Superhard Boron-Rich Boron Carbide with Controlled Degree of Crystallinity
Abstract
:1. Introduction
2. Materials and Methods
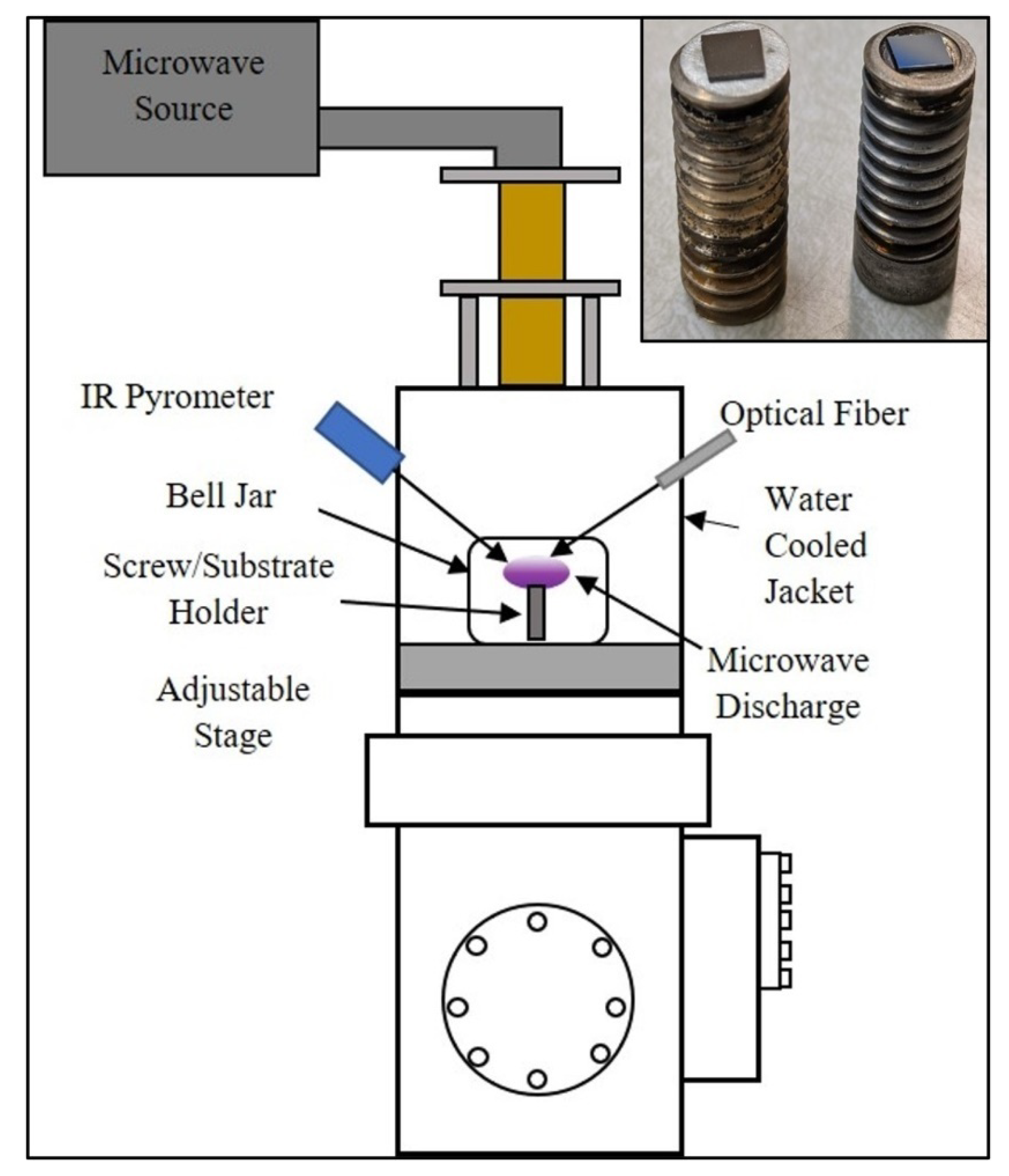
2.1. MPCVD Process
2.2. Characterization Techniques
2.3. Density Functional Theory
3. Results
3.1. X-ray Photoelectron Spectroscopy
3.2. X-ray Diffraction
3.3. Raman Spectroscopy
3.4. Fourier Transform Infrared Spectroscopy (FTIR)
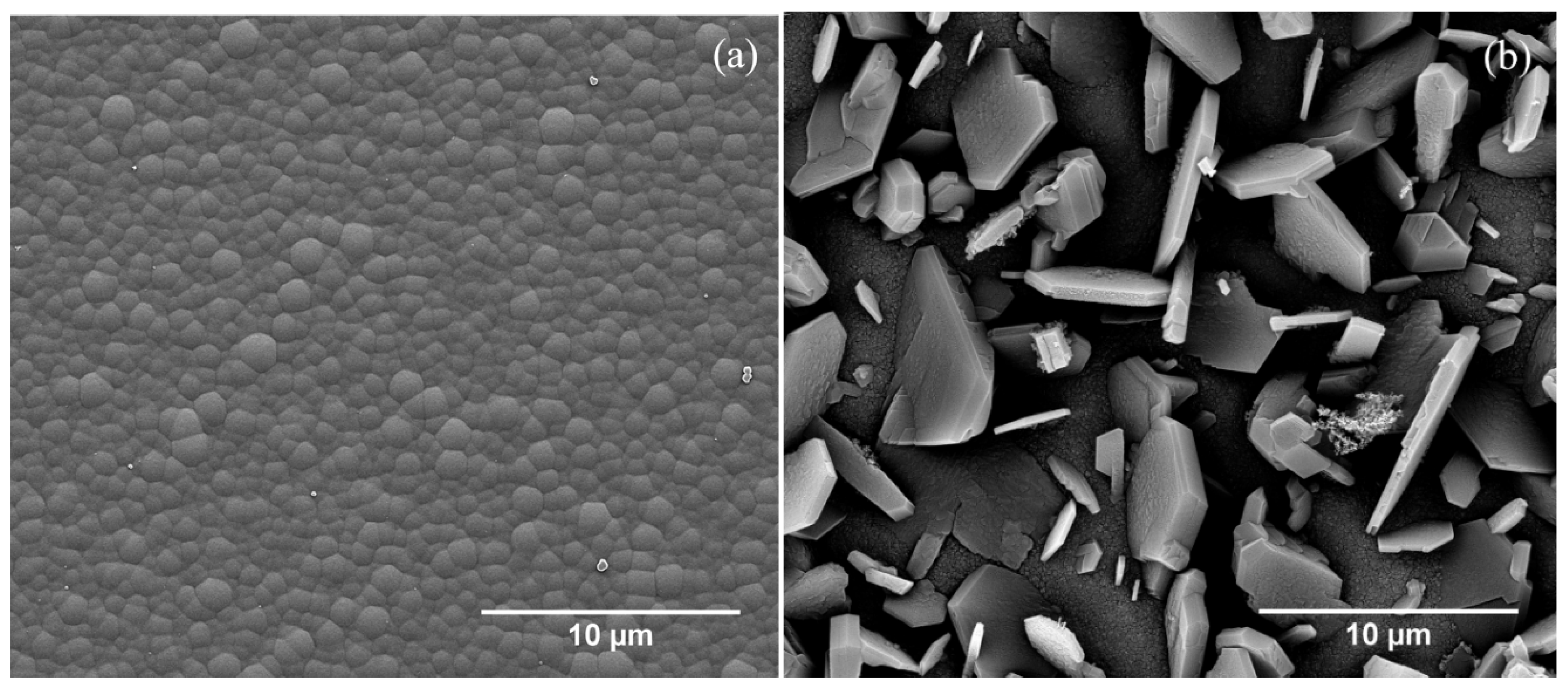
3.5. Scanning Electron Microscopy (SEM)
3.6. Nanoindentation
3.7. Density Functional Theory Calculations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hall, H.T.; Compton, L.A. Group IV Analogs and High Pressure, High Temperature Synthesis of B2O. Inorg. Chem. 1965, 4, 1213–1216. [Google Scholar] [CrossRef]
- Han, S.; Ihm, J.; Louie, S.G.; Cohen, M.L. Enhancement of Surface Hardness: Boron on Diamond (111). Phys. Rev. Lett. 1998, 80, 995–998. [Google Scholar] [CrossRef] [Green Version]
- Velamakanni, A.; Ganesh, K.J.; Zhu, Y.; Ferreira, P.J.; Ruoff, R.S. Catalyst-Free Synthesis and Characterization of Metastable Boron Carbide Nanowires. Adv. Funct. Mater. 2009, 19, 3926–3933. [Google Scholar] [CrossRef]
- Tallant, D.R.; Aselage, T.L.; Campbell, A.N.; Emin, D. Boron carbide structure by Raman spectroscopy. Phys. Rev. B 1989, 40, 5649–5656. [Google Scholar] [CrossRef]
- Bouchacourt, M.; Thevenot, F. The properties and structure of the boron carbide phase. J. Less Common Met. 1981, 82, 227–235. [Google Scholar] [CrossRef]
- Saal, J.E.; Shang, S.; Liu, Z.-K. The structural evolution of boron carbide via ab initio calculations. Appl. Phys. Lett. 2007, 91, 231915. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Y. Shear-Driven Chemical Decomposition of Boron Carbide. J. Phys. Chem. C 2019, 123, 23145–23150. [Google Scholar] [CrossRef]
- Amberger, W.S.E.; Buschbec, B. Boron Compounds. In Gmelin Handbook of Inorganic Chemistry, 8th ed.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar]
- Baker, P.A.; Chen, W.C.; Chen, C.C.; Catledge, S.A.; Vohra, Y.K. First-Principles Predictions and Synthesis of B50C2 by Chemical Vapor Deposition. Sci. Rep. 2020, 10, 4454. [Google Scholar] [CrossRef] [PubMed]
- Beauvy, M. Stoichiometric limits of carbon-rich boron carbide phases. J. Less Common Met. 1983, 90, 169–175. [Google Scholar] [CrossRef]
