Zr4+-Doped Anatase TiO2 Nanotube Array Electrode for Electrocatalytic Reduction of L-cystine
Abstract
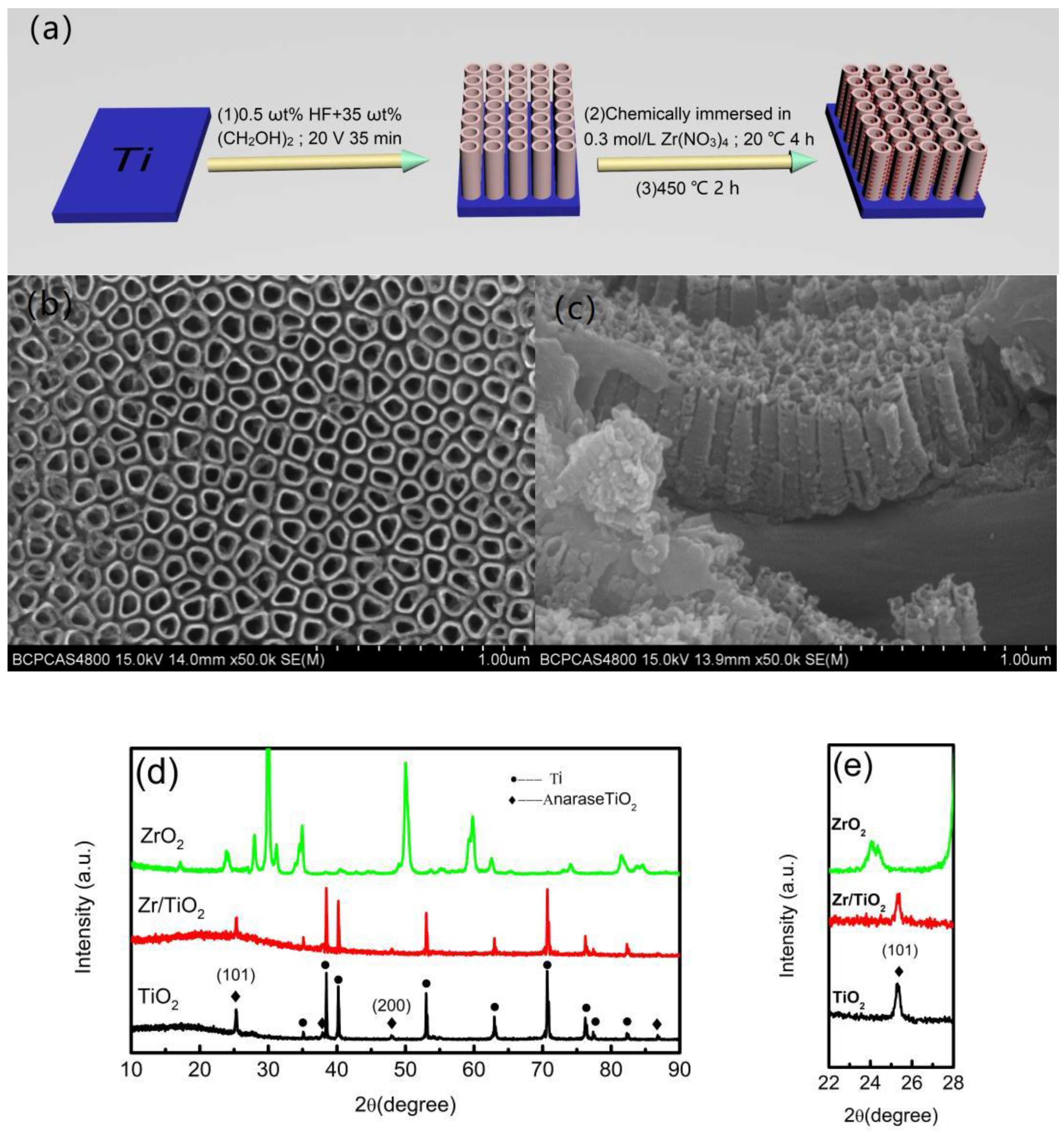
:1. Introduction
2. Materials and Methods
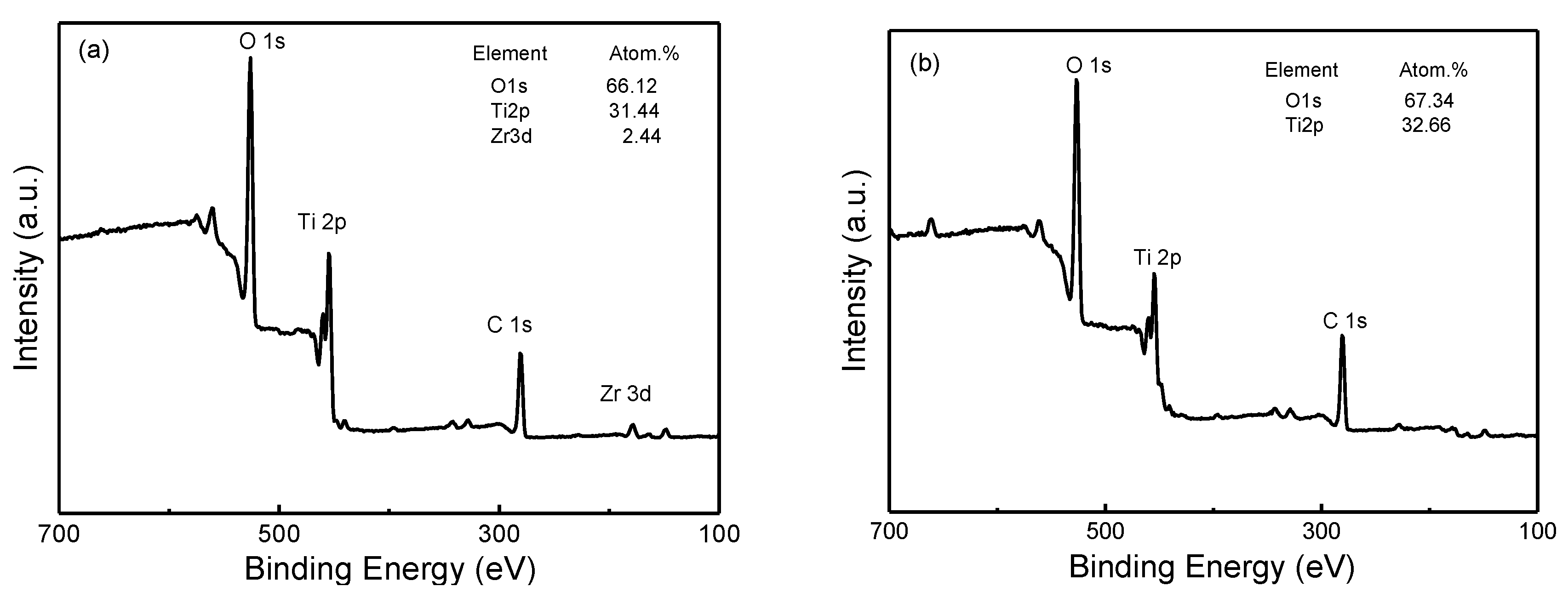
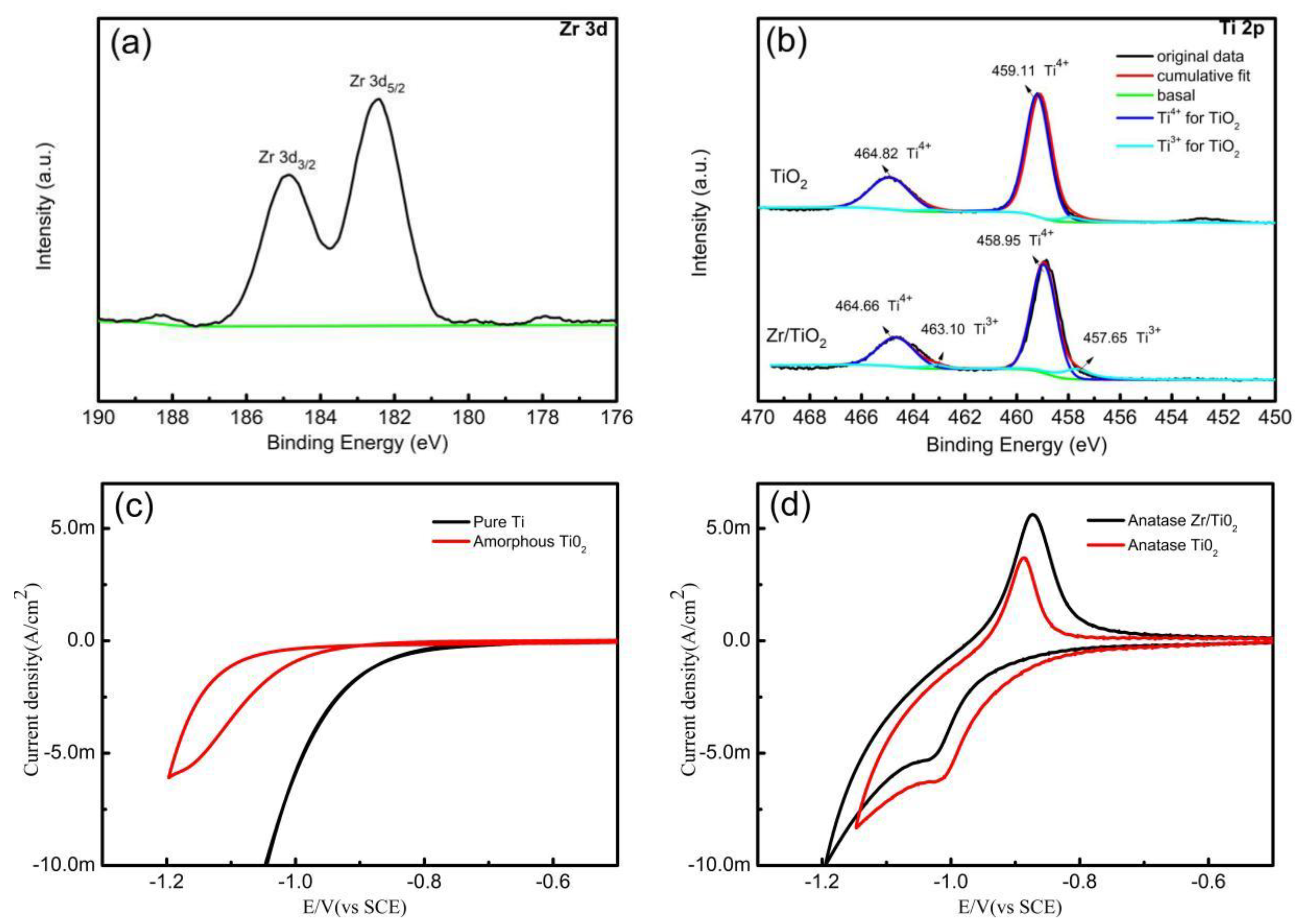
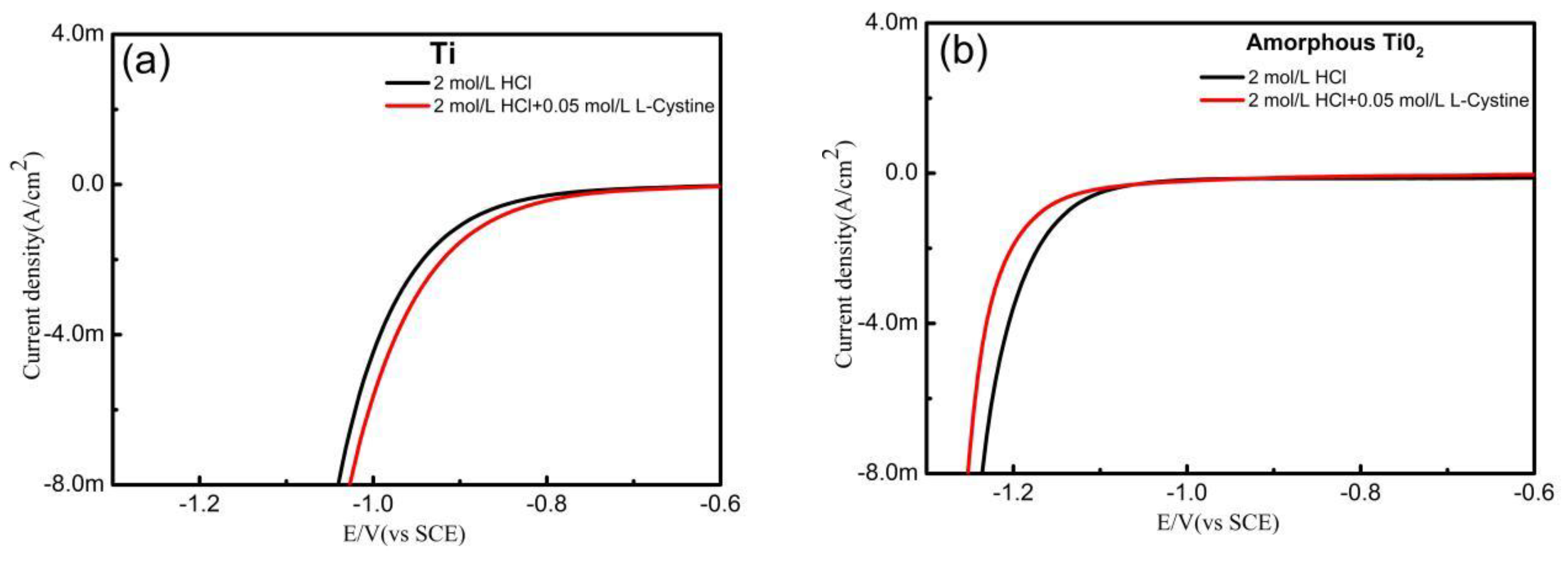
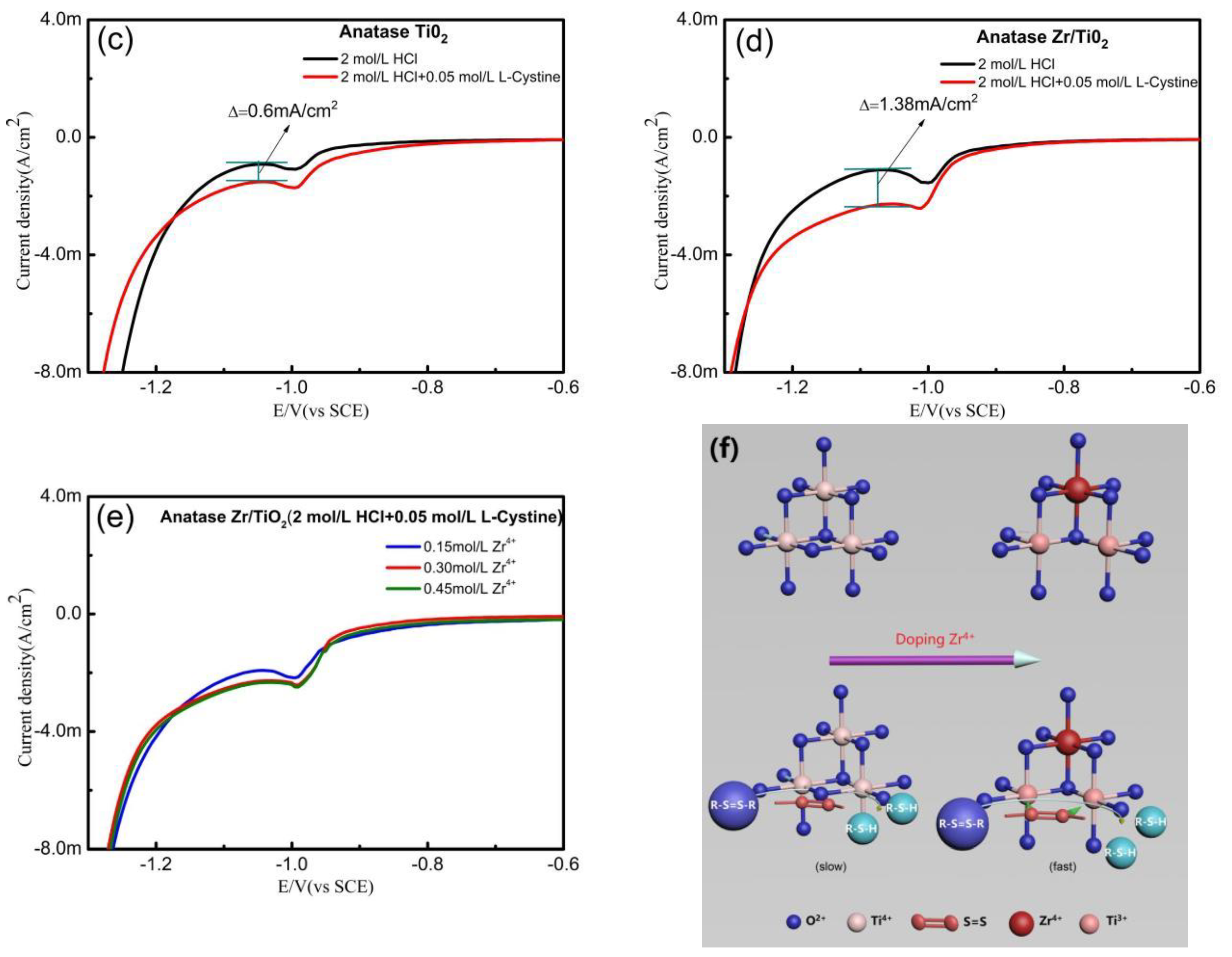
3. Results
(R=CH2(NH3·HCl)COOH)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ralph, T.R.; Hitchman, M.L.; Millington, J.P.; Walsh, F.C. The reduction of l-cystine hydrochloride at stationary and rotating disc mercury electrodes. Electrochim. Acta 2005, 51, 133–145. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Liu, L.; Wang, G.; Wang, D.; Qu, L. Preparation of nickel oxide nanoparticles on N-doped reduced graphene oxide: A two-dimensional hybrid for electrocatalytic sensing of L-cysteine. J. Alloy. Comp. 2017, 691, 834–840. [Google Scholar] [CrossRef]
