Expansion Properties and Diffusion of Blowing Agent for Vinylidene Chloride Copolymer Thermally Expandable Microspheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Vinylidene Chloride Copolymer Thermally Expandable Microspheres
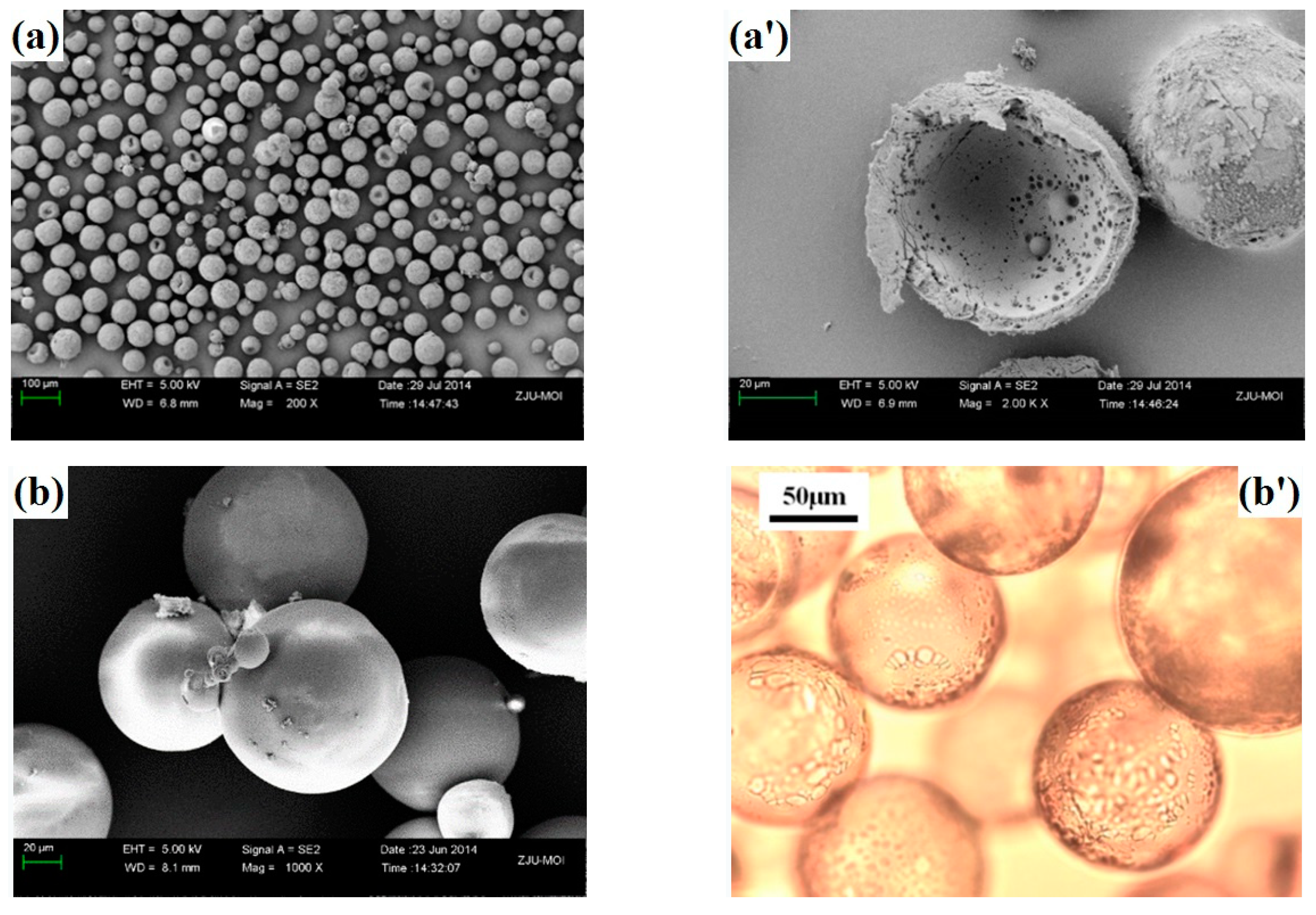
2.3. Characterization
3. Results and Discussion
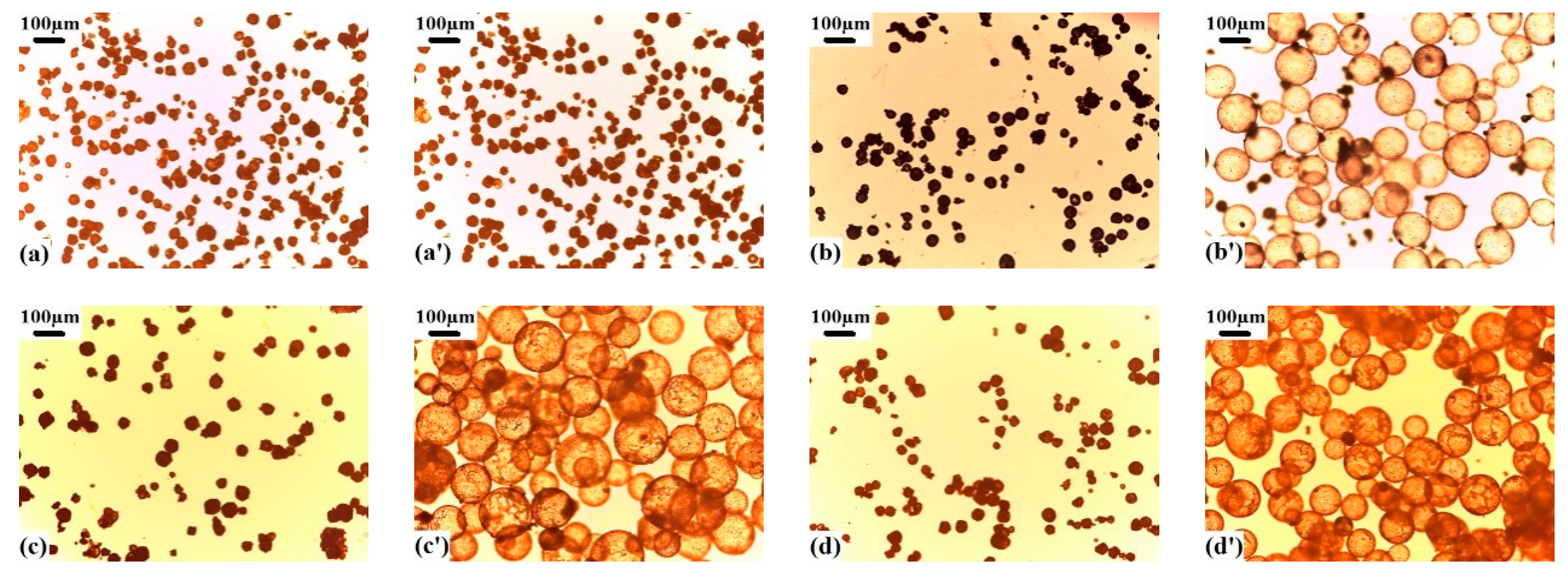
3.1. Effects of the Shell Polymer Composition and Tg on the Expansion Properties of Polymer Microspheres
3.2. Effects of the Shell Polymer Crosslinking Degree on the Expansion Properties of Polymer Microspheres
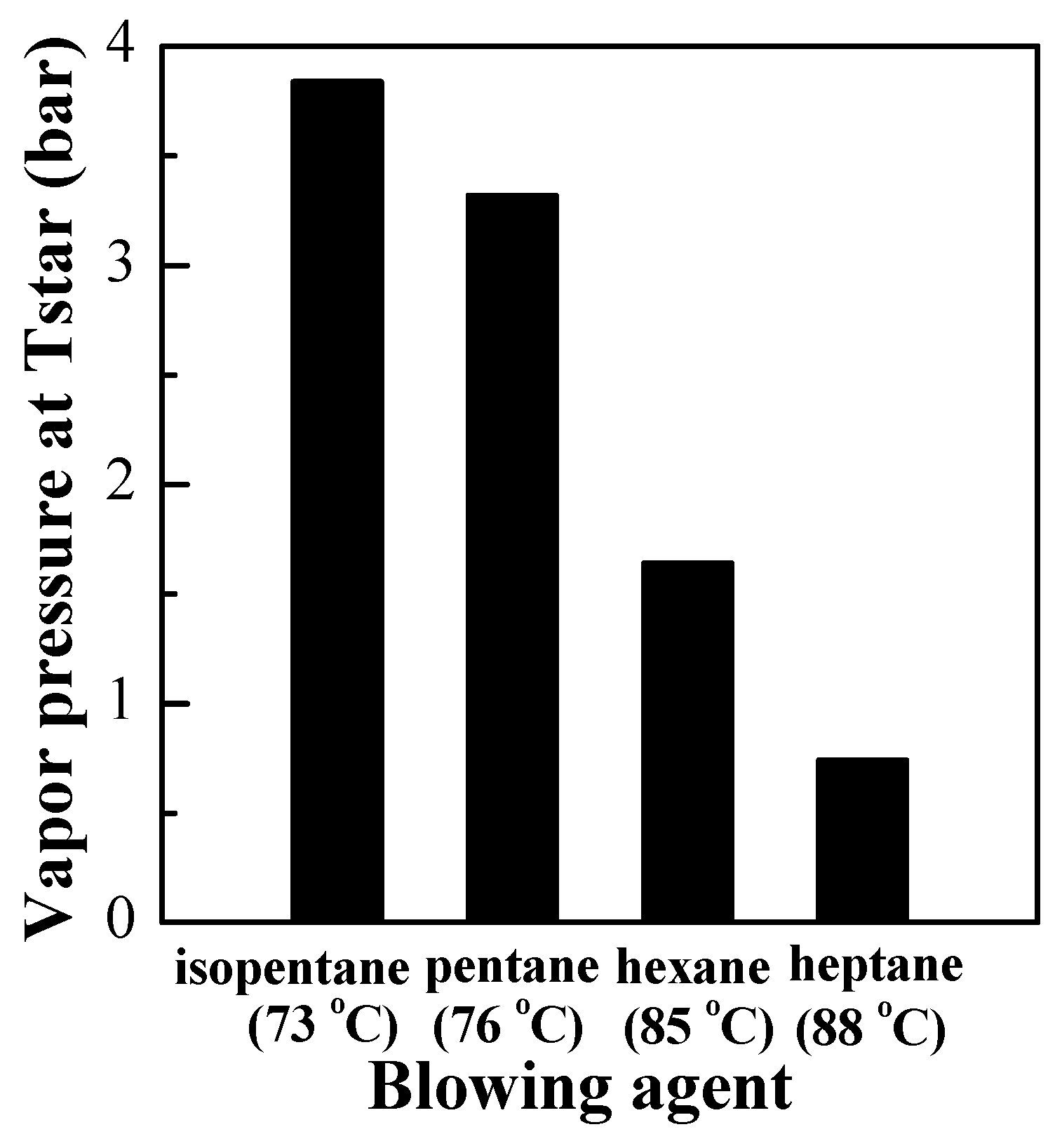
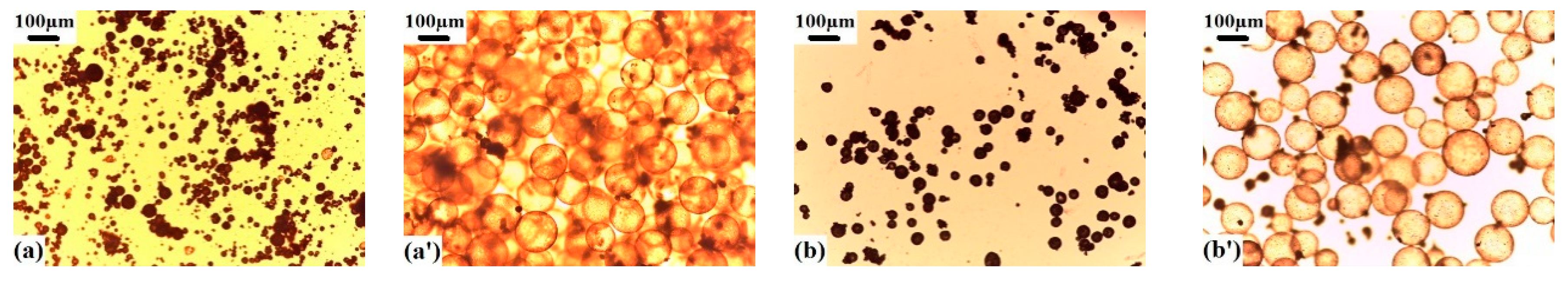
3.3. Influences of the Type and Er of Blowing Agent on the Expansion Properties of Polymer Microspheres
3.4. Expansion Properties of the VDC-MMA-AN Copolymer Thermally Expandable Microspheres
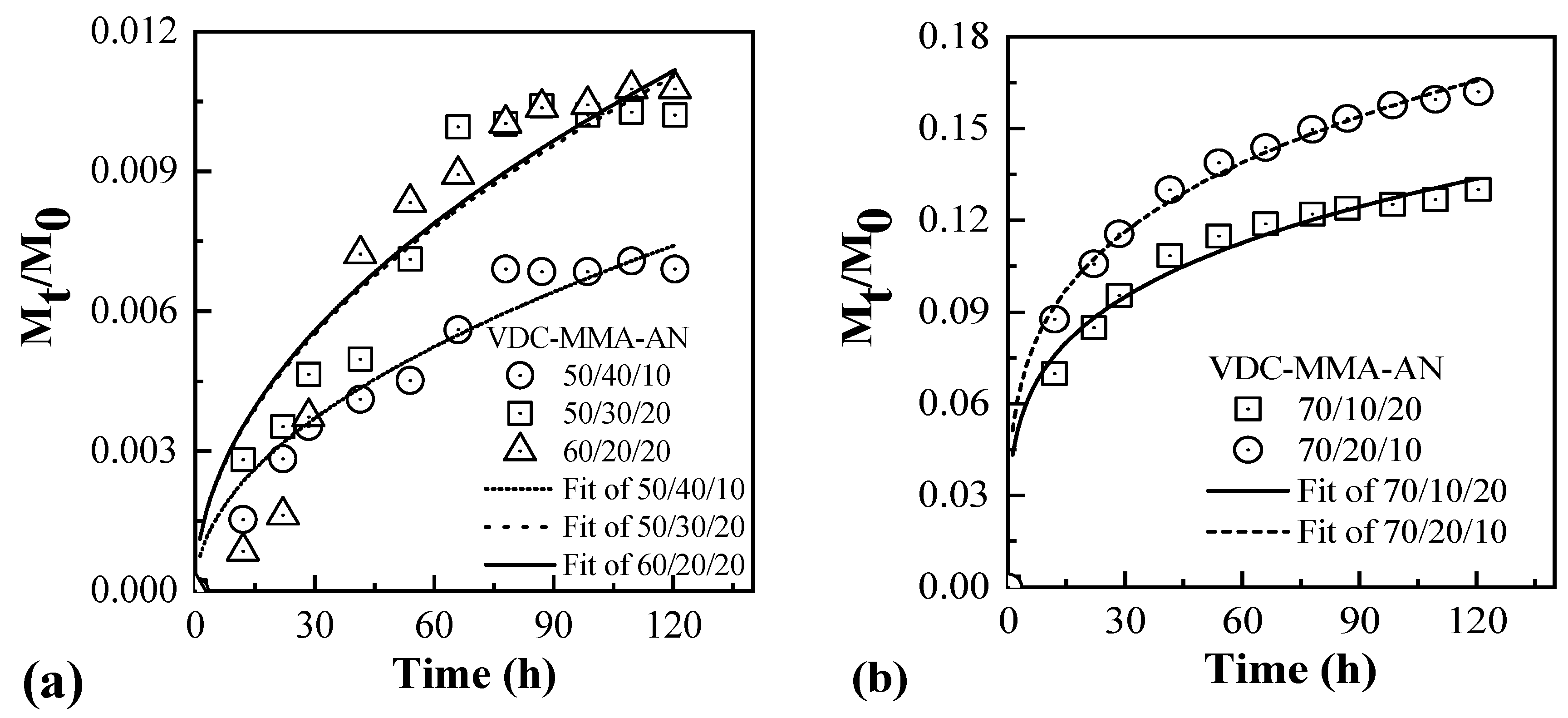
3.5. Diffusion of Blowing Agent in the VDC-MMA-AN Copolymer Thermally Expandable Microspheres
3.6. Thermally Expandable Microspheres Designation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morehouse, D.S.J.; Tetreault, R.J. Expandable Thermoplastic Polymer Particles Containing Volatile Fluid Foaming Agent and Method of Foaming the Same. U.S. Patent 3,615,972, 26 October 1971. [Google Scholar]
- Jonsson, M.; Nordin, O.; Malmström, E.; Hammer, C. Suspension polymerization of thermally expandable core/shell particles. Polymer 2006, 47, 3315–3324. [Google Scholar] [CrossRef]