- Shirai, K. Electronic structures and mechanical properties of boron and boron-rich crystals (Part I). J. Superhard Mater. 2010, 32, 205–225. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.E.; McCauley, J.W.; Wright, T.W. The effects of stoichiometry on the mechanical properties of icosahedral boron carbide under loading. J. Phys. Condens. Matter 2012, 24, 505402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golubeva, N.A.; Plyasunkova, L.A.; Kelina, I.Y.; Antonova, E.S.; Zhuravlev, A.A. Study of Reaction-Bonded Boron Carbide Properties. Refract. Ind. Ceram. 2015, 55, 414–418. [Google Scholar] [CrossRef]
- Mukhanov, V.A.; Kurakevych, O.O.; Solozhenko, V.L. The interrelation between hardness and compressibility of substances and their structure and thermodynamic properties. J. Superhard Mater. 2008, 30, 368–378. [Google Scholar] [CrossRef]
- Niihara, K.; Nakahira, A.; Hirai, T. The Effect of Stoichiometry on Mechanical Properties of Boron Carbide. J. Am. Ceram. Soc. 2006, 67, C-13–C-14. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron Carbide: Structure, Properties, and Stability under Stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Gan, F. Structure and properties of amorphous thin film for optical data storage. J. Non-Cryst. Solids 2008, 354, 1089–1099. [Google Scholar] [CrossRef]
- Hu, T.; Steihl, L.; Rafaniello, W.; Fawcett, T.; Hawn, D.D.; Mashall, J.G.; Rozeveld, S.J.; Putzig, C.L.; Blackson, J.H.; Cermignani, W.; et al. Structures and properties of disordered boron carbide coatings generated by magnetron sputtering. Thin Solid Film. 1998, 332, 80–86. [Google Scholar] [CrossRef]
- Shirai, K. Electronic structures and mechanical properties of boron and boron-rich crystals (Part 2). J. Superhard Mater. 2010, 32, 336–345. [Google Scholar] [CrossRef]
- Vast, N.; Sjakste, J.; Betranhandy, E. Boron carbides from first principles. J. Phys. Conf. Ser. 2009, 176. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, L.; Qian, Y.; Zhang, W.; Ma, J. Synthesis of Nanocrystalline Boron Carbide via a Solvothermal Reduction of CCl4 in the Presence of Amorphous Boron Powder. J. Am. Ceram. Soc. 2004, 88, 225–227. [Google Scholar] [CrossRef]
- Thévenot, F. Boron carbide—A comprehensive review. J. Eur. Ceram. Soc. 1990, 6, 205–225. [Google Scholar] [CrossRef]
- Jansson, U. Chemical Vapor Deposition of Boron Carbides. Mater. Manuf. Process. 1991, 6, 481–500. [Google Scholar] [CrossRef]
