Effect of Rare Earth on Microstructure and Wear Resistance of In-Situ-Synthesized Mo2FeB2 Ceramics-Reinforced Fe-Based Cladding
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
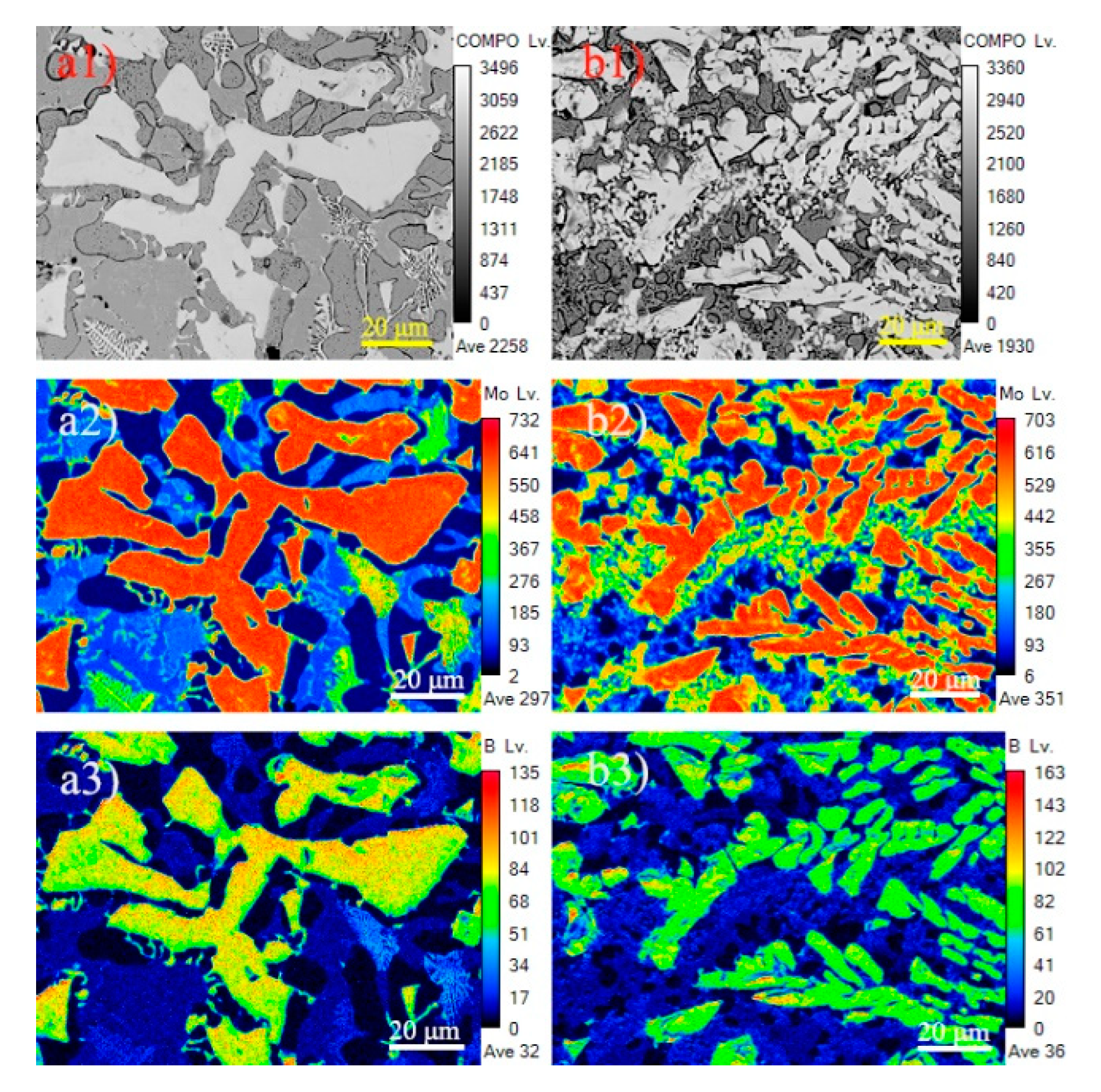
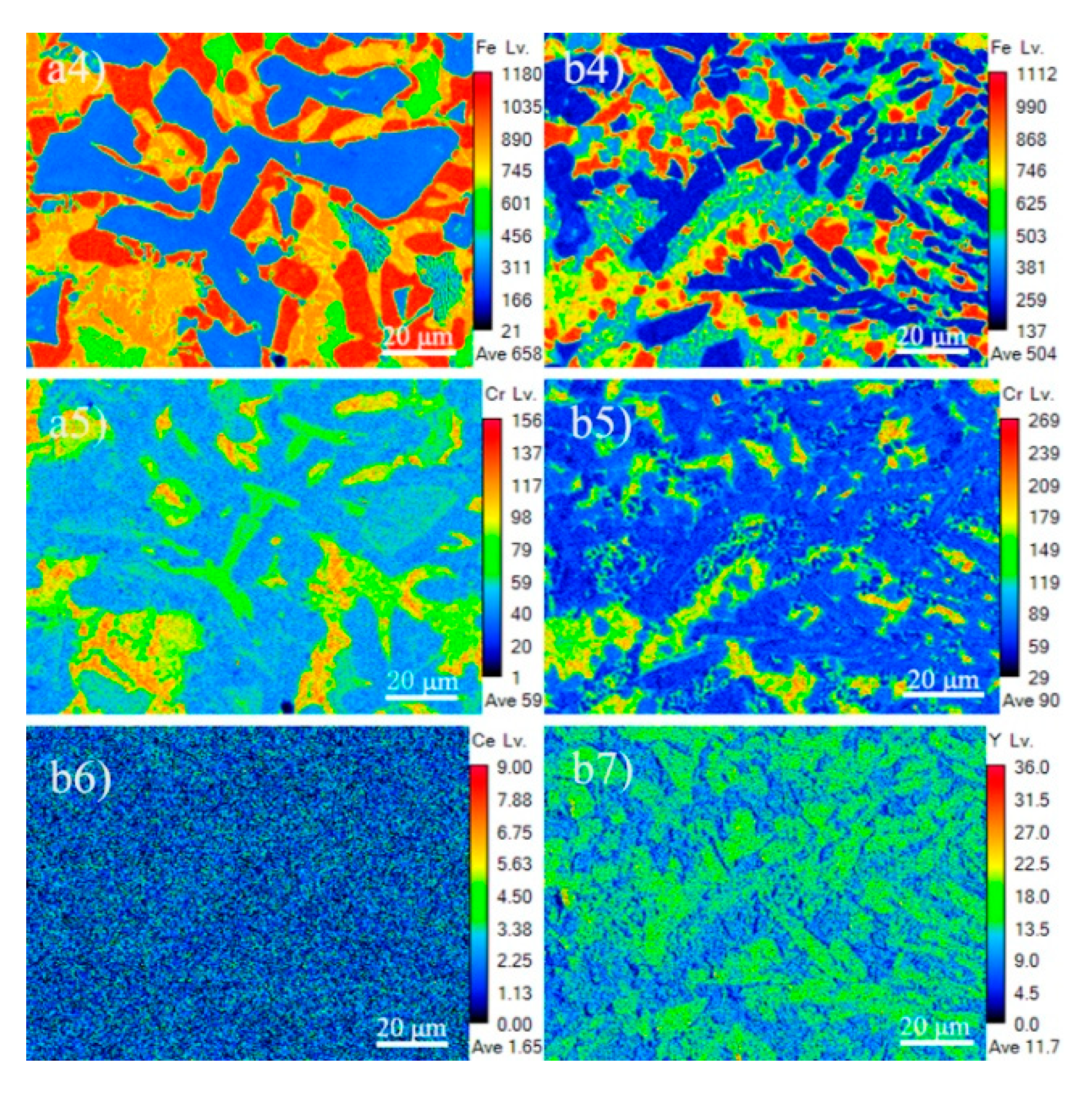
3.1. Microstructure and Composition
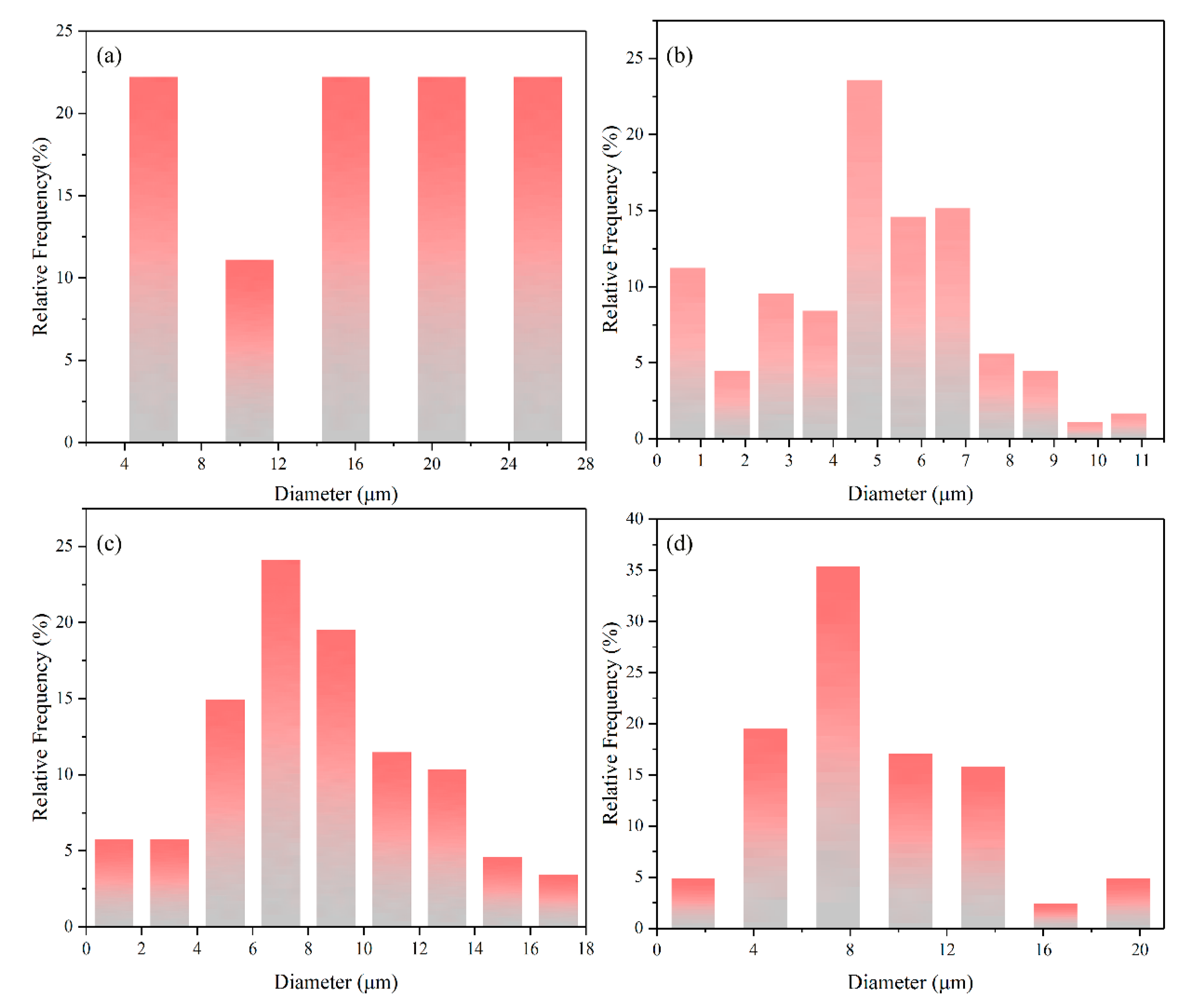