- Hou, Z.; Xia, Y.; Qu, W.; Kan, C. Preparation and Properties of Thermoplastic Expandable Microspheres With P(VDC-AN-MMA) Shell by Suspension Polymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 427–431. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Huang, M.; Zhang, J.; Luo, G.; Wang, B.; Li, M.; Shen, Q.; Zhang, L. Synthesis and compressive response of microcellular foams fabricated from thermally expandable microspheres. Chin. J. Polym. Sci. 2019, 37, 279–288. [Google Scholar] [CrossRef]
- Yao, J.; Barzegari, M.R.; Rodrigue, D. Polyethylene foams produced under a temperature gradient with Expancel and blends thereof. Cell. Polym. 2010, 29, 259–281. [Google Scholar] [CrossRef]
- Wang, L.; Yang, X.; Zhang, J.; Zhang, C.; He, L. The compressive properties of expandable microspheres/epoxy foams. Compos. Part B Eng. 2014, 56, 724–732. [Google Scholar] [CrossRef]
- Vaikhanski, L.; Nutt, S.R. Fiber-reinforced composite foam from expandable PVC microspheres. Compos. Part A Appl. Sci. Manuf. 2003, 34, 1245–1253. [Google Scholar] [CrossRef]
- Tomalino, M.; Bianchini, G. Heat-expandable microspheres for car protection production. Prog. Org. Coat. 1997, 32, 17–24. [Google Scholar] [CrossRef]
- Ahmad, M. Flexible vinyl resiliency property enhancement with hollow thermoplastic microspheres. J. Vinyl Addit. Technol. 2001, 7, 156–161. [Google Scholar] [CrossRef]
- Gehlsen, M.D.; Momchilovich, B.S. Articles that Include a Polymer Foam and Method for Preparing Same. U.S. Patent 6,103,152, 15 August 2004. [Google Scholar]
- Cravens, T.E. Syntactic foams utilizing saran microspheres. J. Cell. Plast. 1973, 9, 260–267. [Google Scholar] [CrossRef]