- Sezer, A.O.; Brand, J.I. Chemical vapor deposition of boron carbide. Mater. Sci. Eng. B 2001, 79, 191–202. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Arnold, I.; Catledge, S.A. Hexagonal boron nitride grown using high atomic boron emission during microwave plasma chemical vapor deposition. J. Vac. Sci. Technol. A 2019, 37, 061507. [Google Scholar] [CrossRef]
- Rau, A.; Chakrabarty, K.; Gullion, W.; Baker, P.A.; Bikmukhametov, I.; Martens, R.L.; Thompson, G.B.; Catledge, S.A. A diffusion approach for plasma synthesis of superhard tantalum borides. J. Mater. Res. 2019, 35, 481–490. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Moulder, J.F. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Waltham, MA, USA, 1992; p. 261. [Google Scholar]
- Vincent, C.; Vincent, H.; Mourichoux, H.; Bouix, J. Characterization by XPS and SEM of reactive chemical vapour deposited boron carbide on carbon fibre. J. Mater. Sci. 1992, 27, 1892–1900. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Kehrer, M.; Duchoslav, J.; Hinterreiter, A.; Cobet, M.; Mehic, A.; Stehrer, T.; Stifter, D. XPS investigation on the reactivity of surface imine groups with TFAA. Plasma Process. Polym. 2019, 16. [Google Scholar] [CrossRef]
- Qu, J.; Li, Q.; Luo, C.; Cheng, J.; Hou, X. Characterization of Flake Boron Nitride Prepared from the Low Temperature Combustion Synthesized Precursor and Its Application for Dye Adsorption. Coatings 2018, 8, 214. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Goto, T.; Hirai, T.; Amano, T. State of boron in chemical vapour-deposited SiC-B composite powders. J. Mater. Sci. Lett. 1990, 9, 997–999. [Google Scholar] [CrossRef]
- Kurakevych, O.O.; Solozhenko, V.L. Crystal structure of dense pseudo-cubic boron allotrope, pc-B52, by powder X-ray diffraction. J. Superhard Mater. 2013, 35, 60–63. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, U.; Werheit, H. Raman effect of boron carbide (B4.3C to B10.37C). J. Alloy. Compd. 1994, 205, 87–91. [Google Scholar] [CrossRef]
- Erdemir, A.; Bindal, C.; Fenske, G.R. Formation of ultralow friction surface films on boron carbide. Appl. Phys. Lett. 1996, 68, 1637–1639. [Google Scholar] [CrossRef]
- Domnich, V.; Gogotsi, Y.; Trenary, M.; Tanaka, T. Nanoindentation and Raman spectroscopy studies of boron carbide single crystals. Appl. Phys. Lett. 2002, 81, 3783–3785. [Google Scholar] [CrossRef]