- Nosal-Wiercińska, A. The catalytic activity of cysteine and cystine on the electroreduction of Bi(III) ions. J. Electroanal. Chem. 2011, 662, 298–305. [Google Scholar] [CrossRef]
- Wei, M.; Guo, J.; Shi, Z.; Yuan, Q.; Pu, M.; Rao, G.; Duan, X. Preparation and characterization of L-cystine and L-cysteine intercalated layered double hydroxides. J. Mater. Sci. 2007, 42, 2684–2689. [Google Scholar] [CrossRef]
- Ke, D.; Liu, H.; Peng, T.; Liu, X.; Dai, K. Preparation and photocatalytic activity of WO3/TiO2 nanocomposite particles. Mater. Lett. 2008, 62, 447–450. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.Y.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting-superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Qin, P.; Paulose, M.; Ibrahim Dar, M.; Moehl, T.; Arora, N.; Gao, P.; Varghese, O.K.; Grätzel, M.; Nazeeruddin, M.K. Stable and efficient perovskite solar cells based on Titania nanotube arrays. Small 2015, 11, 5533–5539. [Google Scholar] [CrossRef]
- Liang, X.P.; Lian, Z.W.; Zhu, J.; Men, K.; Wei, F. The preparation and properties of TiO2 nano-porous layers for perovskite solar cells. AIP Adv. 2020, 10, 015058. [Google Scholar] [CrossRef]
- Ozkan, S.; Ghanem, H.; Mohajernia, S.; Hejazi, S.; Fromm, T.; Borchardt, R.; Rosiwal, S.; Schmuki, P. Boron-doped diamond as an efficient back contact to thermally grown TiO2 photoelectrodes. ChemElectroChem 2019, 6, 4545–4549. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C. TiO2/graphene composite from thermal reaction of graphene oxide and its photocatalytic activity in visible light. J. Mater. Sci. 2011, 46, 2622–2626. [Google Scholar] [CrossRef]
- Zhang, W.; Shironita, S.; Umeda, M. Low Pt loading and high hydrogen oxidation reaction performance at Pt/TiO2–SiO2 investigated by a porous microelectrode. Catal. Lett. 2014, 144, 112–116. [Google Scholar] [CrossRef]
