High Thermally Stable and Melt Processable Polyimide Resins Based on Phenylethynyl-Terminated Oligoimides Containing Siloxane Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
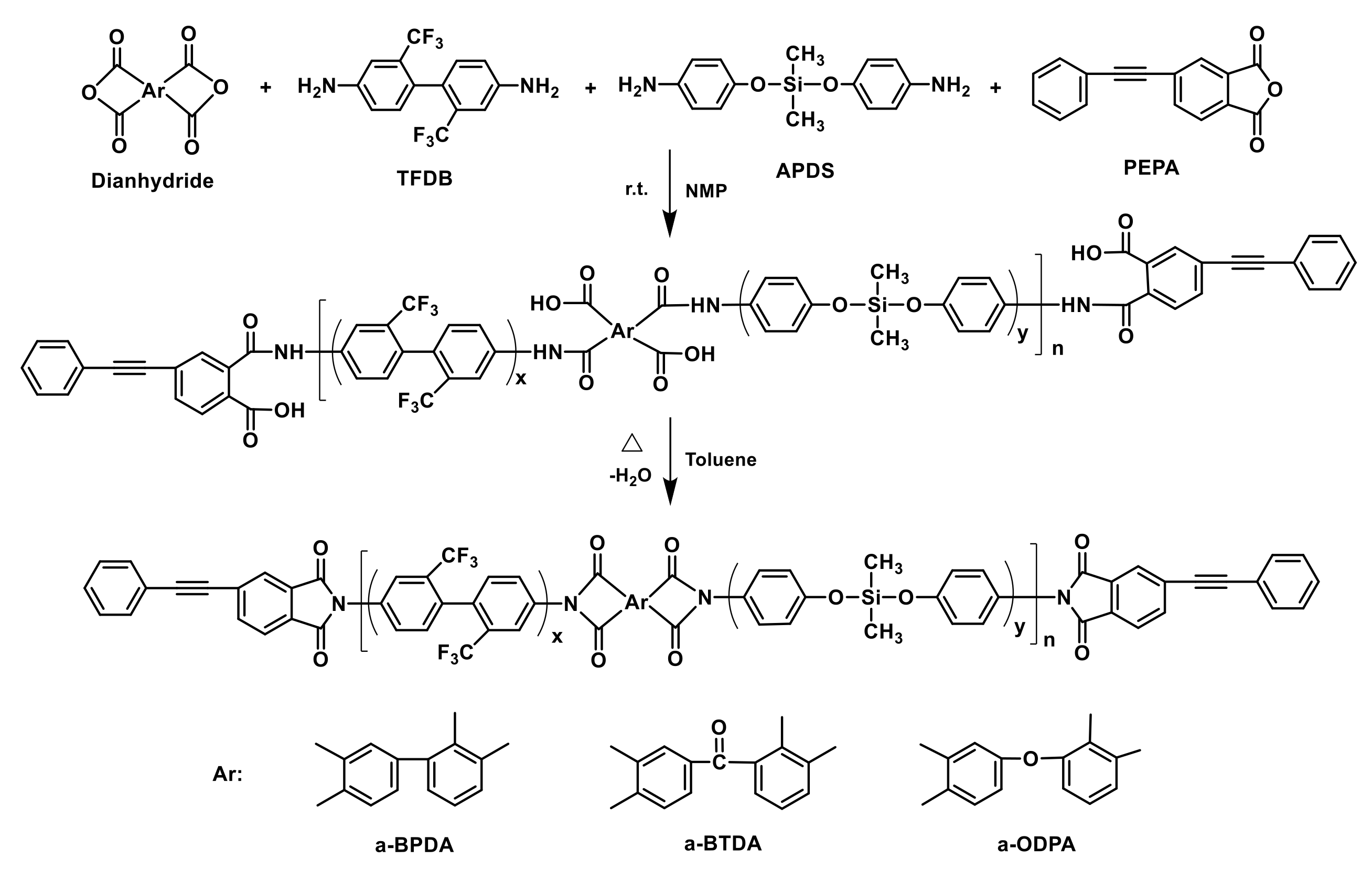
2.2. Synthesis of PEPA-Terminated Oligoimides Containing Siloxane Structure
2.3. Preparation of Cured Polyimides
2.4. Characterization and Measurements
3. Results and Discussion
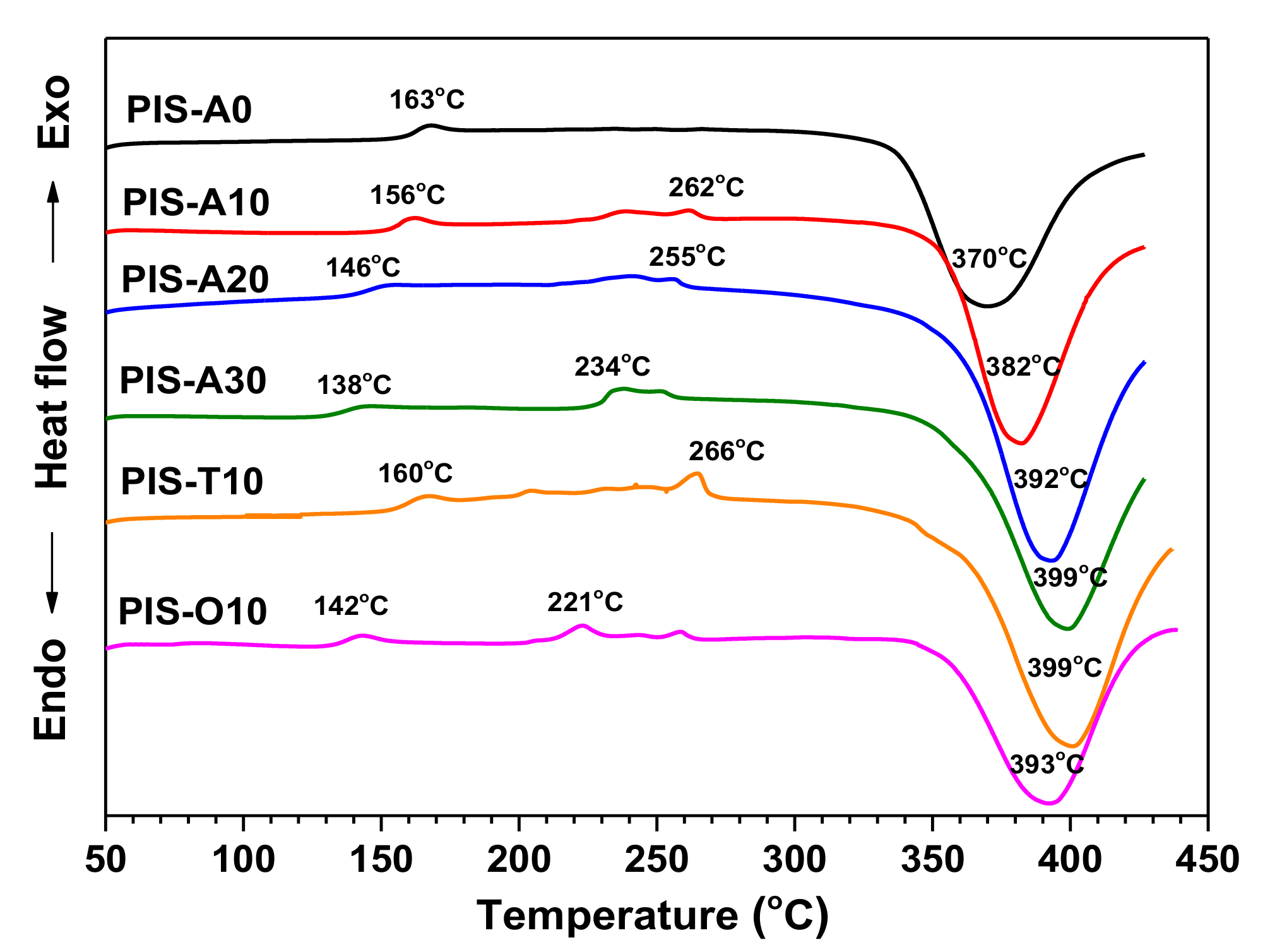
3.1. Characterization of PEPA-Terminated Oligoimides Containing Siloxane Structure
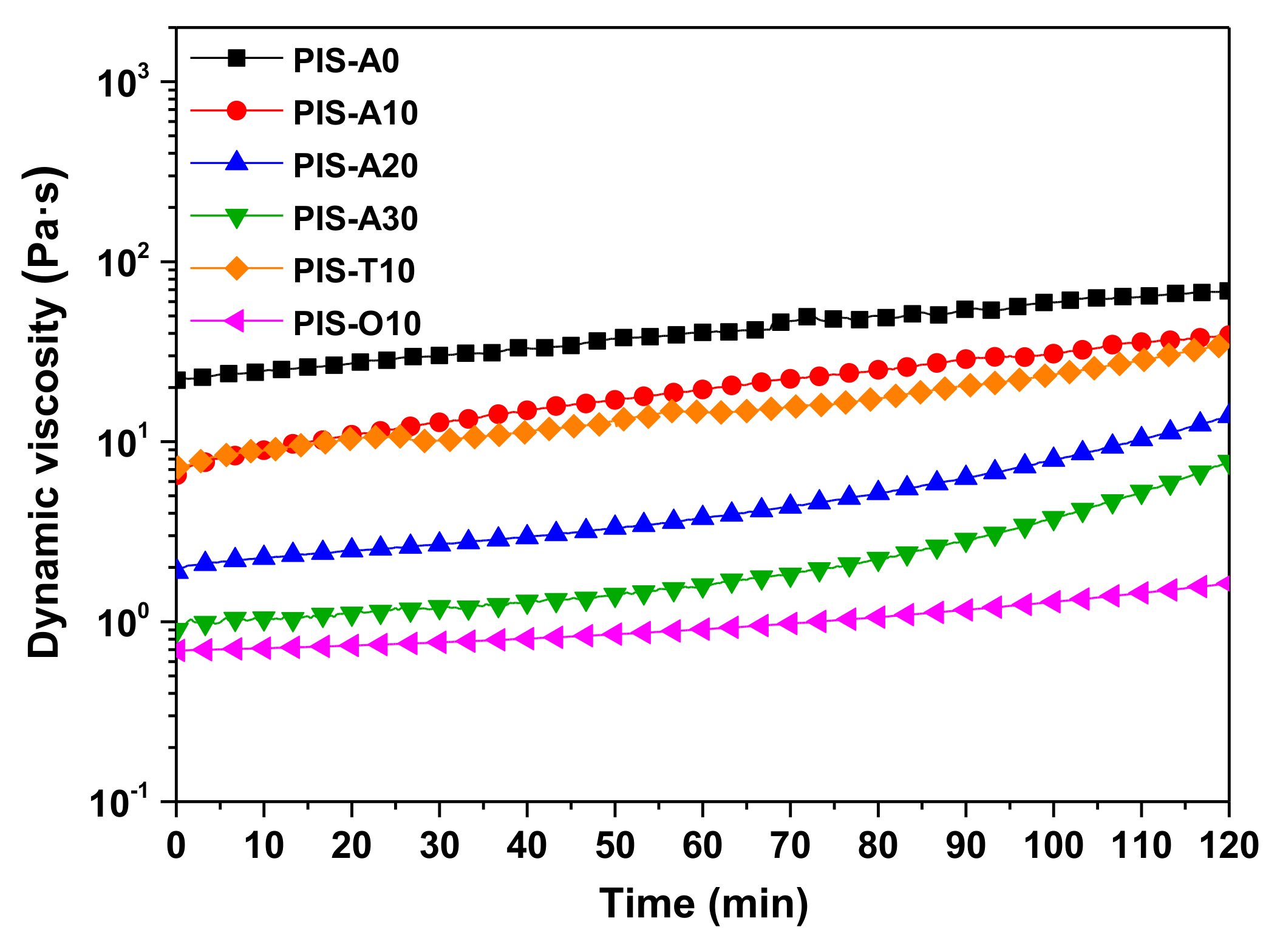
3.2. Processability of PEPA-Terminated Oligoimides Containing Siloxane Structure
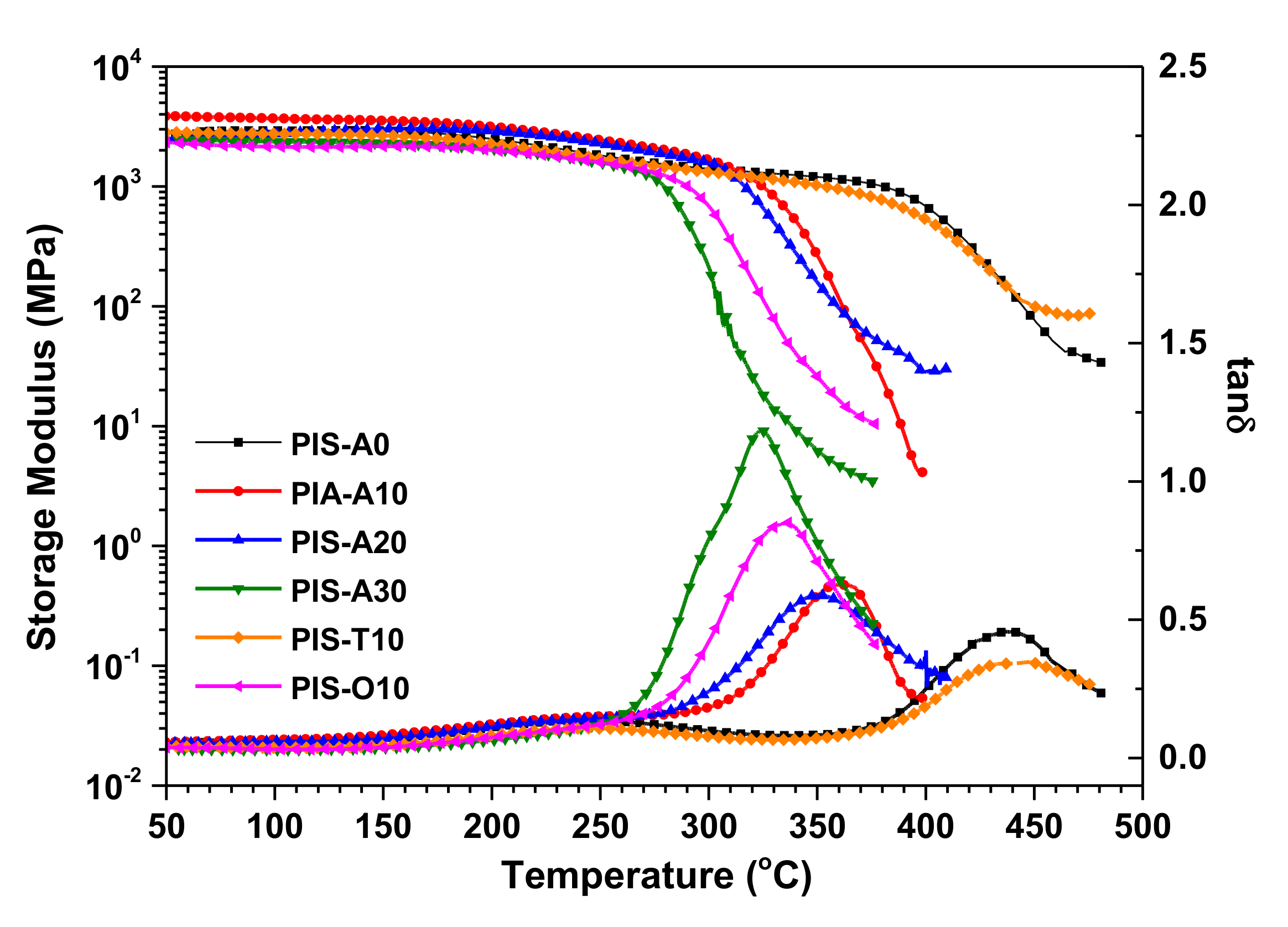
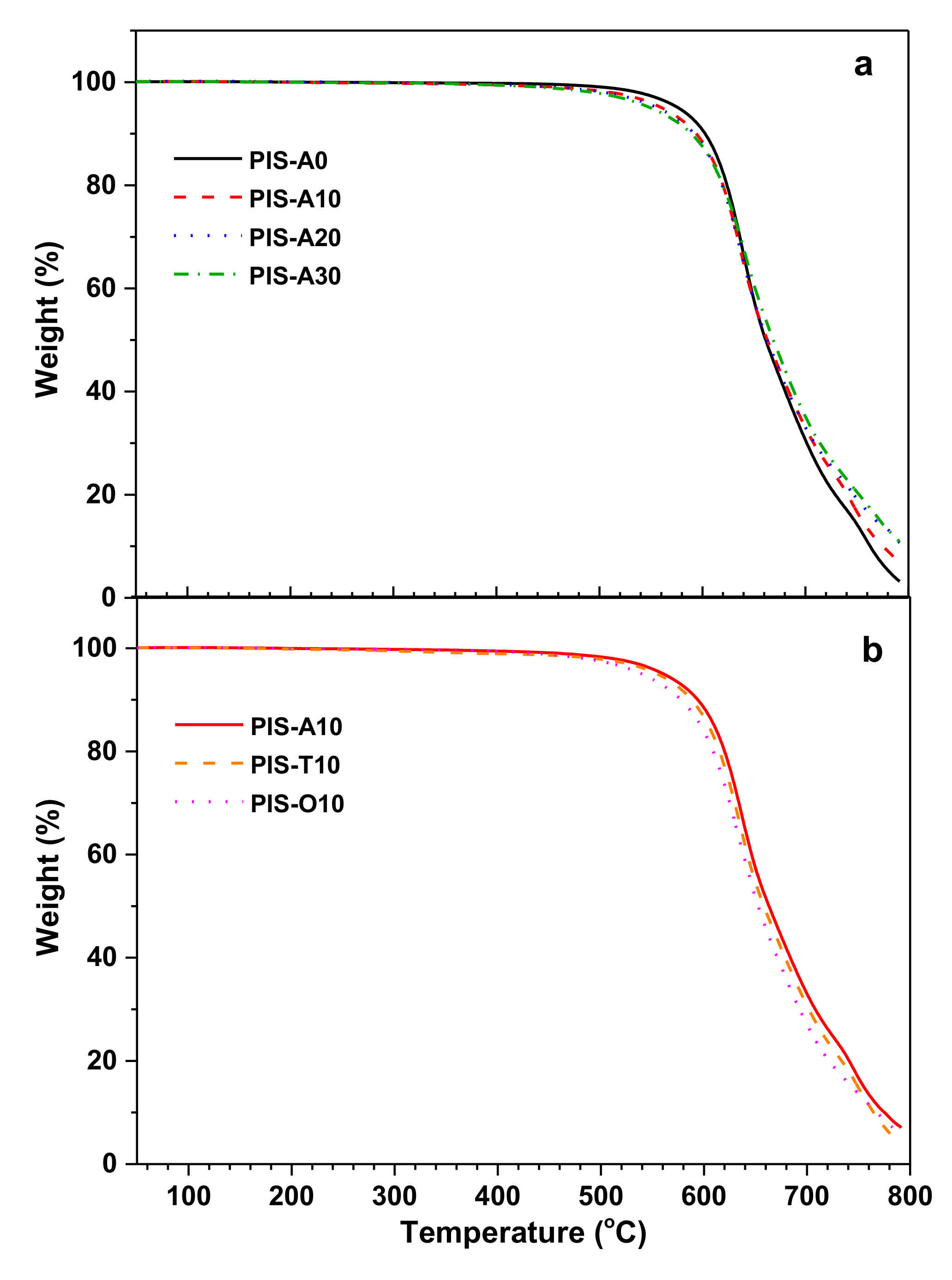
3.3. Mechanical and Thermal Properties of the Cured Polyimides
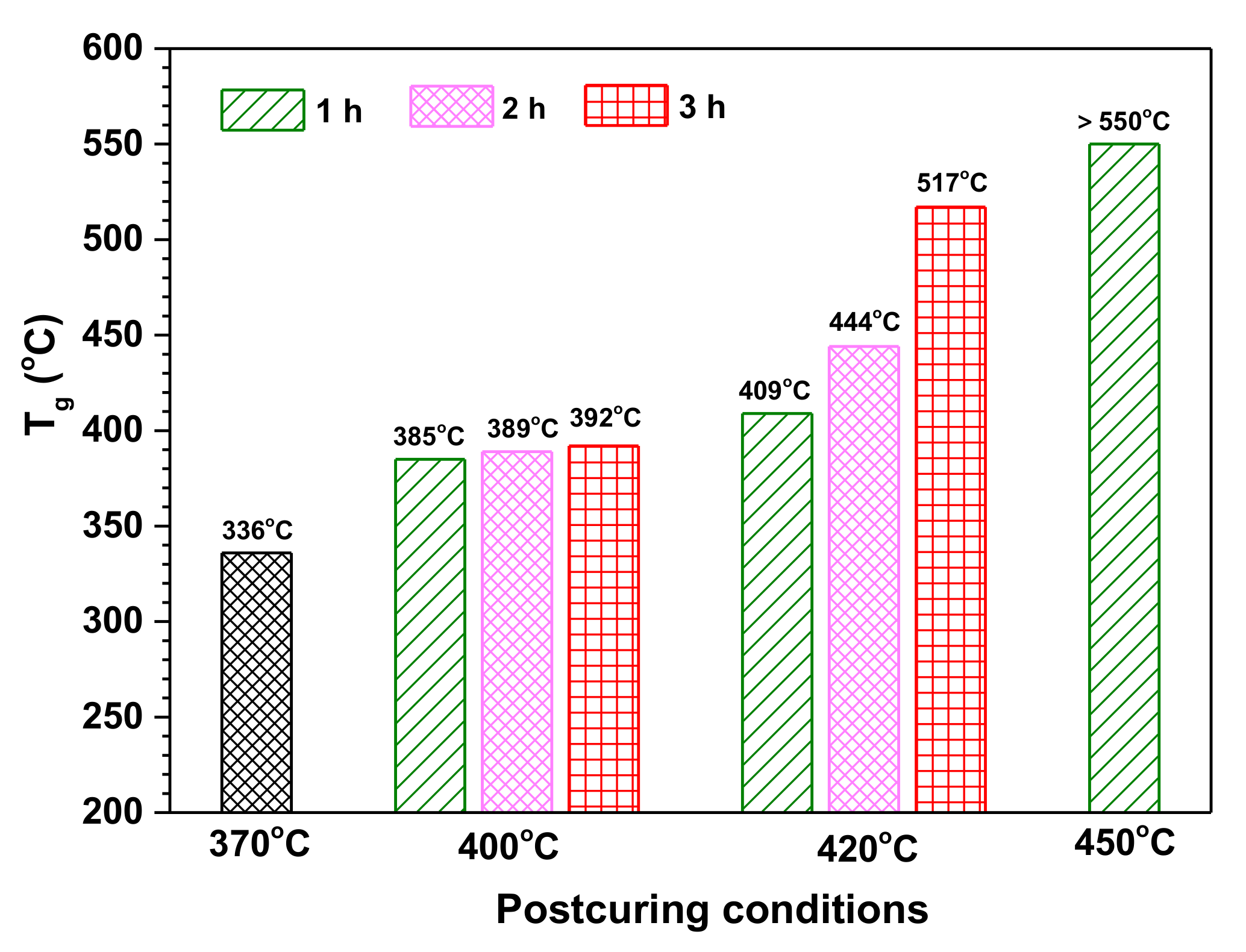
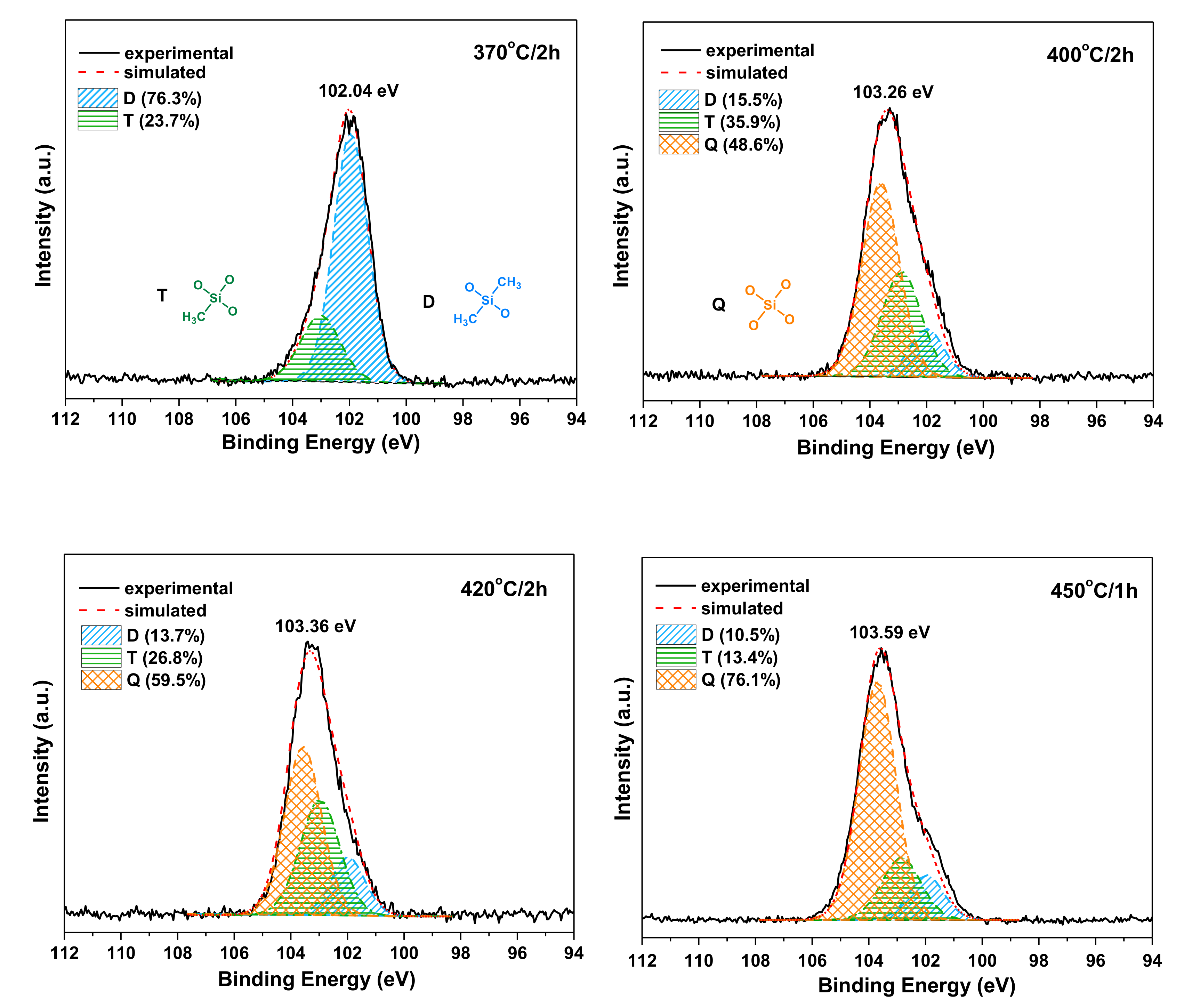
3.4. The Effect of Postcuring Conditions on the Thermal Properties of Cured Polyimides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harvey, B.G.; Yandek, G.R.; Lamb, J.T. Synthesis and characterization of a high temperature thermosetting polyimide oligomer derived from a non-toxic, sustainable bisaniline. RSC Adv. 2017, 7, 23149–23156. [Google Scholar] [CrossRef] [Green Version]
- Flores-Bonano, S.; Vargas-Martinez, J.; Suárez, O.M.; Silva-Araya, W. Tortuosity index based on dynamic mechanical properties of polyimide foam for aerospace applications. Materials 2019, 12, 1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggles-Wrenn, M.B.; Noomen, M. Fatigue of unitized polymer/ceramic matrix composites with 2D and 3D fiber architecture at elevated temperature. Polym. Test. 2018, 72, 244–256. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, G.; Zhan, M. High heat resistant carbon fiber/polyimide composites with neutron shielding performance. Prog. Org. Coat. 2019, 132, 184–190. [Google Scholar] [CrossRef]
- Donghwan, C.; Lawrence, T.D. Characterization, properties, and processing of LaRC™ PETI-5 as a high-temperature sizing material. I. FTIR studies on imidization and phenylethynyl end-group reaction behavior. J. Appl. Polym. Sci. 2000, 76, 190–200. [Google Scholar]
- Wang, W.; Chen, G.; Fang, X. Phenylethynyl-terminated oligoimides with ultra-low melt viscosity derived from 1, 4-bis (3, 4-dicarboxy phenoxy) benzene dianhydride. High. Perform. Polym. 2019, 31, 580–589. [Google Scholar] [CrossRef]
- Smith, J.G., Jr.; Connell, J.W.; Hergenrother, P.M. Resin transfer moldable phenylethynyl containing imide oligomers. J. Compos. Mater. 2002, 36, 2255–2265. [Google Scholar] [CrossRef]
- Hergenrother, P.M.; Smith, J.G., Jr. Chemistry and properties of imide oligomers end-capped with phenylethynylphthalic anhydrides. Polymer 1994, 35, 4857–4864. [Google Scholar] [CrossRef]
- Fang, X.; Rogers, D.F.; Scola, D.A. A study of the thermal cure of a phenylethynyl-terminated imide model compound and a phenylethynyl-terminated imide oligomer (PETI-5). J. Polym. Sci. Pol. Chem. 1998, 36, 461–470. [Google Scholar] [CrossRef]
- Fang, X.; Xie, X.Q.; Simone, C.D. A solid-state 13C NMR study of the cure of 13C-labeled phenylethynyl end-capped polyimides. Macromolecules 2000, 33, 1671–1681. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Jin, Z. Preparation of new phenylethynyl terminated imide oligomers from pyromellitic dianhydride. High Perform. Polym. 2012, 24, 180–187. [Google Scholar] [CrossRef]