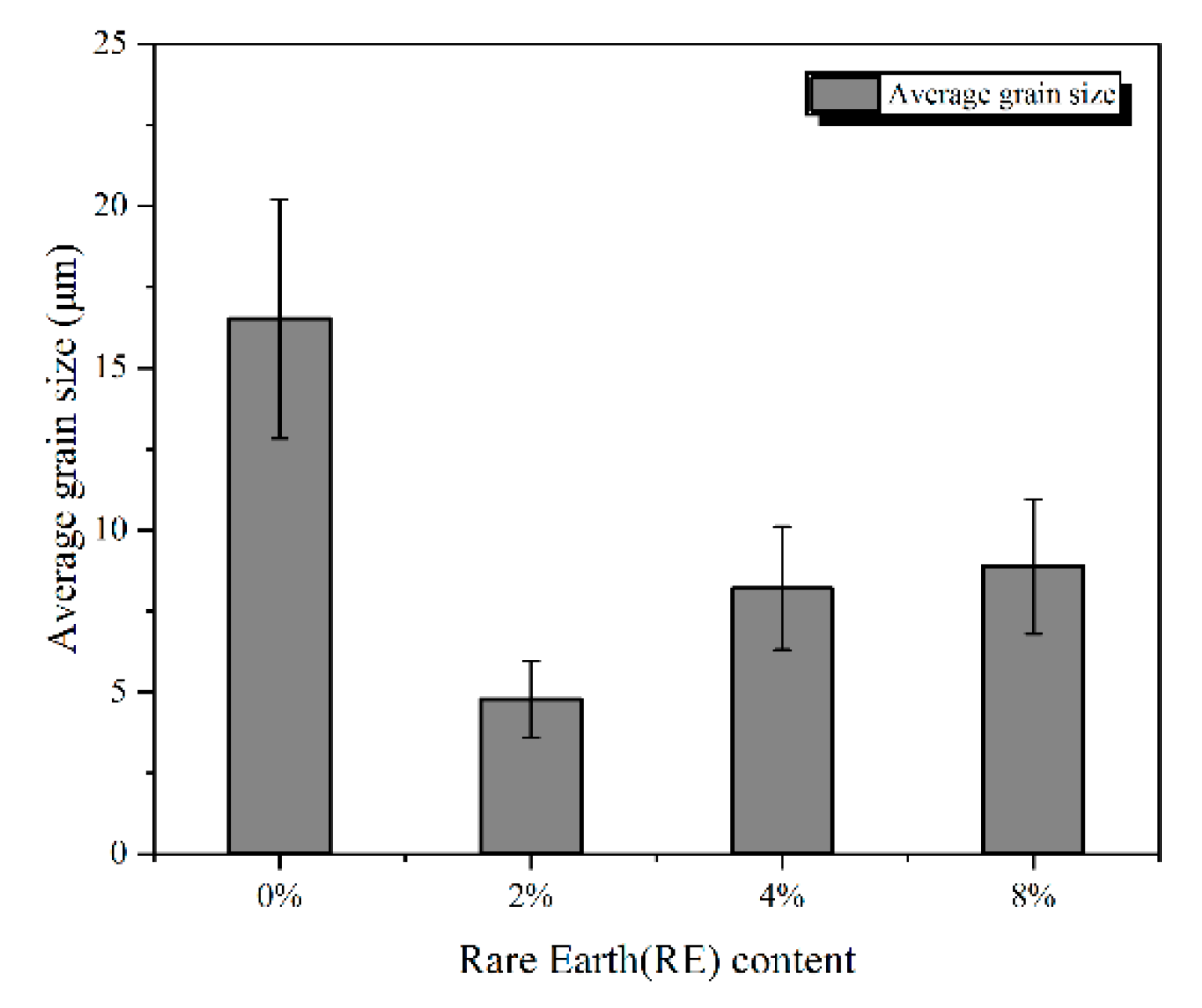
3.2. Statistics of Phase Fraction and Grain Size
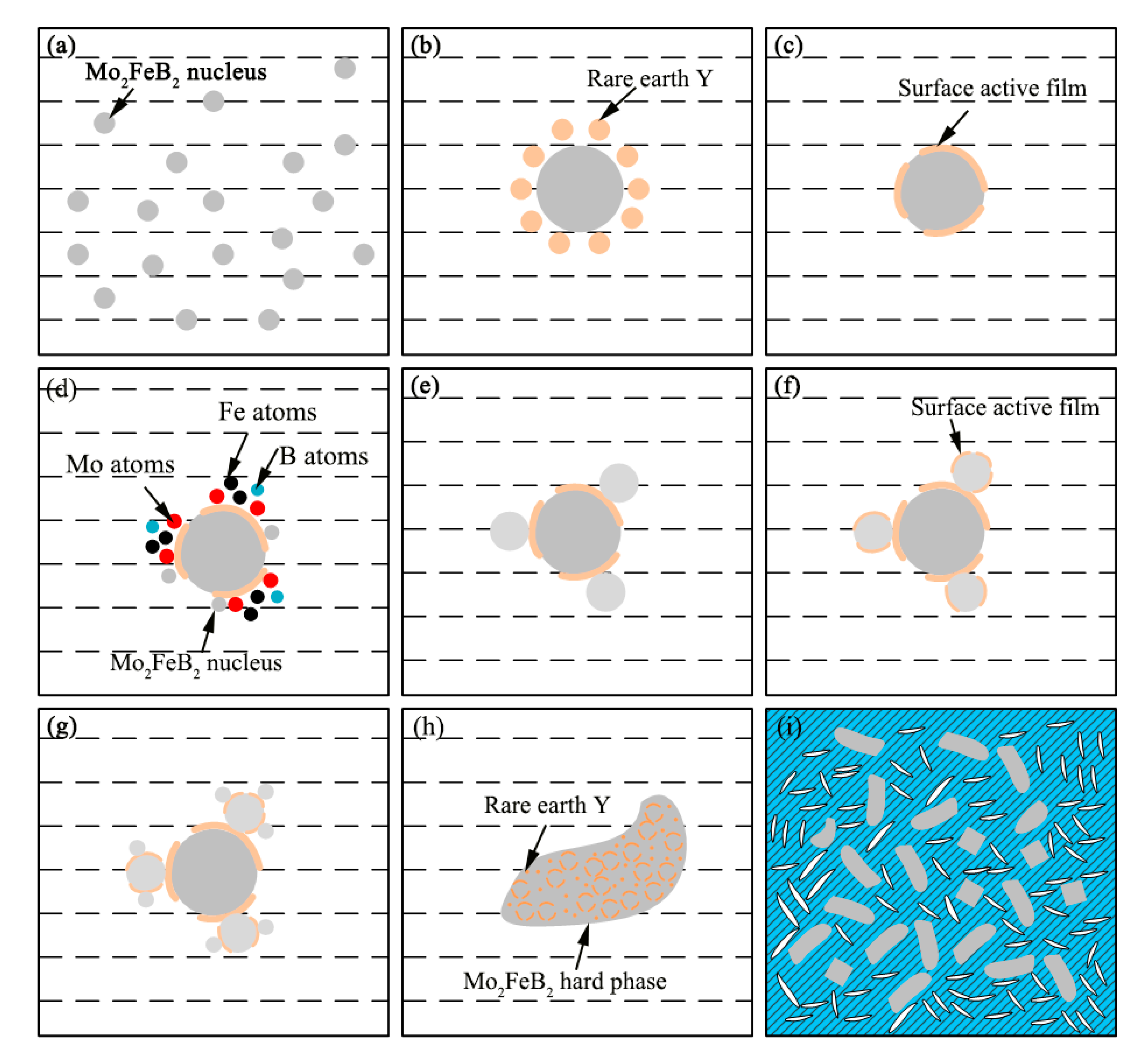
3.3. Refining Mechanism of Rare Earth
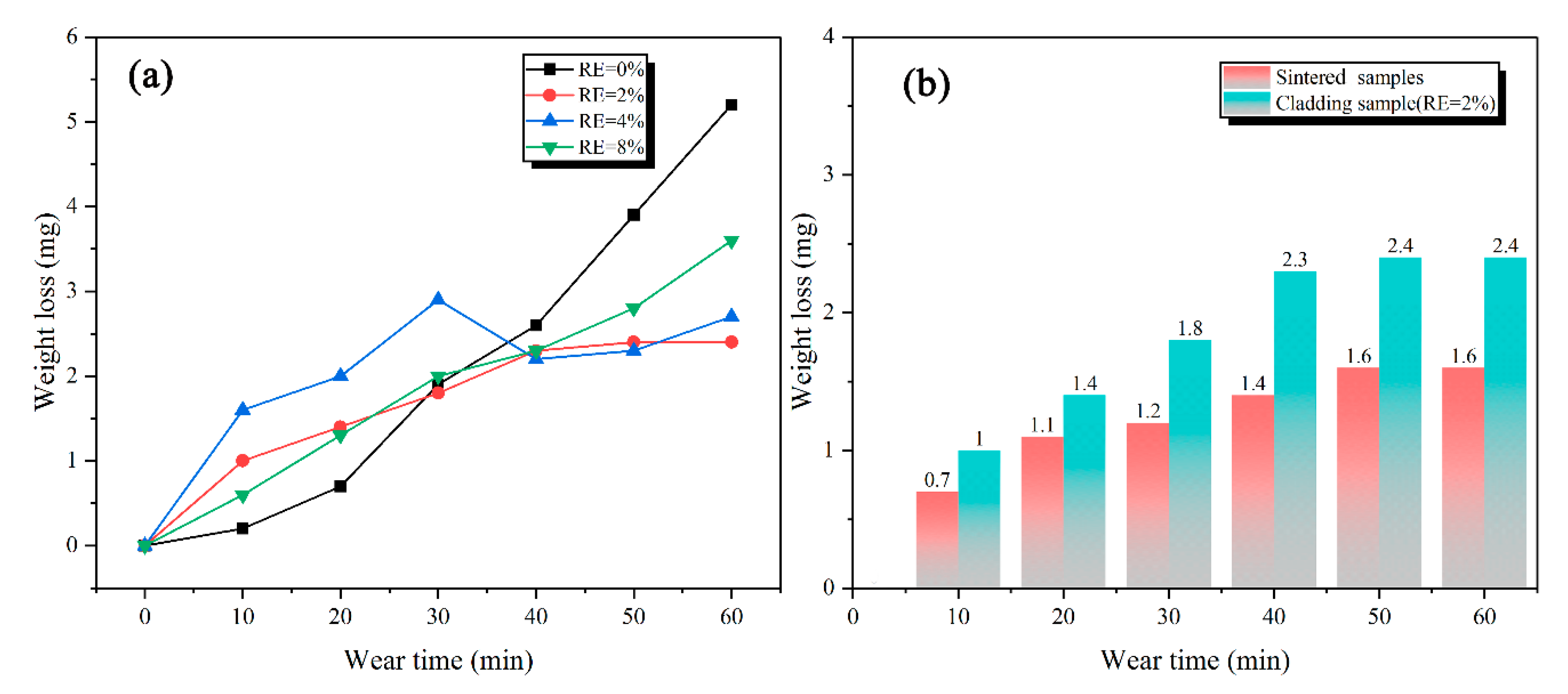
3.4. Wear Characteristics
4. Conclusions
- Rare earth Y has a good refinement effect on Mo2FeB2 grains. When the RE content is 2%, average grain size of Mo2FeB2 phase is 4.78 μm, which is 1/3.44 of the cladding with 0% RE content.
- Rare earth Y rapidly diffuses around Mo2FeB2 particles and serves as surface active film to hinder the growth of the Mo2FeB2 nucleus. Meanwhile, Mo atoms, B atoms and Fe atoms are easily enriched in the surface active film to form the Mo2FeB2 nucleus, which increases the number of Mo2FeB2 phases as well as refining Mo2FeB2 grains. In addition, rare earth Y reduces the specific surface energy and more liquid atoms reach the nucleation work through energy fluctuations, so that the nucleation rate of Mo2FeB2 is also improved.
