A Simple and Cost-Effective Method for Producing Stable Surfactant-Coated EGaIn Liquid Metal Nanodroplets
Abstract
:1. Introduction
2. Materials and Methods
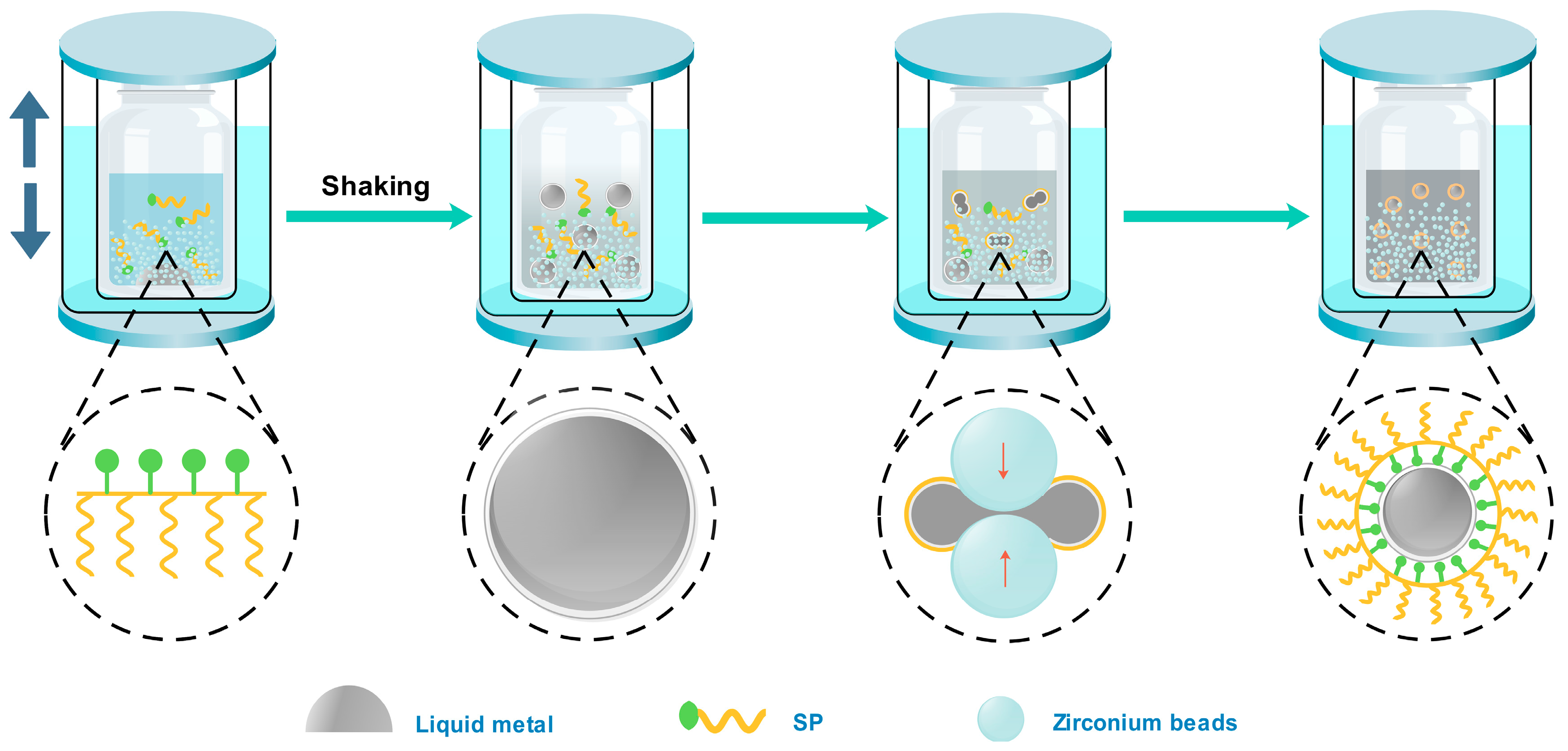
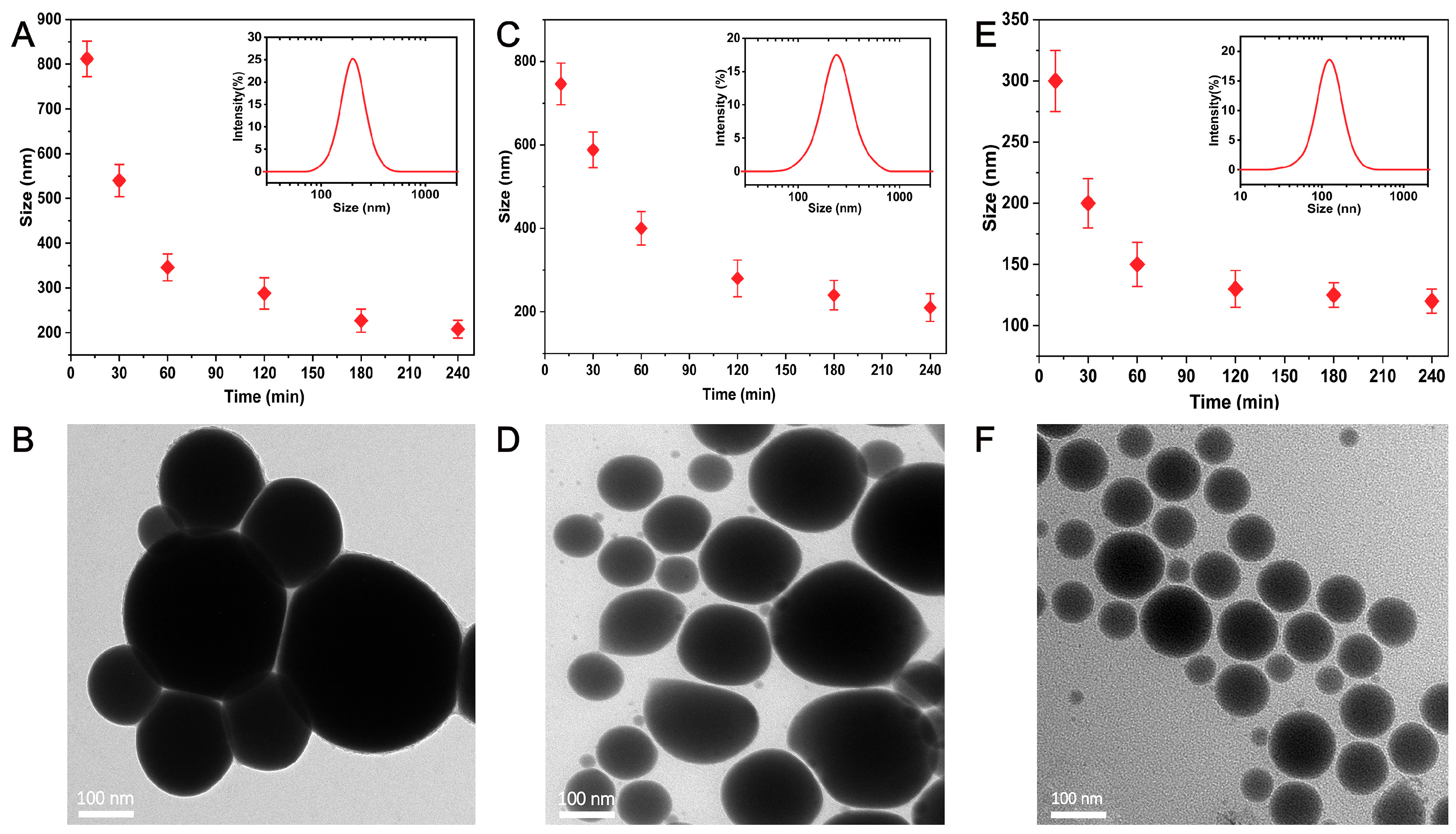
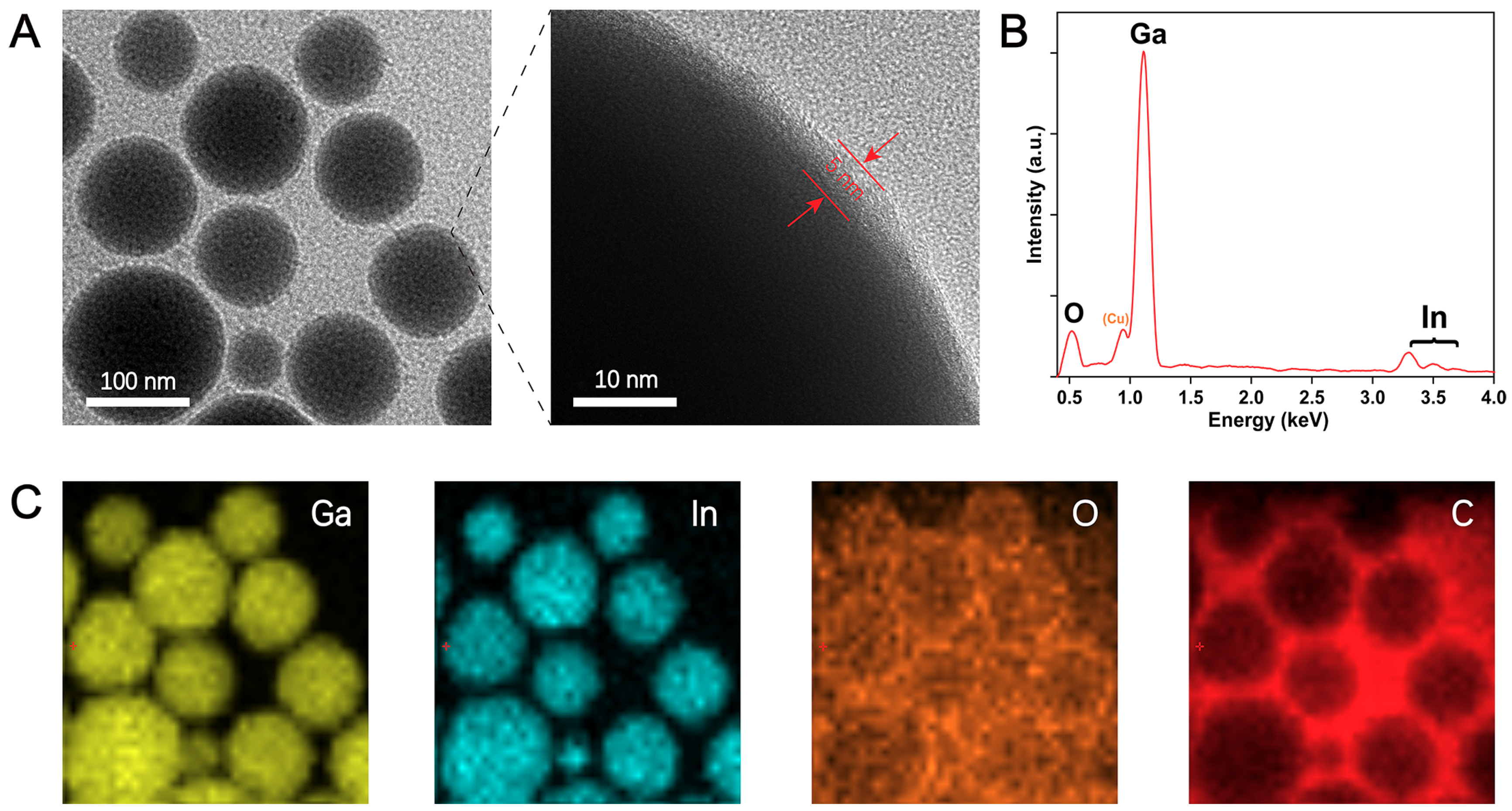
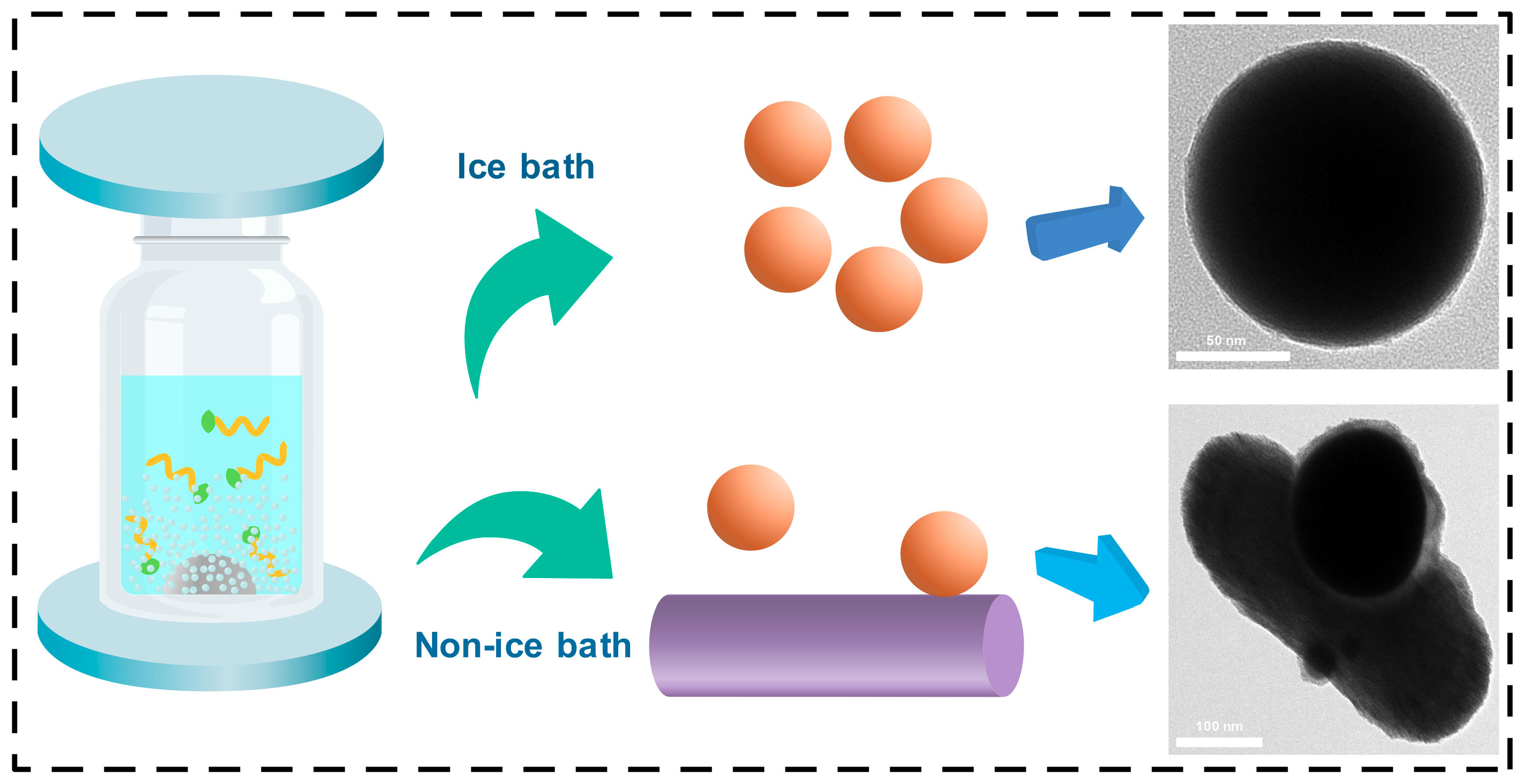
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, H.S.; Baek, J.; Park, T.H.; Oh, S.; Lee, H.; Koo, M.; Park, C. Rewritable, Printable Conducting Liquid Metal Hydrogel. ACS Nano 2019, 13, 9122–9130. [Google Scholar] [CrossRef]