- Vaikhanksi, L.; Nutt, S.R. Synthesis of composite foam from thermoplastic microspheres and 3D long fibers. Compos. Part A Appl. Sci. Manuf. 2003, 34, 755–763. [Google Scholar] [CrossRef]
- Andersson, L.; BergstrÖm, L. Gas-filled microspheres as an expandable sacrificial template for direct casting of complex-shaped macroporous ceramics. J. Eur. Ceram. Soc. 2008, 28, 2815–2821. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Uto, N.; Sato, C.; Sakurai, H. Dismantlement behavior and strength of dismantlable adhesive including thermally expansive particles. Int. J. Adhes. Adhes. 2003, 23, 377–382. [Google Scholar] [CrossRef]
- Aglan, H.; Shebl, S.; Morsy, M.; Calhoun, M.; Harding, H.; Ahmad, M. Strength and toughness improvement of cement binders using expandable thermoplastic microspheres. Constr. Build. Mater. 2009, 23, 2856–2861. [Google Scholar] [CrossRef]
- Kim, D.; Chung, I.; Kim, G. Dismantlement studies of dismantlable polyurethane adhesive by controlling thermal property. J. Adhes. Sci. Technol. 2012, 26, 2571–2589. [Google Scholar] [CrossRef]
- Cingil, H.E.; Balmer, J.A.; Armes, S.P.; Bain, P.S. Conducting polymer-coated thermally expandable microspheres. Polym. Chem. 2010, 1, 1323–1331. [Google Scholar] [CrossRef]
- Jiao, S.; Sun, Z.; Li, F.; Yan, M.; Cao, M.; Li, D.; Liu, Y.; Li, L. Preparation and Application of Conductive Polyaniline-Coated Thermally Expandable Microspheres. Polymers 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Andersson, H.; Griss, P.; Stemme, G. Expandable microspheres-surface immobilization techniques. Sens. Actuators B Chem. 2002, 84, 290–295. [Google Scholar] [CrossRef]