- Zuo, H.J.; Chen, J.S.; Yang, H.X.; Hu, A.J.; Fan, L.; Yang, S.Y. Synthesis and characterization of melt-processable polyimides derived from 1,4-bis(4-amino-2-trifluoromethylphenoxy)benzene. J. Appl. Polym. Sci. 2008, 107, 755–765. [Google Scholar] [CrossRef]
- Smith, J.G., Jr.; Connell, J.W.; Hergenrother, P.M. Resin transfer molding imide resins based on 2,3,3’,4’-biphenyltetracarboxylic dianhydride. Macromol. Symp. 2003, 199, 401–418. [Google Scholar] [CrossRef]
- Su, C.N.; Ji, M.; Fan, L.; Yang, S.Y. Phenylethynyl-endcapped oligomides with low melt viscosities and high Tgs: Effects of the molecular weights. High Perform. Polym. 2011, 23, 352–361. [Google Scholar] [CrossRef]
- Yu, P.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z.M. Synthesis and characterization of phenylethynyl-terminated polyimide oligomers derived from 2,3,3’,4’-diphenyl ether tetracarboxylic acid dianhydride and 3,4’-oxydianiline. Chin. J. Polym. Sci. 2016, 34, 122–134. [Google Scholar] [CrossRef]
- Chuang, K.C.; Criss, J.M.; Mintz, E.A. Polyimides Based on Asymmetric Dianhydrides (II) (a-BPDA vs. a-BTDA) for Resin Transfer Molding (RTM); Technical Report; National Aeronautics and Space Administration: Cleveland, OH, USA, 2010. [Google Scholar]
- Fernberg, P.; Gong, G.; Mannberg, P. Development of novel high Tg polyimide-based composites. Part I: RTM processing properties. J. Compos. Mater. 2018, 52, 253–260. [Google Scholar] [CrossRef]
