Plog, C.; Knezevic, A.; Leye, H.; Irion, E.; Braun, T.; Marquardt, K.-J.; Binder, K. Selective ammonia exhaust gas sensor for automotive applications. Sens. Actuators B Chem. 2002, 83, 181–189. [Google Scholar] [CrossRef]
- Jiang, Q.; Kresin, F.; Bregt, A.K.; Kooistra, L.; Pareschi, E.; Van Putten, E.; Volten, H.; Wesseling, J. Citizen sensing for improved urban environmental monitoring. J. Sens. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef]
- Seekaew, Y.; Phokharatkul, D.; Wisitsoraat, A.; Wongchoosuk, C. Highly sensitive and selective room-temperature NO2 gas sensor based on bilayer transferred chemical vapor deposited graphene. Appl. Surf. Sci. 2017, 404, 357–363. [Google Scholar] [CrossRef]
- Niu, F.; Tao, L.-M.; Deng, Y.-C.; Wang, Q.-H.; Song, W. Phosphorus doped graphene nanosheets for room temperature NH3 sensing. New J. Chem. 2014, 38, 2269. [Google Scholar] [CrossRef]
- Lv, R.; Chen, G.; Li, Q.; McCreary, A.; Botello-Méndez, A.R.; Morozov, S.V.; Liang, L.; Declerck, X.; Perea-López, N.; Cullen, D.A.; et al. Ultrasensitive gas detection of large-area boron-doped graphene. Proc. Natl. Acad. Sci. USA 2015, 112, 14527–14532. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Yoon, Y.-G.; Choi, K.; Kang, J.H.; Shim, Y.-S.; Kim, Y.H.; Chang, H.J.; Lee, J.-H.; Park, C.R.; Kim, S.Y.; et al. Role of oxygen functional groups in graphene oxide for reversible room-temperature NO2 sensing. Carbon 2015, 91, 178–187. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Berry, V. How do the electrical properties of graphene change with its functionalization? Small 2012, 9, 341–350. [Google Scholar] [CrossRef]