- Griss, P.; Andersson, H.; Stemme, G. Expandable microspheres for the handling of liquids. Lab. Chip. 2002, 2, 117–120. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Oishi, T. Synthesis and properties of thermoplastic expandable microspheres: The relation between crosslinking density and expandable property. J. Appl. Polym. Sci. 2004, 93, 505–512. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Ito, D.; Kosaka, Y.; Nakachi, T.; Kake, H.; Kim, J.K.; Shikuma, H.; Ohshima, M. Thermally expandable microcapsules for polymer foaming-relationship between expandability and viscoelasticity. Polym. Eng. Sci. 2010, 50, 835–842. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Itamura, Y.; Onimura, K.; Oishi, T. Effects of the chemical structure on the heat resistance of thermoplastic expandable microspheres. J. Appl. Polym. Sci. 2005, 96, 1306–1312. [Google Scholar] [CrossRef]
- Jonsson, M.; Nordin, O.; Kron, A.L.; Malmström, E. Thermally expandable microspheres with excellent expansion characteristics at high temperature. J. Appl. Polym. Sci. 2010, 117, 384–392. [Google Scholar] [CrossRef]
- Jonsson, M.; Nordin, O.; Kron, A.L.; Malmström, E. Influence of crosslinking on the characteristics of thermally expandable microspheres expanding at high temperature. J. Appl. Polym. Sci. 2010, 118, 1219–1229. [Google Scholar] [CrossRef]
- Jonsson, M.; Nordin, O.; Malmström, E. Increased onset temperature of expansion in thermally expandable microspheres through combination of crosslinking agents. J. Appl. Polym. Sci. 2011, 121, 369–375. [Google Scholar] [CrossRef]