- Tsampas, S.; Fernberg, P.; Joffe, R. Development of novel high Tg polyimide-based composites. Part II: Mechanical characterisation. J. Compos. Mater. 2018, 52, 261–274. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, S.; He, M.H.; Fan, L. Phenylethynyl-terminated oligoimides based on bis (p-aminophenoxy) dimethyl silane: Effect of siloxane structure on processability and thermal stability. High Perform. Polym. 2018, 36, 651–661. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, L.; Qu, X.M.; Jia, M.; Yang, S. Fluorinated phenylethynylterminated imide oligomers with reduced melt viscosity and enhanced melt stability. Polymer 2011, 52, 138–148. [Google Scholar] [CrossRef]
- Chen, W.; Ji, M.; Yang, S. High thermal stable polyimide resins derived from phenylethynyl-endcapped fluorenyl oligoimides with low melt viscosities. Chin. J. Polym. Sci. 2016, 34, 933–948. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, S.Y.; Fan, L. Preparation and characterization of highly transparent and colorless semi-aromatic polyimide films derived from alicyclic dianhydride and aromatic diamines. Polymer 2012, 53, 3529–3539. [Google Scholar] [CrossRef]
- Babanzadeh, S.; Mahjoub, A.R.; Mehdipour-Ataei, S. Novel soluble thermally stable silane-containing aromatic polyimides with reduced dielectric constant. Polym. Degrad. Stabil. 2010, 95, 2492–2498. [Google Scholar] [CrossRef]
- Pei, X.L.; Chen, G.F.; Fang, X.Z. Synthesis and properties of poly(imide siloxane) block copolymers with different block lengths. J. Appl. Polym. Sci. 2013, 129, 3718–3727. [Google Scholar] [CrossRef]
- Song, N.; Yao, D.; Wang, Z.Y. A unique crystallization and double melting behavior of a polyimide derived from 3,3′,4,4′-biphenyltetracarboxylic dianhydride and 1,4-bis (3-aminopropyl) piperazine. Polymer 2005, 46, 3831–3837. [Google Scholar] [CrossRef]