- Han, Z.; Li, G.; Tian, J.; Gu, M. Microstructure and mechanical properties of boron carbide thin films. Mater. Lett. 2002, 57, 899–903. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Stalla, D.; Tekeei, A.; Suppes, G.; Jalisatgi, S.; Lee, M.; Hawthorne, F.; Robertson, J.D.; Firlej, L.; et al. Infrared study of boron—Carbon chemical bonds in boron-doped activated carbon. Carbon 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Pascual, E.; Martínez, E.; Esteve, J.; Lousa, A. Boron carbide thin films deposited by tuned-substrate RF magnetron sputtering. Diam. Relat. Mater. 1999, 8, 402–405. [Google Scholar] [CrossRef]
- Philip, J.; Launer, B.A. Infrared Analysis of Organosilicon Compounds: Spectra-Structure Correlations. In Silicon Compounds: Silanes & Silicones; Gelest, Inc.: Morrisville, PA, USA, 2013; pp. 175–178. [Google Scholar]
- Ploog, K.; Schmidt, H.; Amberger, E.; Will, G.; Kossobutzki, K.H. B48B2C2 und B48B2N2, zwei Nichtmetallboride mit der Struktur des sog. I tetragonalen Bors. J. Less Common Met. 1972, 29, 161–169. [Google Scholar] [CrossRef]
- Will, G.; Kossobutzki, K.H. X-ray diffraction analysis of B50C2 and B50N2 crystal-lizing in the “tetragonal” boron lattice. J. Less Common Met. 1976, 47, 33–38. [Google Scholar] [CrossRef]
- Yang, X.; Goddard, W.A.; An, Q. Structure and Properties of Boron-Very-Rich Boron Carbides: B12 Icosahedra Linked through Bent CBB Chains. J. Phys. Chem. C 2018, 122, 2448–2453. [Google Scholar] [CrossRef] [Green Version]
- Clark, H.K.; Hoard, J.L. The Crystal Structure of Boron Carbide. J. Am. Chem. Soc. 1943, 65, 2115–2119. [Google Scholar] [CrossRef]
- Wood, C.; Emin, D. Conduction mechanism in boron carbide. Phys. Rev. B 1984, 29, 4582–4587. [Google Scholar] [CrossRef]
- Van Schilfgaarde, M.; Harrison, W.A. Electronic structure of boron. J. Phys. Chem. Solids 1985, 46, 1093–1100. [Google Scholar] [CrossRef]
- Yakel, H.L. Recent developments in the structural crystallography of boron and the higher borides. Aip Conf. Proc. 1986, 140, 97–108. [Google Scholar] [CrossRef]
- Larson, A.C. Comments concerning the crystal structure of B4C. Aip Conf. Proc. 1986, 140, 5. [Google Scholar] [CrossRef]
- Kirfel, A.; Gupta, A.; Will, G. The nature of the chemical bonding in boron carbide, B13C2. I. Structure refinement. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 1052–1059. [Google Scholar] [CrossRef] [Green Version]
- Uemura, N.; Shirai, K.; Eckert, H.; Kunstmann, J. Structure, nonstoichiometry, and geometrical frustration ofα-tetragonal boron. Phys. Rev. B 2016, 93, 104101. [Google Scholar] [CrossRef] [Green Version]