- Xie, G.; Pan, P.; Bao, Y. Morphology and blowing agent encapsulation efficiency of vinylidene chloride copolymer microspheres synthesized by suspension polymerization in the presence of a blowing agent. J. Appl. Polym. Sci. 2017, 134, 44376. [Google Scholar] [CrossRef]
- Hou, Z.; Kan, C. Preparation and properties of thermoexpandable polymeric microspheres. Chin. Chem. Lett. 2014, 25, 1279–1281. [Google Scholar] [CrossRef]
- GB/T7139-2002. Plastics-Vinyl Chloride Homopolymers and Copolymers-Determination of Chlorine Content; China Standard Press: Beijing, China, 2002; pp. 1–10. [Google Scholar]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A.; Bloch, D.R. Polymer Handbook, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1972; pp. 428–430. [Google Scholar]
- Perry, R.H.; Green, D.W.; Maloney, J.O. Perry’s Chemical Engineers Handbook, 7th ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 1999; pp. 1–374. [Google Scholar]
- Lide, D.R. CRC Press-Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Harogoppad, S.B.; Aminabhavi, T.M. Diffusion and sorption of organic liquids through polymer membranes. 5. Neoprene, styrene-butadiene-rubber, ethylene-propylene-diene terpolymer, and natural rubber versus hydrocarbons (C8-C16). Macromolecules 1991, 24, 2598–2605. [Google Scholar] [CrossRef]
- Khinnavar, R.S.; Aminabhavi, T.M. Diffusion and sorption of organic liquids through polymer membranes. VI. Polyurethane, neoprene, natural rubber, nitrile butadiene rubber, styrene butadiene rubber, and ethylene propylene diene terpolymer versus organic esters. J. Appl. Polym. Sci. 1992, 46, 909–920. [Google Scholar] [CrossRef]