- Li, Y.T.; Murphy, L.A.; Lincoln, J.E.; Morgan, R.J. Phenylethynyl end-capped fluorinated imide oligomer AFR-PEPA-N: Morphology and processibility characteristics. Macromol Mater. Eng. 2007, 292, 78–84. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, L.; Ji, M.; Yang, S.Y. Phenylethynylnaphthalic endcapped imide oligomers with reduced cure temperatures. Eur. Polym. J. 2010, 46, 2145–2155. [Google Scholar] [CrossRef]
- Meng, X.; Zheng, Y.; Yan, J.; Li, Y.; Wang, Z.; Li, G. 2,3’,3,4’-Oxydiphthalic dianhydride-based phenylethynyl-terminated imide oligomers for low-temperature resin transfer molding applications. High Perform. Polym. 2016, 28, 962–970. [Google Scholar] [CrossRef]
- Sun, L.; Wang, W.; Xu, L. Phenylethynyl-terminated imide oligomers derived from thioetherdiphthalic anhydride isomers with decreased melt viscosities. High Perform. Polym. 2016, 28, 927–935. [Google Scholar] [CrossRef]
- Brostow, W.; Lobland, H.E.; Narkis, M. Sliding wear, viscoelasticity and brittleness of polymers. J. Mater. Res. 2006, 21, 2422–2428. [Google Scholar] [CrossRef] [Green Version]
- Brostow, W.; Lobland, H.E.; Hong, H.J.; Lohse, S.; Osmanson, A.T. Flexibility of polymers defined and related to dynamic friction. J. Mater. Sci. Res. 2019, 8, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Coburn, J.C.; Soper, P.D.; Auman, B.C. Relaxation behavior of polyimides based on 2,2′-disubstituted benzidines. Macromolecules 1995, 28, 3253–3260. [Google Scholar] [CrossRef]