- Schmechel, R.; Werheit, H. Correlation between structural defects and electronic properties of icosahedral boron-rich solids. J. Phys. Condens. Matter 1999, 11, 6803–6813. [Google Scholar] [CrossRef]








| Sample | Peaks | Binding Energy | Peak Area (%) | Assignment |
|---|---|---|---|---|
| BC-1 | B1s | 186.2 | 75 | B-C |
| B1s | 187.4 | 25 | B-B | |
| C1s | 283.3 | 69 | C-B | |
| C1s | 284.9 | 22 | C-C | |
| C1s | 286.5 | 9 | C-O | |
| O1s | 533.3 | 100 | C-O | |
| BC-2 | B1s | 187.0 | 39 | B-C |
| B1s | 188.5 | 48 | B-B | |
| B1s | 190.5 | 10 | B-N | |
| B1s | 192.0 | 3 | B-O | |
| C1s | 282.0 | 26 | C-B | |
| C1s | 284.5 | 36 | C-C | |
| C1s | 287.0 | 38 | C-O | |
| O1s | 532.3 | 90 | C-O | |
| O1s | 533.6 | 10 | B-O | |
| N1s | 399.0 | 100 | B-N |
| Transmittance Frequency (cm−1) | Assignment |
|---|---|
| 1022 | Si-O stretching vibrations (from the silicon substrate) |
| 1097 | B-C stretching vibrations |
| 1305 | B-O stretching vibrations |
| 1450 | C=C stretching vibrations |
| 1622 | OH bending modes of C-OH and B-OH groups |
| 2857 | CH symmetric stretching vibrations |
| 2910 | CH asymmetric stretching vibrations |
| 3272 | B-OH stretching vibrations |
| 3440 | C-OH stretching vibrations |
| Transmittance Frequency (cm−1) | Assignment |
|---|---|
| 1058 | Si-O stretching vibrations (from the silicon substrate) |
| 1107 | B-C stretching vibrations |
| 1236 | C-O stretching vibrations |
| 1292 | C-H bending vibrations |
| 1340 | B-O stretching vibrations |
| 1450 | C=C stretching vibrations |
| 1616 | OH bending modes of C-OH and B-OH groups |
| 2750 | CH symmetric stretching vibrations |
| 2879 | CH asymmetric stretching vibrations |
| 3274 | B-OH stretching vibrations |
| 3442 | C-OH stretching vibrations |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakrabarty, K.; Chen, W.-C.; Baker, P.A.; Vijayan, V.M.; Chen, C.-C.; Catledge, S.A. Superhard Boron-Rich Boron Carbide with Controlled Degree of Crystallinity. Materials 2020, 13, 3622. https://doi.org/10.3390/ma13163622
Chakrabarty K, Chen W-C, Baker PA, Vijayan VM, Chen C-C, Catledge SA. Superhard Boron-Rich Boron Carbide with Controlled Degree of Crystallinity. Materials. 2020; 13(16):3622. https://doi.org/10.3390/ma13163622
Chicago/Turabian StyleChakrabarty, Kallol, Wei-Chih Chen, Paul A. Baker, Vineeth M. Vijayan, Cheng-Chien Chen, and Shane A. Catledge. 2020. "Superhard Boron-Rich Boron Carbide with Controlled Degree of Crystallinity" Materials 13, no. 16: 3622. https://doi.org/10.3390/ma13163622