| VDC/MMA/AN (Mass) | Shell Composition of PVDC/PMMA/PAN | Er (%) | Tg (oC) | Tst (oC) | Tsh (oC) | Tr (oC) | Ev |
|---|---|---|---|---|---|---|---|
| 50/50/0 | 46.1/53.9/0 | 1.7 | 56.3 | - | - | - | 0 |
| 60/40/0 | 54.6/45.4/0 | 6.8 | 53.6 | 80 | 85 | 5 | 3 |
| 70/30/0 | 61.0/39.0/0 | 27.2 | 53.5 | - | - | - | 0 |
| 50/40/10 | 48.5/42.0/9.5 | 17.5 | 63.8 | 80 | 105 | 25 | 19 |
| 70/20/10 | 61.5/29.3/9.2 | 17.2 | 51.4 | 55 | 65 | 10 | 7.9 |
| 50/30/20 | 41.2/41.0/17.8 | 20.6 | 63.4 | 76 | 100 | 24 | 30.3 |
| 60/20/20 | 48.8/31.5/19.7 | 20.4 | 61.5 | 70 | 90 | 20 | 28.9 |
| 70/10/20 | 61.7/17.9/20.4 | 20.2 | 42.4 | 65 | 75 | 10 | 27 |
| DVB (wt%) | Gel Content (%) | Er (%) | Tst (oC) | Tsh (oC) | Tr (oC) | Ev |
|---|---|---|---|---|---|---|
| 0 | 5.9 | 12.0 | 80 | 80 | 0 | 0 |
| 0.2 | 64.1 | 20.6 | 76 | 100 | 24 | 30.3 |
| 0.4 | 69.6 | 21.8 | 75 | 105 | 30 | 37.1 |
| 0.6 | 75.8 | 18.2 | 69 | 95 | 27 | 29.3 |
| VDC-MMA-AN (Mass) | Blowing Agent | Er (%) | Tst (oC) | Tsh (oC) | Tr (oC) | Ev |
|---|---|---|---|---|---|---|
| 70/30/0 | pentane | 27.2 | - | - | - | 0 |
| 70/30/0 | hexane | 32.1 | - | - | - | 0 |
| 70/30/0 | heptane | 24.2 | - | - | - | 0 |
| 50/30/20 | isopentane | 19.8 | 73 | 105 | 32 | 28.6 |
| 50/30/20 | pentane | 20.6 | 76 | 100 | 24 | 30.3 |
| 50/30/20 | hexane | 21.0 | 85 | 100 | 15 | 29.8 |
| 50/30/20 | heptane | 22.2 | 88 | 100 | 12 | 28.2 |
| Blowing Agent | Boiling Point (°C) | Solubilitypara Meter (Cal/cm3)1/2 | Vapor Pressure Constants | ||||
|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | |||
| isopentane | 28 | 6.7 | 72.350 | −5010.9 | −7.8830 | 8.9795 × 10−6 | 2 |