- The addition of rare-earth Y element plays an important role in improving the wear resistance of cladding. When the RE content was 2%, the wear resistance of cladding was 2.4 mg, which is about 70% of the sintering sample with similar wear-track morphology.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, J.J.; Zheng, Y.; Chen, J.X.; Zhou, W.; Zhao, Y.J.; Feng, P. Microstructures and mechanical properties of Mo2FeB2-based cermets prepared by two-step sintering technique. Int. J. Refract. Met. Hard Mater. 2018, 72, 56–62. [Google Scholar] [CrossRef]
- Hu, Z.W.; Li, W.G. Research review of Mo-Ni-B ternary boride preparation and properties. Surf. Technol. 2016, 45, 1–9. [Google Scholar]
- Ren, X.J.; Fan, Z.S. Research progress of boride cermet coatings. Mater. Prot. 2018, 51, 90–95. [Google Scholar]
- Jian, Y.X.; Huang, Z.F.; Liu, X.T.; Xing, J.D. Comparative investigation on the stability, electronic structures and mechanical properties of Mo2FeB2 and Mo2NiB2 ternary borides by first-principles calculations. Results Phys. 2019, 15, 102698. [Google Scholar] [CrossRef]
- Takagi, K. High tough boride base cermets produced by reaction sintering. Mater. Chem. Phys. 2001, 67, 214–219. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.G.; Zhong, J.; Xiang, Y. Feasibility of preparing Mo2FeB2 -based cermet coating by electrospark deposition on high speed steel. Surf. Coat. Technol. 2016, 296, 58–64. [Google Scholar] [CrossRef]
- Ivanov, M.B.; Vershinina, T.N.; Ivanisenko, V.V. The effect of composition and microstructure on hardness and toughness of Mo2FeB2 based cermets. Mater. Sci. Eng. A 2019, 763, 138117. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Y.; Zhang, J.J.; Zhang, G.T.; Ke, Z.; Xu, X.Y.; Xu, X.P.; Zhou, W. Preparation of Mo2FeB2-based cermets with a core/rim structure by multi-step sintering approach. Ceramics Int. 2019, 45, 22371–22375. [Google Scholar] [CrossRef]
- Takagi, K. Development and application of high strength ternary boride base cermets. J. Solid State Chem. 2006, 179, 2809–2818. [Google Scholar] [CrossRef]
- Yu, H.Z.; Liu, W.J.; Feng, P.; Zheng, Y. Synthesis and microstructure evolution during vacuum sintering of Mo2FeB2 based cermets. Int. J. Refract. Met. Hard Mater. 2014, 45, 48–52. [Google Scholar]
- Yu, H.Z.; Liu, W.J.; Zheng, Y. Microstructure and mechanical properties of liquid phase sintered Mo2FeB2 based cermets. Mater. Des. 2011, 32, 3521–3525. [Google Scholar] [CrossRef]
- Yang, F.H.; Wu, Y.Z.; Han, J.S.; Meng, J.H. Microstructure, mechanical and tribological properties of Mo2FeB2 based cermets with Mn addition. J. Alloys Compd. 2016, 665, 373–380. [Google Scholar] [CrossRef]
- Yu, H.Z.; Zheng, Y.; Liu, W.J.; Zheng, J.Z.; Xiong, W.H. Effect of V content on the microstructure and mechanical properties of Mo2FeB2 based cermets. Mater. Des. 2010, 31, 2680–2683. [Google Scholar] [CrossRef]
- Yu, H.; Liu, W.; Zheng, Y. Effect of carbon content on the microstructure and mechanical properties of Mo2FeB2 based cermets. Int. J. Refract. Metals Hard Mater. 2011, 29, 724–728. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, Y.; Liu, W.; Pang, X.; Zheng, J.; Xiong, W. Effect of Mo/B atomic ratio on the microstructure and mechanical properties of Mo2FeB2 based cermets. Int. J. Refract. Metals Hard Mater. 2010, 28, 338–342. [Google Scholar] [CrossRef]
- Liu, W.; Cao, F.; Chang, L.; Zhang, Z.; Zhang, J. Effect of rare earth element Ce and La on corrosion behavior of AM60 magnesium alloy. Corros. Sci. 2009, 51, 1334–1343. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, F.; Song, G.L.; Liu, M.; Atrens, A. Corrosion behaviour in salt spray and in 3.5% NaCl solution saturated with Mg(OH)2 of as-cast and solution heat-treated binary MgeRE alloys: RE¼Ce, La, Nd, Y, Gd. Corros. Sci. 2013, 76, 98–118. [Google Scholar] [CrossRef]
- Wang, C.L.; Gao, Y.; Zeng, Z.C.; Fu, Y.K. Effect of rare-earth on friction and wear properties of laser cladding Ni-based coatings on 6063Al. J. Alloys Compd. 2017, 727, 278–285. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Feng, K.; Yao, C.W.; Li, Z.G. Effect of LaB6 addition on the microstructure and properties of (Ti3Al + TiB)/Ti composites by laser cladding. Mater. Des. 2019, 181, 107959. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z. Development of ternary-boride-based hard cladding material. Mater. Res. Bull. 2002, 37, 417–423. [Google Scholar]
- Takagi, K.; Koike, W.; Momozawa, A.; Fujima, T. Effects of Cr on the properties of Mo2NiB2 ternary boride. Solid State Sci. 2012, 14, 1643–1647. [Google Scholar] [CrossRef]
- Li, W.H.; Liu, F.T. Sintering thermodynamic analysis of Mo2FeB2 cermet prepared by in-situ reaction. Powder Metall. Technol. 2010, 28, 192–195. [Google Scholar]
- Feng, Q.; Wang, C.S.; Han, L.Y.; Yu, Q. Microstructure and properties of WC/Co-Y2O3 coating fabricated by laser alloying on 38CrMoAl steel substrate. Chin. J. Lasers. 2015, 42, 0803001. [Google Scholar] [CrossRef]
- Lu, W.J.; Zhang, D.; Zhang, X.N.; Guo, S.L.; Wu, R.J. Growth mechanism of reinforcements in in-situ synthesized (TiB+TiC)/Ti composites. Trans. Nonferr. Metal. Soc. 2001, 11, 67–71. [Google Scholar]
- Cao, W.; Chen, S.L.; Zhang, F.; Wu, F.K.; Yang, Y. PANDAT software with PanEngine, PanOptimizer and PanPrecipitation for multi-component phase diagram calculation and materials property simulation. CALPHAD 2009, 33, 328–342. [Google Scholar] [CrossRef]
- SGTE Pure Elements (unary) Database, Version 5.1 Scientific Group; Thermodata Europe: Teddington, UK, 2010.