- Chen, N.; Huang, X.; Qu, L. Heteroatom substituted and decorated graphene: Preparation and applications. Phys. Chem. Chem. Phys. 2015, 17, 32077–32098. [Google Scholar] [CrossRef]
- Sturala, J.; Luxa, J.; Pumera, M.; Sofer, Z. Chemistry of graphene derivatives: Synthesis, applications, and perspectives. Chem. Eur. J. 2018, 24, 5992–6006. [Google Scholar] [CrossRef]
- Bouša, D.; Luxa, J.; Mazánek, V.; Jankovský, O.; Sedmidubský, D.; Klímová, K.; Pumera, M.; Sofer, Z. Toward graphene chloride: Chlorination of graphene and graphene oxide. RSC Adv. 2016, 6, 66884–66892. [Google Scholar] [CrossRef]
- Jankovský, O.; Lojka, M.; Luxa, J.; Sedmidubský, D.; Tomanec, O.; Zbořil, R.; Pumera, M.; Sofer, Z. Selective bromination of graphene oxide by the hunsdiecker reaction. Chem. A Eur. J. 2017, 23, 10473–10479. [Google Scholar] [CrossRef]
- Mazánek, V.; Jankovský, O.; Luxa, J.; Sedmidubský, D.; Janoušek, Z.; Šembera, F.; Mikulics, M.; Sofer, Z. Tuning of fluorine content in graphene: Towards large-scale production of stoichiometric fluorographene. Nanoscale 2015, 7, 13646–13655. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Okotrub, A.V. Electronic Structure of Fluorinated Graphene In New Fluorinated Carbons: Fundamentals and Applications; Boltalina, O.V., Nakajima, T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 177–213. [Google Scholar]