- Chuang, K.C.; Criss, J.M.; Mintz, E.A. Polyimide Composites Based on Asymmetric Dianhydrides (a-ODPA vs. a-BPDA). In Proceedings of the 54th International SAMPE Symposium, Baltimore, MD, USA, 18–21 May 2009. [Google Scholar]
- Tiptipakorn, S.; Damrongsakkul, S.; Ando, S.; Hemvichian, K.; Rimdusit, S. Thermal degradation behaviors of polybenzoxazine and silicon-containing polyimide blends. Polym. Degrad. Stabil. 2007, 92, 1265–1278. [Google Scholar] [CrossRef]
- Li, X.C.; Miyauchi, M.; Gonzalez, C.; Nutt, S. Thermal oxidation of PEPA-terminated polyimide. High Perform. Polym. 2019, 31, 707–718. [Google Scholar] [CrossRef]
- Lias, S.G.; Bartmess, J.E.; Lieman, J.F.; Holmes, J.L.; Levin, R.D.; Mallard, W.G. Gas-phase ion and neutral thermochemistry. J. Phys. Chem. Ref. Data. 1988, 17, 1–861. [Google Scholar]
- Liu, Y.; Fan, L.; Xu, X.Z.; Mo, S.; Peng, D.; Mu, Q.T.; Zhu, C.Z.; Li, C.H.; Xu, J. Melt fluidity and thermal property of thermosetting siloxane-containing polyimide resins and their organic/inorganic hybrid characteristics. Mater. Today Commun. 2020, 25, 101443. [Google Scholar] [CrossRef]
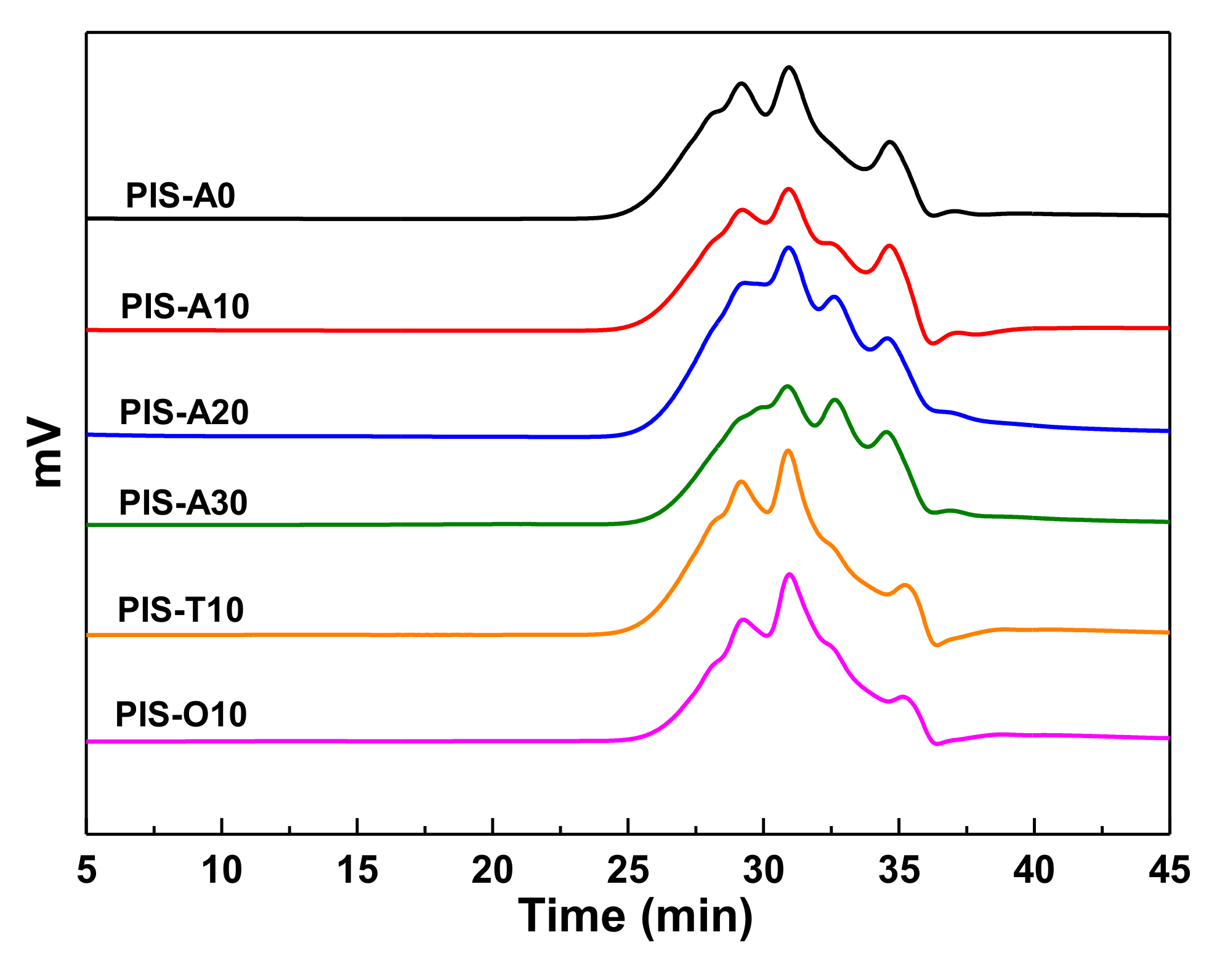




| Samples | APDS in Diamines (mol%) | Siloxane in Oligoimide (wt%) | Theoretical Mn (g/mol) | GPC Molecular Weight | ||
|---|---|---|---|---|---|---|
| Mn (g/mol) | Mw (g/mol) | Mw/Mn | ||||
| PIS-A0 | 0 | 0.00 | 1364 | 1687 | 2491 | 1.48 |
| PIS-A10 | 10 | 1.26 | 1357 | 1529 | 2244 | 1.47 |
| PIS-A20 | 20 | 2.56 | 1350 | 1429 | 2078 | 1.45 |
| PIS-A30 | 30 | 3.85 | 1344 | 1356 | 1933 | 1.43 |
| PIS-T10 | 10 | 1.24 | 1399 | 1543 | 2333 | 1.51 |
| PIS-O10 | 10 | 1.25 | 1381 | 1398 | 1992 | 1.42 |
| Samples | Minimum Melt Viscosity (Pa·s@°C) | Dynamic Viscosity (Pa·s) | Melt Viscosity Variation a @260 °C for 2 h (Pa·s) | ||