- Wang, H.Q.; Sun, J.S.; Li, C.N.; Geng, S.N.; Sun, H.G.; Wang, G.L. Microstructure and mechanical properties of molybdenum-iron-boron-chromium cladding using argon arc welding. Mater. Sci. Technol. Ser. 2016, 32, 1694–1701. [Google Scholar] [CrossRef]
- Jin, J.; Sun, J.S.; Wang, G.L. Effect of Mo content on microstructure and wear resistance of Mo-Fe-B claddings. Int. J. Refract. Metals Hard Mater. 2019, 81, 233–241. [Google Scholar] [CrossRef]
- Liu, T.Y. Study on Strengthening Mechanism of Al-30wt.% Mg2Si Composite Based on First-Principle Calculation. Ph.D. Thesis, Shenyang University of Technology, Shenyang, China, 1 December 2018. (In Chinese). [Google Scholar]
- Shi, L.M. Study on the Effect of B and Y on the Microstructure and Properties of High Temperature Titanium Alloys. Master’s Thesis, Harbin Institude of Technology, Harbin, China, 1 July 2014. (In Chinese). [Google Scholar]
- Ding, W.W.; Zhao, X.Y.; Chen, T.L.; Zhang, H.X.; Liu, X.X.; Cheng, Y.; Lei, D.K. Effect of rare earth Y and Al-Ti-B master alloy on the microstructure and mechanical properties of 6063 Aluminum alloy. J. Alloys Compd. 2020, 830, 154685. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Chen, G.N. Microstructure and properties of the MoSi2 powder coating clad by laser beam. Heat Treat. Metal. 2002, 27, 10–13. [Google Scholar]