- Feng, W.; Long, P.; Feng, Y.; Li, Y. Two-dimensional fluorinated graphene: Synthesis, Structures, properties and applications. Adv. Sci. 2016, 3, 1500413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Li, Z.; Gong, P.; Liu, X.; Zhang, L.; Ren, J.; Wang, H.; Yang, S. Synthesis of fluorinated graphene with tunable degree of fluorination. Carbon 2012, 50, 5403–5410. [Google Scholar] [CrossRef]
- Nebogatikova, N.A.; Antonova, I.; Prinz, V.; Kurkina, I.I.; Vdovin, V.I.; Aleksandrov, G.N.; Timofeev, V.B.; Smagulova, S.A.; Zakirov, E.R.; Kesler, V.G. Fluorinated graphene dielectric films obtained from functionalized graphene suspension: Preparation and properties. Phys. Chem. Chem. Phys. 2015, 17, 13257–13266. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Suk, J.W.; Chou, H.; Lee, J.; Hao, Y.; Wu, Y.; Piner, R.; Akinwande, D.; Kim, H.R.; Ruoff, R.S. Selective-area fluorination of graphene with fluoropolymer and laser irradiation. Nano Lett. 2012, 12, 2374–2378. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, L.; Dong, H.; Zhang, P.; Nie, K.; Zhong, J.; Li, Y.; Guo, J.; Sun, X. Spectroscopic investigation of plasma-fluorinated monolayer graphene and application for gas sensing. ACS Appl. Mater. Interfaces 2016, 8, 8652–8661. [Google Scholar] [CrossRef]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.; et al. Fluorographene: A two-dimensional counterpart of teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Fedoseeva, Y.; Flahaut, E.; Rio, J.; Ewels, C.; Koroteev, V.O.; Van Lier, G.; Vyalikh, D.V.; Okotrub, A.V. Effect of the fluorination technique on the surface-fluorination patterning of double-walled carbon nanotubes. Beilstein J. Nanotechnol. 2017, 8, 1688–1698. [Google Scholar] [CrossRef]