- Skúlason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J.K. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic conversion of nitrogen to ammonia with water on surface oxygen vacancies of titanium dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, R.; Zang, S.; He, H.; Su, Y.; Qiu, W.; Sun, X. Activity of selective catalytic reduction of NO over V2O5/TiO2 catalysts preferentially exposed anatase {001} and {101} facets. Catal. Lett. 2017, 147, 934–945. [Google Scholar] [CrossRef]
- Krbal, M.; Sopha, H.; Pohl, D.; Benes, L.; Damm, C.; Rellinghaus, B.; Kupčík, J.; Bezdička, P.; Šubrt, J.; Macak, J.M. Self-organized TiO2 nanotubes grown on Ti substrates with different crystallographic preferential orientations: Local structure of TiO2 nanotubes vs. photo-electrochemical response. Electrochim. Acta 2018, 264, 393–399. [Google Scholar] [CrossRef]
- Saputera, W.H.; Mul, G.; Hamdy, M.S. Ti3+-containing titania: Synthesis tactics and photocatalytic performance. Catal. Today 2015, 246, 60–66. [Google Scholar] [CrossRef]
- Cao, N.; Chen, Z.; Zang, K.; Xu, J.; Zhong, J.; Luo, J.; Xu, X.; Zheng, G. Doping strain induced bi-Ti3+ pairs for efficient N2 activation and electrocatalytic fixation. Nat. Commun. 2019, 10, 2877. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef]
- Lucky, R.A.; Charpentier, P.A. N-doped ZrO2/TiO2 bimetallic materials synthesized in supercritical CO2: Morphology and photocatalytic activity. Appl. Catal. B Environ. 2010, 96, 516–523. [Google Scholar] [CrossRef]
- Reddy, M.V.; Sharma, N.; Adams, S.; Rao, R.P.; Peterson, V.K.; Chowdari, B.V.R. Evaluation of undoped and M-doped TiO2, where M = Sn, Fe, Ni/Nb, Zr, V, and Mn, for lithium-ion battery applications prepared by the molten-salt method. RSC Adv. 2015, 5, 29535. [Google Scholar] [CrossRef] [Green Version]
- Macak, J.M.; Gong, B.G.; Hueppe, M.; Schmuki, P. Filling of TiO2 nanotubes by self-doping and electrodeposition. Adv. Mater. 2010, 19, 3027–3031. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, G.; Zhao, X.; Zhang, X.; Tang, Y.; Zuo, Y. Zr4+-Doped Anatase TiO2 Nanotube Array Electrode for Electrocatalytic Reduction of L-cystine. Materials 2020, 13, 3572. https://doi.org/10.3390/ma13163572
Wang W, Wang G, Zhao X, Zhang X, Tang Y, Zuo Y. Zr4+-Doped Anatase TiO2 Nanotube Array Electrode for Electrocatalytic Reduction of L-cystine. Materials. 2020; 13(16):3572. https://doi.org/10.3390/ma13163572
Chicago/Turabian StyleWang, Weizhen, Guangxin Wang, Xuhui Zhao, Xiaofeng Zhang, Yuming Tang, and Yu Zuo. 2020. "Zr4+-Doped Anatase TiO2 Nanotube Array Electrode for Electrocatalytic Reduction of L-cystine" Materials 13, no. 16: 3572. https://doi.org/10.3390/ma13163572