|---|---|---|---|---|---|
| 260 °C | 270 °C | 280 °C | |||
| PIS-A0 | 0.55@321 | 20.68 | 5.27 | 2.48 | 21.92–69.02 |
| PIS-A10 | 0.26@323 | 6.10 | 2.32 | 1.20 | 6.52–39.01 |
| PIS-A20 | 0.15@323 | 1.82 | 0.82 | 0.53 | 1.89–13.89 |
| PIS-A30 | 0.12@322 | 0.84 | 0.48 | 0.34 | 0.90–7.74 |
| PIS-T10 | 0.58@327 | 7.21 | 2.40 | 1.59 | 7.19–34.76 |
| PIS-O10 | 0.09@333 | 0.64 | 0.40 | 0.28 | 0.69–1.63 |
| Samples | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) | Flexural Strength (MPa) | Flexural Modulus (GPa) |
|---|---|---|---|---|---|
| PIS-A0 | 70.48 ± 1.92 | 2.62 ± 0.12 | 3.90 ± 0.28 | 121.52 ± 2.97 | 4.08 ± 0.13 |
| PIS-A10 | 62.82 ± 0.97 | 2.53 ± 0.12 | 3.37 ± 0.38 | 102.78 ± 3.36 | 4.05 ± 0.18 |
| PIS-A20 | 62.28 ± 1.24 | 2.35 ± 0.11 | 2.77 ± 0.18 | 99.37 ± 2.53 | 3.63 ± 0.09 |
| PIS-T10 | 54.69 ± 4.19 | 2.71 ± 0.14 | 2.41 ± 0.23 | 114.07 ± 3.18 | 4.62 ± 0.13 |
| PIS-O10 | 58.44 ± 3.28 | 2.51 ± 0.15 | 2.43 ± 0.14 | 108.13 ± 5.27 | 3.84 ± 0.09 |
| Samples | Siloxane in Oligoimide (wt%) | DMA a | TGA in Air b | |||
|---|---|---|---|---|---|---|
| E’ (°C) | tanδ (°C) | T5 (°C) | T10 (°C) | R700 (%) | ||
| PIS-A0 | 0.00 | 388 | 442 | 576.7 | 602.5 | 30.5 |
| PIS-A10 | 1.26 | 330 | 361 | 560.8 | 593.9 | 32.9 |
| PIS-A20 | 2.56 | 322 | 347 | 556.5 | 591.9 | 33.0 |
| PIS-A30 | 3.85 | 287 | 323 | 549.2 | 589.4 | 35.1 |
| PIS-T10 | 1.24 | 379 | 437 | 554.1 | 588.2 | 30.7 |
| PIS-O10 | 1.25 | 303 | 336 | 539.7 | 577.8 | 27.1 |
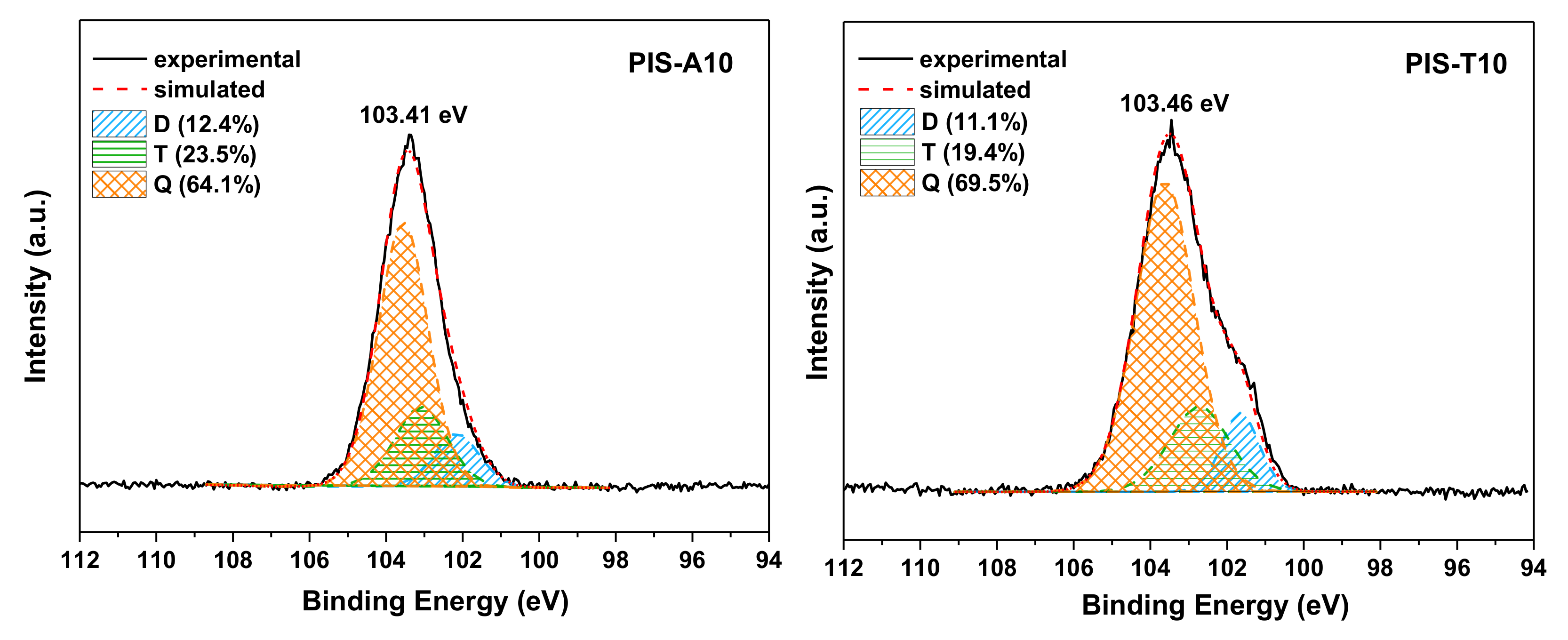
| Curing and Postcuring Conditions | D[(CH3)2SiO2/2] | T[CH3SiO3/2] | Q[SiO4/2] |
|---|---|---|---|
| (at.%) | (at.%) | (at.%) | |