- Chekhova, G.; Pinakov, D.; Shubin, Y.; Fadeeva, V.; Tikhova, V.; Okotrub, A.; Bulusheva, L. Room temperature synthesis of fluorinated graphite intercalation compounds with low fluorine loading of host matrix. J. Fluor. Chem. 2020, 232, 109482. [Google Scholar] [CrossRef]
- Vyalikh, A.; Bulusheva, L.; Chekhova, G.N.; Pinakov, D.; Okotrub, A.V.; Scheler, U. Fluorine patterning in room-temperature fluorinated graphite determined by solid-state NMR and DFT. J. Phys. Chem. C 2013, 117, 7940–7948. [Google Scholar] [CrossRef]
- Katkov, M.V.; Sysoev, V.I.; Gusel’Nikov, A.V.; Asanov, I.P.; Bulusheva, L.G.; Okotrub, A.V. A backside fluorine-functionalized graphene layer for ammonia detection. Phys. Chem. Chem. Phys. 2015, 17, 444–450. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Asanov, I.P.; Yudanov, N.F.; Babin, K.S.; Gusel’Nikov, A.V.; Nedoseikina, T.I.; Gevko, P.N.; Bulusheva, L.G.; Osváth, Z.; Biró, L.P. Development of graphene layers by reduction of graphite fluoride C2F surface. Phys. Status Solidi B 2009, 246, 2545–2548. [Google Scholar] [CrossRef]
- Okotrub, A.V.; Yudanov, N.F.; Asanov, I.P.; Vyalikh, D.V.; Bulusheva, L. Anisotropy of chemical bonding in semifluorinated graphite C2F revealed with angle-resolved X-ray absorption spectroscopy. ACS Nano 2012, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Asanov, I.; Bulusheva, L.; Dubois, M.; Yudanov, N.; Alexeev, A.; Makarova, T.; Okotrub, A.V. Graphene nanochains and nanoislands in the layers of room-temperature fluorinated graphite. Carbon 2013, 59, 518–529. [Google Scholar] [CrossRef]