| pentane | 36.1 | 7.0 | 78.741 | −5420.3 | −8.8253 | 9.6171 × 10−6 | 2 |
| hexane | 68.7 | 7.3 | 104.650 | −6995.5 | −12.7020 | 1.2381 × 10−5 | 2 |
| heptane | 99 | 7.4 | 87.829 | −6996.4 | −9.8802 | 7.2099 × 10−6 | 2 |
| Er (%) of Pentane | Tst (oC) | Tsh (oC) | Tr (oC) | Ev |
|---|---|---|---|---|
| 3.4 | 87 | 110 | 23 | 16.2 |
| 10.0 | 80 | 105 | 25 | 32.2 |
| 20.6 | 76 | 100 | 24 | 30.3 |
| 28.6 | 70 | 93 | 23 | 41.3 |
| VDC-MMA-AN | t | n | Adj. R-Square | ||
|---|---|---|---|---|---|
| Value | Standard Error | Value | Standard Error | ||
| 50/40/10 | 6.76 × 10−4 | 1.87 × 10−5 | 0.50 | 2.51 × 10−7 | 0.93 |
| 50/30/20 | 1.01 × 10−3 | 3.93 × 10−5 | 0.50 | 2.10 × 10−7 | 0.88 |
| 60/20/20 | 1.02 × 10−3 | 5.75 × 10−5 | 0.50 | 2.37 × 10−7 | 0.83 |
| 70/10/20 | 0.041 | 0.0032 | 0.25 | 0.018 | 0.96 |
| 70/20/10 | 0.049 | 0.0022 | 0.26 | 0.011 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, G.; Wang, Z.; Bao, Y. Expansion Properties and Diffusion of Blowing Agent for Vinylidene Chloride Copolymer Thermally Expandable Microspheres. Materials 2020, 13, 3673. https://doi.org/10.3390/ma13173673
Xie G, Wang Z, Bao Y. Expansion Properties and Diffusion of Blowing Agent for Vinylidene Chloride Copolymer Thermally Expandable Microspheres. Materials. 2020; 13(17):3673. https://doi.org/10.3390/ma13173673
Chicago/Turabian StyleXie, Guiming, Zhiyang Wang, and Yongzhong Bao. 2020. "Expansion Properties and Diffusion of Blowing Agent for Vinylidene Chloride Copolymer Thermally Expandable Microspheres" Materials 13, no. 17: 3673. https://doi.org/10.3390/ma13173673