- Shan, J.G.; Ding, J.C.; Ren, J.L. Microstructure and strengthening mechanism of light beam cladding layer with iron-based self-fluxing alloy powder. China Weld. 2001, 22, 1–4. [Google Scholar]
- Yin, K.; Ma, C.; Liao, P.; Li, M.Q.; Li, M.B. Effects of rare earth and alloy elements on wear resistance of powder block hardfacing layer. Weld. Join. 1998, 1, 9–12. [Google Scholar]
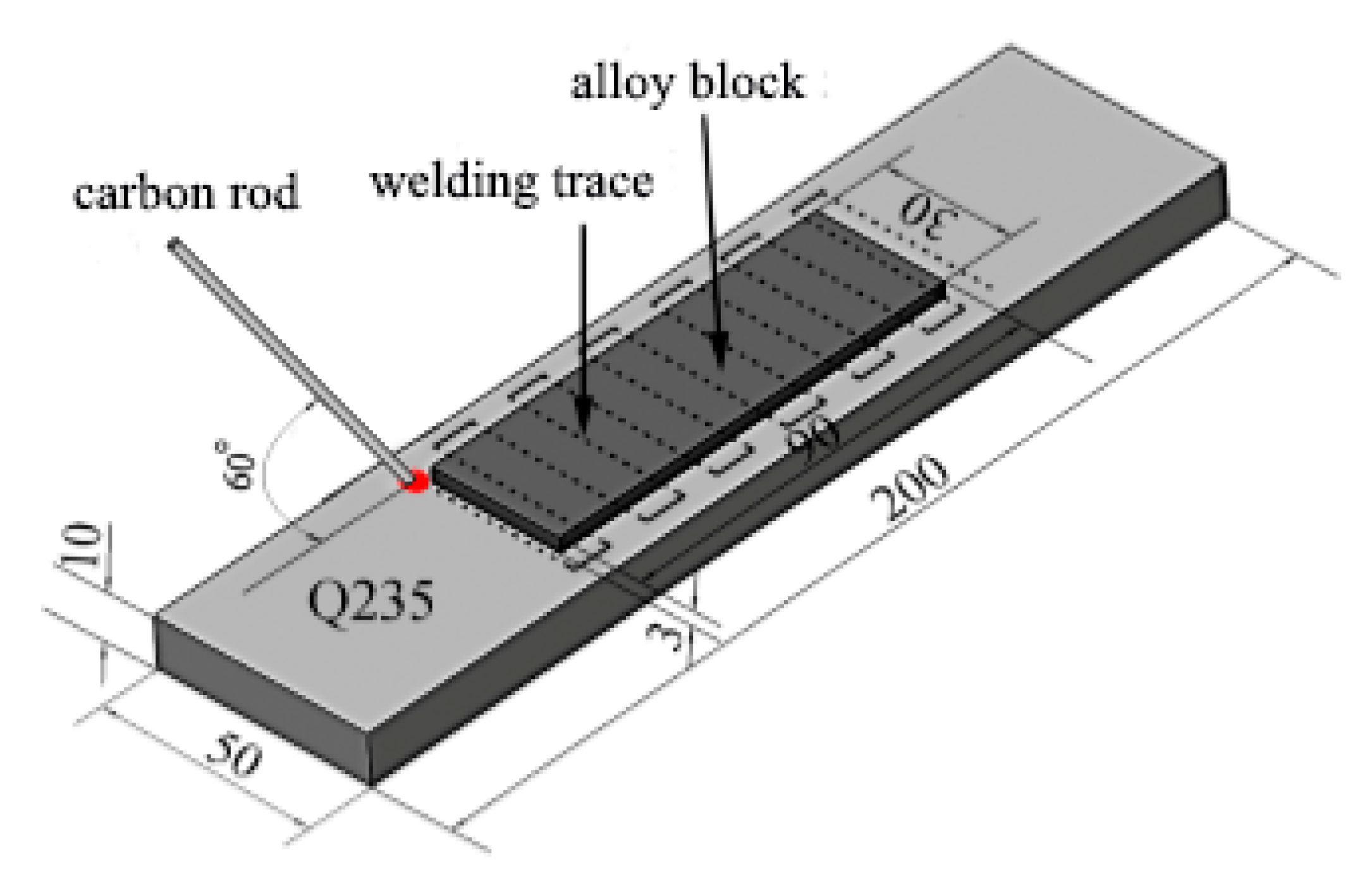
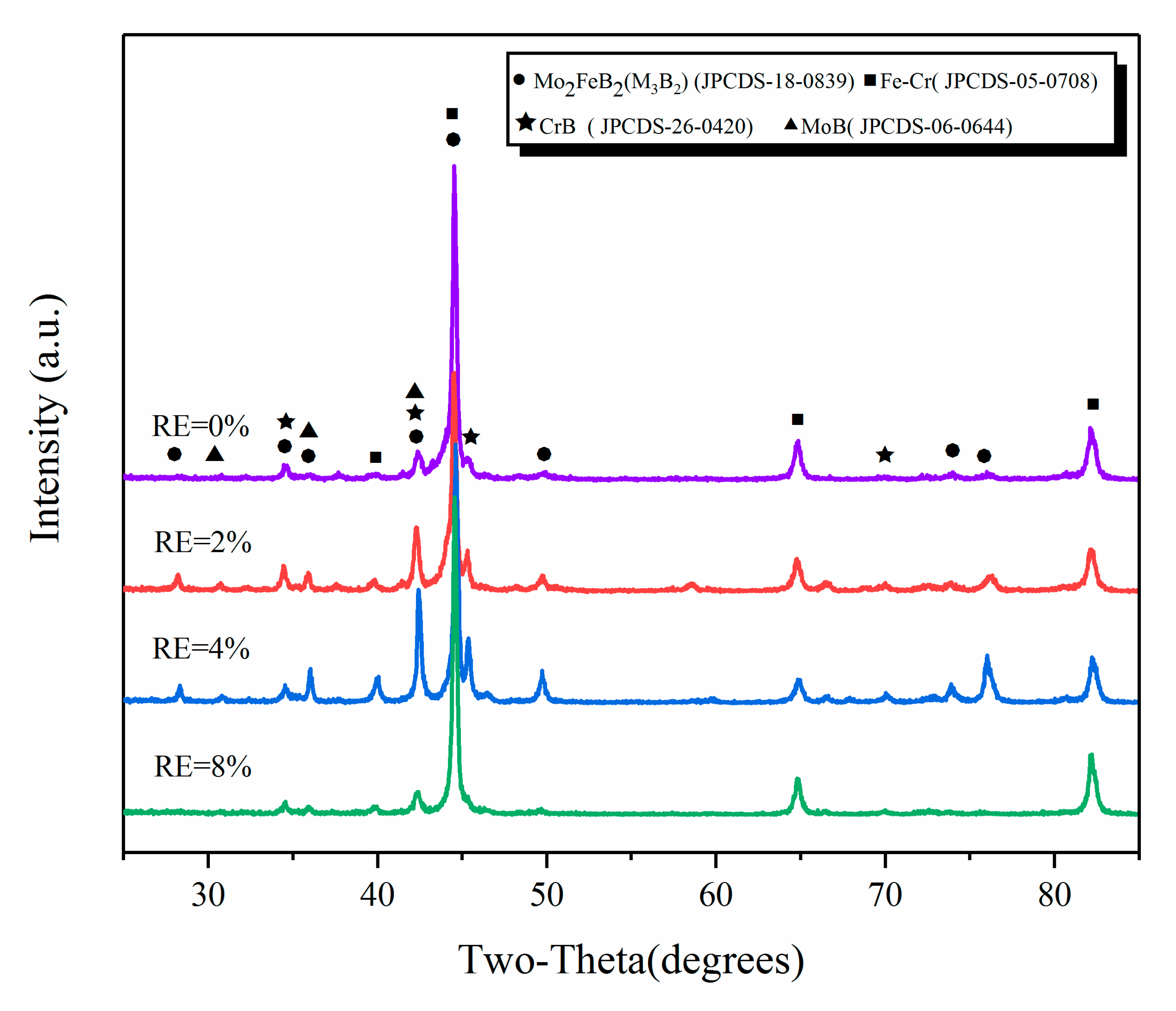
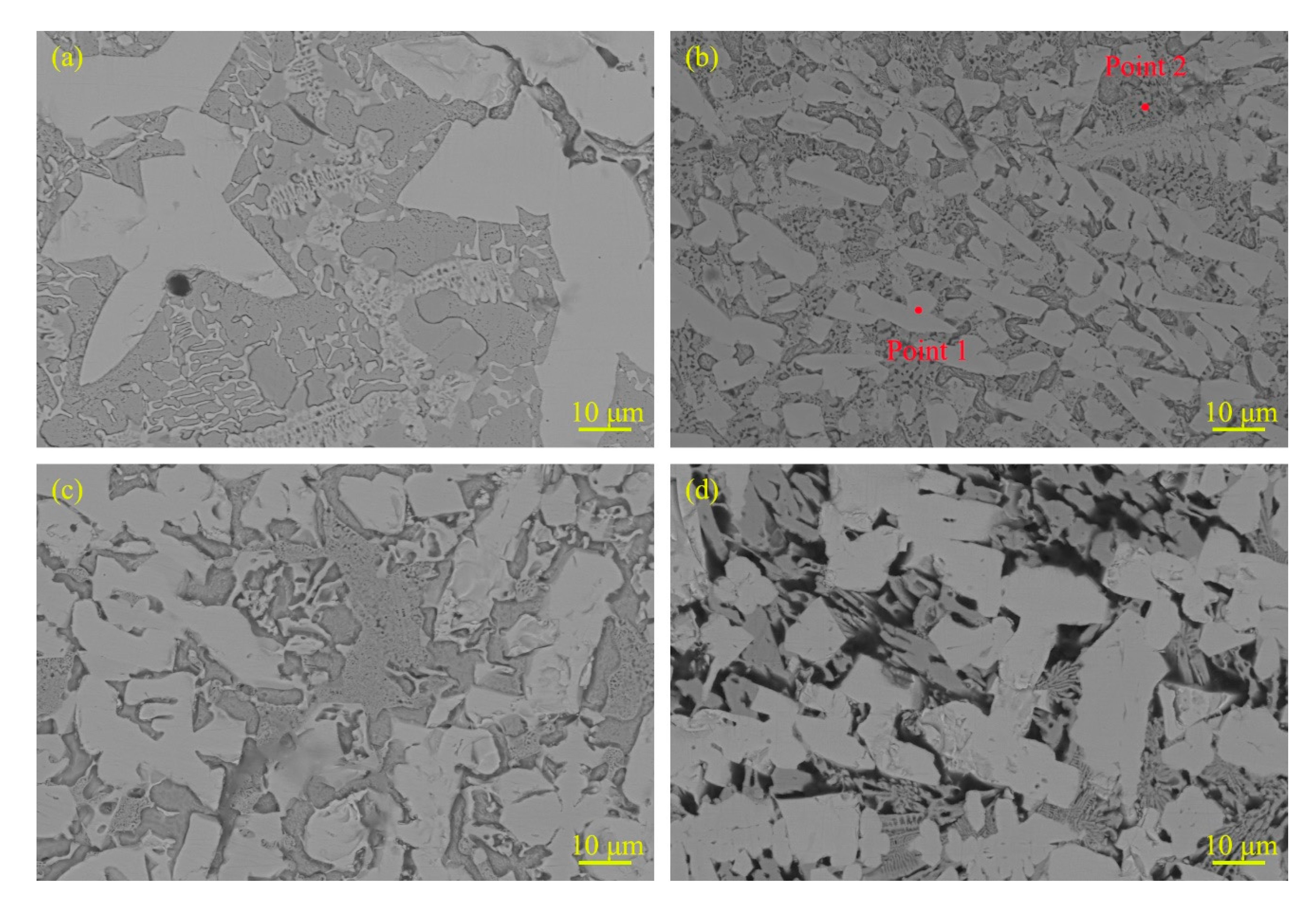
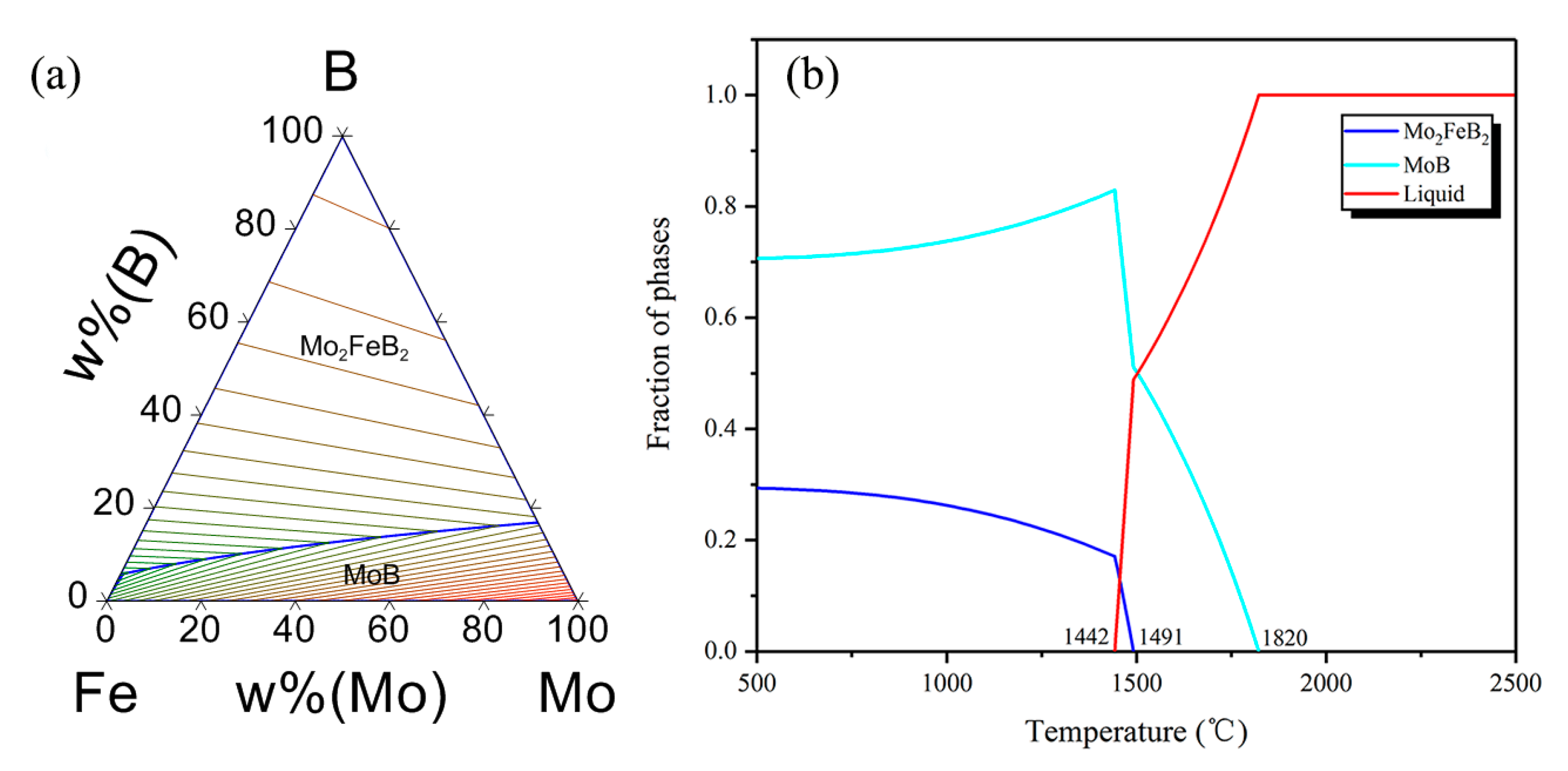



| Powder | Mean Particle Size (μm) | Chemical Composition (wt.%) |
|---|---|---|
| Mo | 100 | Fe < 0.002, O < 0.1, Si < 0.001, Bal Mo |
| Fe | 90 | C < 0.1, N < 0.1, O < 0.2, Bal Fe |
| Cr | 110 | O < 0.2, Fe < 0.18, N < 0.045, Bal Cr |
| FeB | 60 | B = 22%, C < 0.27, Si < 0.71, Bal Fe |
| RE | 100 | Mg = 8%, RE = 8.1% (Y = 4.2%), Si = 41%, Bal Fe |
| Samples | Mo | Cr | B | RE | Fe |
|---|---|---|---|---|---|
| No.1 | 47.5 | 10 | 6 | 0 | Bal. |
| No.2 | 47.5 | 10 | 6 | 2 | Bal. |
| No.3 | 47.5 | 10 | 6 | 4 | Bal. |
| No.4 | 47.5 | 10 | 6 | 8 | Bal. |
| Carbon Rod Size/mm | Polarity | Swing Way | Voltage/V | Current/A | Welding Speed mm/min |
|---|---|---|---|---|---|
| Φ8 × 300 | DC-positive connection | Rectangular swing | 20~25 | 230~250 | 100 |