- Tressaud, A.; Durand, E.; Labrugere, C. Surface modification of several carbon-based materials: Comparison between CF4 rf plasma and direct F2-gas fluorination routes. J. Fluor. Chem. 2004, 125, 1639–1648. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Zhu, J. Fluorination of graphene: A spectroscopic and microscopic study. ACS Nano 2014, 8, 1862–1870. [Google Scholar] [CrossRef]
- Fedoseeva, Y.; Bulusheva, L.G.; Okotrub, A.V.; Vyalikh, D.V.; Fonseca, A. A comparative study of argon ion irradiated pristine and fluorinated single-wall carbon nanotubes. J. Chem. Phys. 2010, 133, 224706. [Google Scholar] [CrossRef]
- Bulusheva, L.; Tur, V.; Fedorovskaya, E.; Asanov, I.; Pontiroli, D.; Riccò, M.; Okotrub, A.V. Structure and supercapacitor performance of graphene materials obtained from brominated and fluorinated graphites. Carbon 2014, 78, 137–146. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Z.; Wang, J.; Wang, H.; Li, Z.; Fan, Z.; Xu, Y.; Han, X.; Yang, S. One-pot sonochemical preparation of fluorographene and selective tuning of its fluorine coverage. J. Mater. Chem. 2012, 22, 16950. [Google Scholar] [CrossRef]
- Zhu, M.; Xie, X.; Guo, Y.; Chen, P.; Ou, X.; Yu, G.; Liu, M. Fluorographene nanosheets with broad solvent dispersibility and their applications as a modified layer in organic field-effect transistors. Phys. Chem. Chem. Phys. 2013, 15, 20992. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Verzhbitskiy, I.A.; Davey, R.; Casiraghi, C. Raman study on defective graphene: Effect of the excitation energy, type, and amount of defects. Phys. Rev. B 2013, 88, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Xie, L. Structural quantification for graphene and related two-dimensional materials by Raman spectroscopy. Anal. Chem. 2018, 91, 468–481. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular doping of graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, S.L.; Liu, G.; Shur, M.; Potyrailo, R.A.; Balandin, A.A. Selective gas sensing with a single pristine graphene transistor. Nano Lett. 2012, 12, 2294–2298. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, S.L.; Liu, G.; Potyrailo, R.A.; Balandin, A.A.; Shur, M. Selective sensing of individual gases using graphene devices. IEEE Sens. J. 2013, 13, 2818–2822. [Google Scholar] [CrossRef]
- Robinson, J.A.; Snow, E.S.; Bădescu Ştefan, C.; Reinecke, T.L.; Perkins, F.K. Role of defects in single-walled carbon nanotube chemical sensors. Nano Lett. 2006, 6, 1747–1751. [Google Scholar] [CrossRef] [PubMed]
- Koleśnik-Gray, M.; Sysoev, V.I.; Gollwitzer, S.; Pinakov, D.V.; Chekhova, G.N.; Bulusheva, L.G.; Okotrub, A.V.; Krstić, V. Electrical transport in devices based on edge-fluorinated graphene. Adv. Electron. Mater. 2018, 4, 1–5. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Okotrub, A.V.; Asanov, I.P.; Gevko, P.N.; Bulusheva, L. Advantage of graphene fluorination instead of oxygenation for restorable adsorption of gaseous ammonia and nitrogen dioxide. Carbon 2017, 118, 225–232. [Google Scholar] [CrossRef]