| 370 ℃/2 h | 76.3 | 23.7 | 0.0 |
| 400 ℃/1 h | 16.4 | 37.9 | 45.7 |
| 400 ℃/2 h | 15.5 | 35.9 | 48.6 |
| 420 ℃/1 h | 14.9 | 27.6 | 57.5 |
| 420 ℃/2 h | 13.7 | 26.8 | 59.5 |
| 42 0℃/3 h | 12.2 | 20.5 | 64.1 |
| 450 ℃/1 h | 10.5 | 13.4 | 76.1 |
| Samples | DMA a | TGA in Air b | |||
|---|---|---|---|---|---|
| E’ (°C) | tanδ (°C) | T5 (°C) | T10 (°C) | R700 (%) | |
| PIS-A10 | 465 | 498 | 577.2 | 599.1 | 27.8 |
| PIS-T10 | 485 | >550 | 573.4 | 596.2 | 26.1 |
| PIS-O10 | 495 | >550 | 572.9 | 595.1 | 28.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, Y.; Lan, B.; Mo, S.; Zhai, L.; He, M.; Fan, L. High Thermally Stable and Melt Processable Polyimide Resins Based on Phenylethynyl-Terminated Oligoimides Containing Siloxane Structure. Materials 2020, 13, 3742. https://doi.org/10.3390/ma13173742
Xu X, Liu Y, Lan B, Mo S, Zhai L, He M, Fan L. High Thermally Stable and Melt Processable Polyimide Resins Based on Phenylethynyl-Terminated Oligoimides Containing Siloxane Structure. Materials. 2020; 13(17):3742. https://doi.org/10.3390/ma13173742
Chicago/Turabian StyleXu, Xiaozhou, Yi Liu, Bangwei Lan, Song Mo, Lei Zhai, Minhui He, and Lin Fan. 2020. "High Thermally Stable and Melt Processable Polyimide Resins Based on Phenylethynyl-Terminated Oligoimides Containing Siloxane Structure" Materials 13, no. 17: 3742. https://doi.org/10.3390/ma13173742
APA StyleXu, X., Liu, Y., Lan, B., Mo, S., Zhai, L., He, M., & Fan, L. (2020). High Thermally Stable and Melt Processable Polyimide Resins Based on Phenylethynyl-Terminated Oligoimides Containing Siloxane Structure. Materials, 13(17), 3742. https://doi.org/10.3390/ma13173742