| Position | Structure | Element Mass Percentage (wt.%) | |||
|---|---|---|---|---|---|
| Mo | Fe | Cr | B | ||
| Point 1 | Hard phase | 56.27 | 14.01 | 4.28 | 25.44 |
| Point 2 | Eutectic structure | 10.77 | 49.54 | 9.07 | 30.61 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Sun, J.; Wang, W.; Song, J.; Xu, H. Effect of Rare Earth on Microstructure and Wear Resistance of In-Situ-Synthesized Mo2FeB2 Ceramics-Reinforced Fe-Based Cladding. Materials 2020, 13, 3633. https://doi.org/10.3390/ma13163633
Jin J, Sun J, Wang W, Song J, Xu H. Effect of Rare Earth on Microstructure and Wear Resistance of In-Situ-Synthesized Mo2FeB2 Ceramics-Reinforced Fe-Based Cladding. Materials. 2020; 13(16):3633. https://doi.org/10.3390/ma13163633
Chicago/Turabian StyleJin, Jun, Junsheng Sun, Weimin Wang, Jijun Song, and Hu Xu. 2020. "Effect of Rare Earth on Microstructure and Wear Resistance of In-Situ-Synthesized Mo2FeB2 Ceramics-Reinforced Fe-Based Cladding" Materials 13, no. 16: 3633. https://doi.org/10.3390/ma13163633
APA StyleJin, J., Sun, J., Wang, W., Song, J., & Xu, H. (2020). Effect of Rare Earth on Microstructure and Wear Resistance of In-Situ-Synthesized Mo2FeB2 Ceramics-Reinforced Fe-Based Cladding. Materials, 13(16), 3633. https://doi.org/10.3390/ma13163633