- Park, M.-S.; Kim, K.H.; Kim, M.-J.; Lee, Y.-S. NH3 gas sensing properties of a gas sensor based on fluorinated graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2016, 490, 104–109. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Graphene-based composite thin films for electronics. Nano Lett. 2009, 9, 814–818. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Bulusheva, L.G.; Asanov, I.P.; Shubin, Y.V.; Okotrub, A.V. Thermally exfoliated fluorinated graphite for NO2 gas sensing. Phys. Status Solidi B 2016, 253, 2492–2498. [Google Scholar] [CrossRef]
- Chen, G.; Paronyan, T.M.; Harutyunyan, A.R. Sub-ppt gas detection with pristine graphene. Appl. Phys. Lett. 2012, 101, 53119. [Google Scholar] [CrossRef]
- Han, T.; Nag, A.; Mukhopadhyay, S.; Xu, Y. Carbon nanotubes and its gas-sensing applications: A review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Prezioso, S.; Perrozzi, F.; Giancaterini, L.; Cantalini, C.; Treossi, E.; Palermo, V.; Nardone, M.; Santucci, S.; Ottaviano, L. Graphene oxide as a practical solution to high sensitivity gas sensing. J. Phys. Chem. C 2013, 117, 10683–10690. [Google Scholar] [CrossRef]
- Donarelli, M.; Ottaviano, L. 2D Materials for gas sensing applications: A review on graphene oxide, MoS2, WS2 and phosphorene. Sensors 2018, 18, 3638. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Rumyantsev, S.L.; Jiang, C.; Shur, M.; Balandin, A.A. Selective gas sensing with h-BN capped MoS2 heterostructure thin-film transistors. IEEE Electron Device Lett. 2015, 36, 1202–1204. [Google Scholar] [CrossRef]
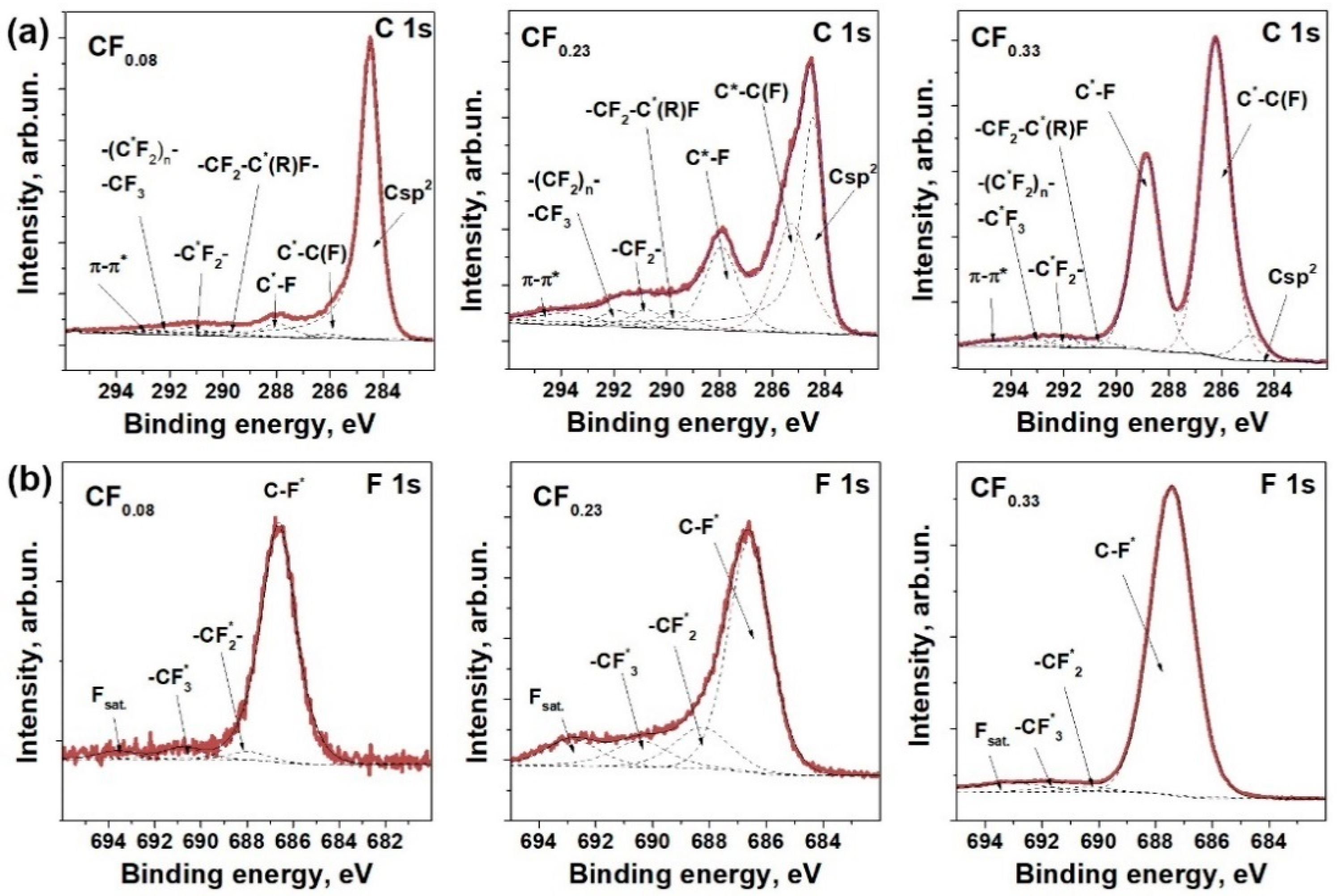
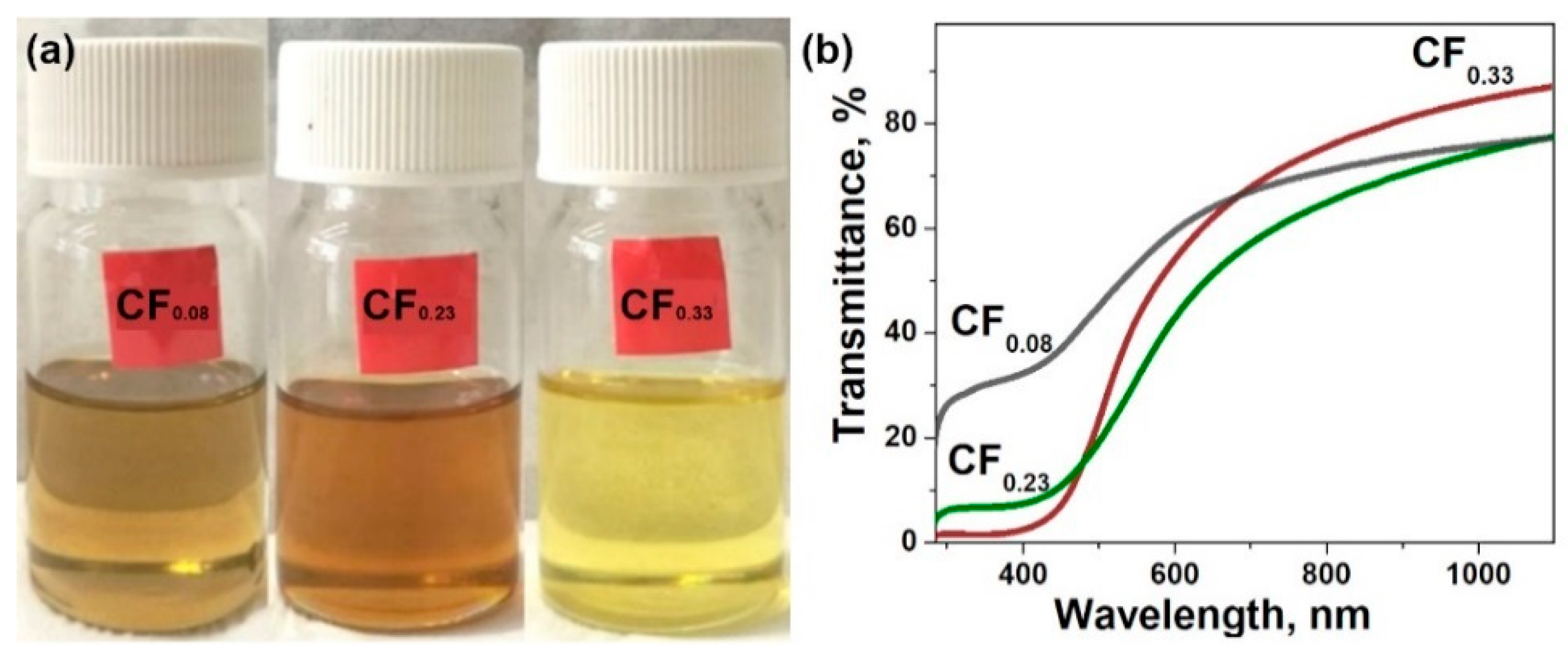
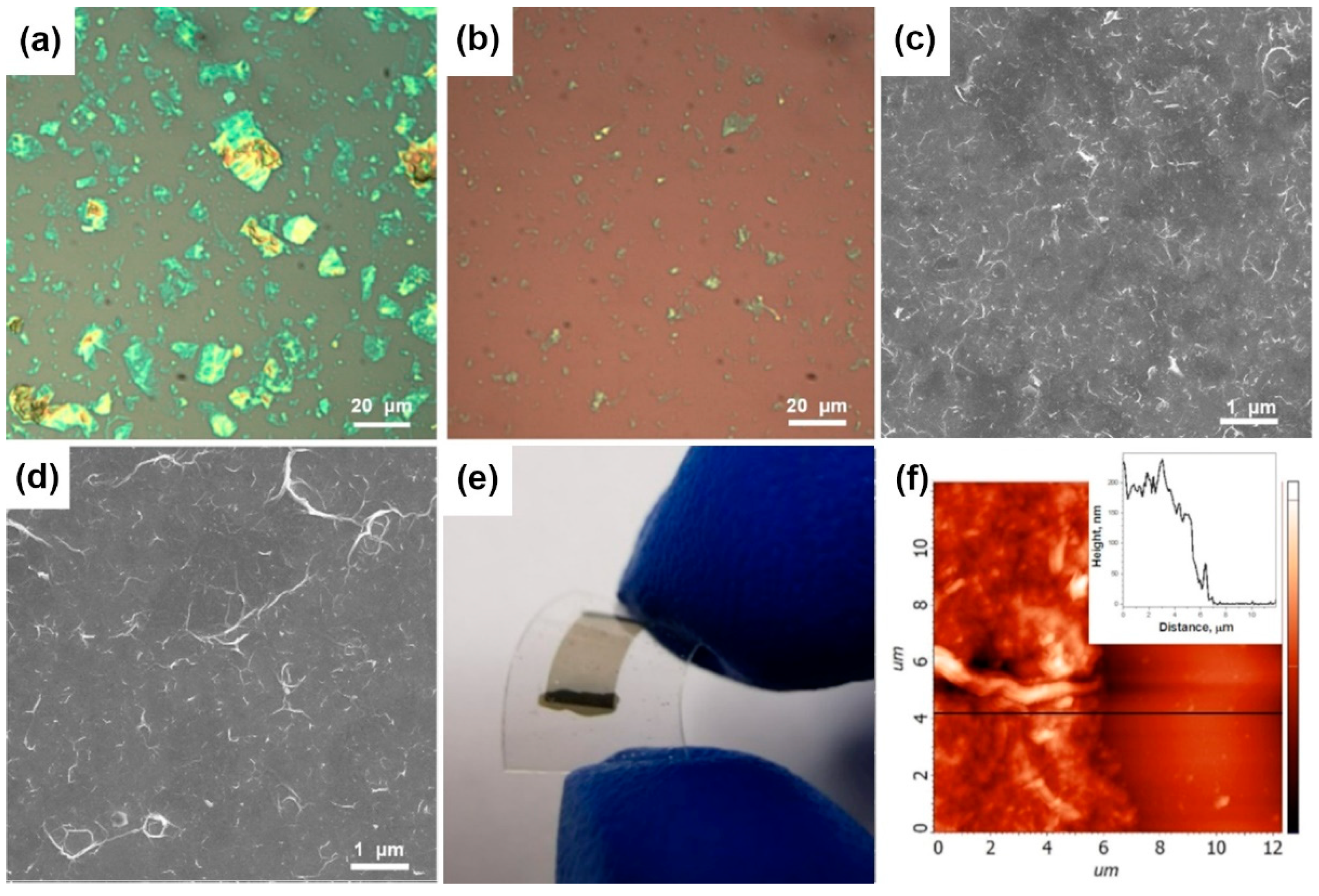
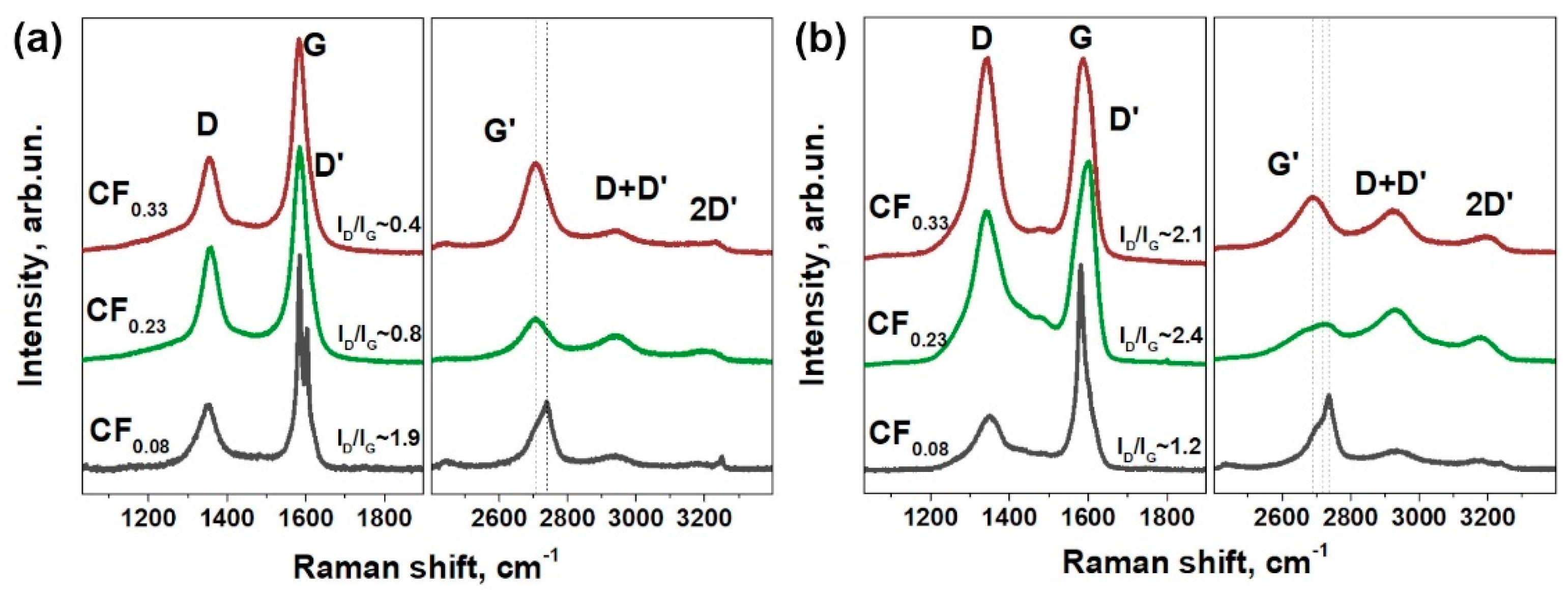
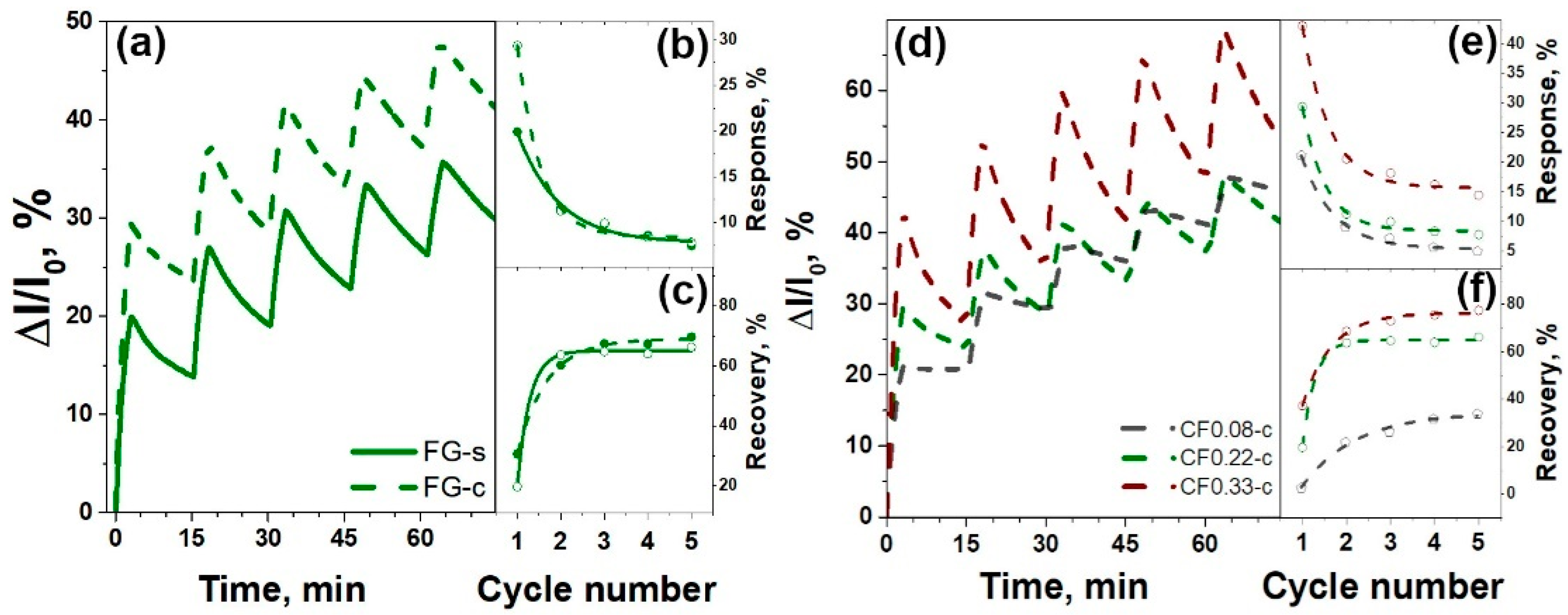
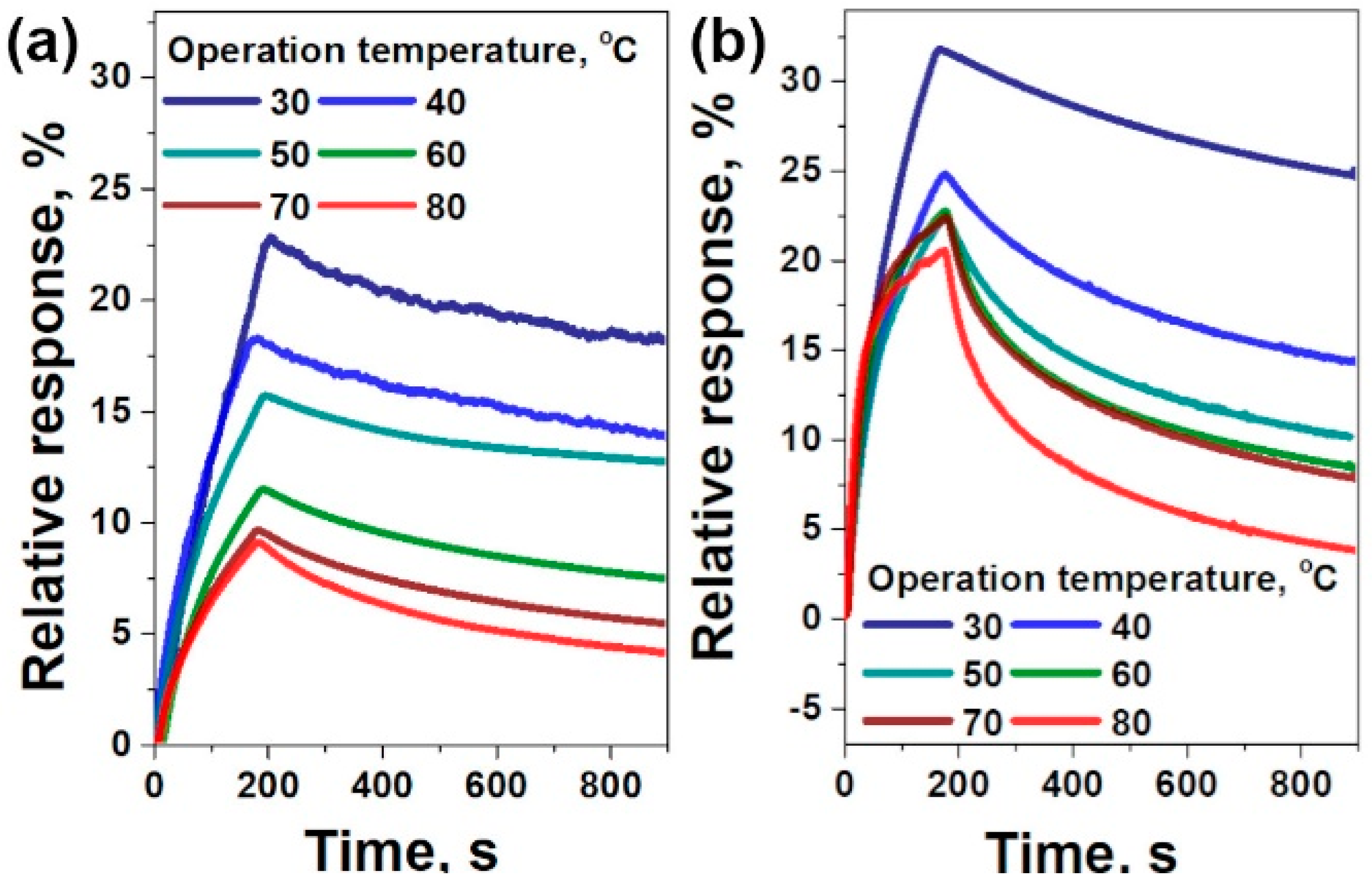
| Sample | F Content, at.% | Csp2, Fraction % | t, nm | Rs, kΩ/□ | ρ, Ω·m | Response, % | Recovery, % | |
|---|---|---|---|---|---|---|---|---|
| CF0.08 | FG-s | 7 | 88 | 146 | 66.9 | 9.8 × 10−2 | 10 | 21 |
| FG-c | 266 | 75.3 | 2.0 × 10−2 | 21 | 2 | |||
| CF0.23 | FG-s | 19 | 69 | 210 | 163.9 | 3.4 × 10−2 | 15 | 30 |
| FG-c | 210 | 120.9 | 2.5 × 10−2 | 29 | 19 | |||
| CF0.33 | FG-c | 25 | 60 | 215 | 81960 | 17.6 | 43 | 37 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sysoev, V.I.; Bulavskiy, M.O.; Pinakov, D.V.; Chekhova, G.N.; Asanov, I.P.; Gevko, P.N.; Bulusheva, L.G.; Okotrub, A.V. Chemiresistive Properties of Imprinted Fluorinated Graphene Films. Materials 2020, 13, 3538. https://doi.org/10.3390/ma13163538
Sysoev VI, Bulavskiy MO, Pinakov DV, Chekhova GN, Asanov IP, Gevko PN, Bulusheva LG, Okotrub AV. Chemiresistive Properties of Imprinted Fluorinated Graphene Films. Materials. 2020; 13(16):3538. https://doi.org/10.3390/ma13163538
Chicago/Turabian StyleSysoev, Vitalii I., Mikhail O. Bulavskiy, Dmitry V. Pinakov, Galina N. Chekhova, Igor P. Asanov, Pavel N. Gevko, Lyubov G. Bulusheva, and Alexander V. Okotrub. 2020. "Chemiresistive Properties of Imprinted Fluorinated Graphene Films" Materials 13, no. 16: 3538. https://doi.org/10.3390/ma13163538
APA StyleSysoev, V. I., Bulavskiy, M. O., Pinakov, D. V., Chekhova, G. N., Asanov, I. P., Gevko, P. N., Bulusheva, L. G., & Okotrub, A. V. (2020). Chemiresistive Properties of Imprinted Fluorinated Graphene Films. Materials, 13(16), 3538. https://doi.org/10.3390/ma13163538