- Hu, Q.; Jiang, T.; Jiang, H. Versatile Movements of Liquid Metal Droplet under Electrostatic Actuation in Alkaline Solutions. Materials 2020, 13, 2122. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lu, Y.; Chen, G.; Yang, M.; Gu, Z. Advances in liquid metals for biomedical applications. Chem. Soc. Rev. 2018, 47, 2518–2533. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, Fabrication, and Applications of Gallium-Based Liquid Metal Particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Lu, C.N.; Dou, M.J.; Zhang, C.L.; Chang, H.; Liu, J.; Rao, W. Soft liquid metal nanoparticles achieve reduced crystal nucleation and ultrarapid rewarming for human bone marrow stromal cell and blood vessel cryopreservation. Acta Biomater. 2020, 102, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Ger, T.R.; Uapipatanakul, B.; Huang, J.C.; Chen, K.H.; Hsiao, C.D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, A.; Daeneke, T.; Zavabeti, A.; Zhang, B.Y.; Orrell-Trigg, R.; Kalantar-Zadeh, K.; Wójcik, A.; Maziarz, W.; Gancarz, T. Investigation of the surface of Ga–Sn–Zn eutectic alloy by the characterisation of oxide nanofilms obtained by the touch-printing method. Nanomaterials 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Chechetka, S.A.; Yu, Y.; Zhen, X.; Pramanik, M.; Pu, K.; Miyako, E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat. Commun. 2017, 8, 15432. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Yu, Y.; Pan, K.; Liu, J. Liquid metal angiography for mega contrast X-ray visualization of vascular network in reconstructing in-vitro organ anatomy. IEEE Trans. Biomed. Eng. 2014, 61, 2161–2166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, C.W.; Li, J.W.; Huang, C.Y.; Yang, S.S.; Soong, Y.C.; Lin, C.L.; Lee, J.C.; Sanchez, W.A.L.; Cheng, C.C.; Suen, M.C. Controlling the Structures, Flexibility, Conductivity Stability of Three-Dimensional Conductive Networks of Silver Nanoparticles/Carbon-Based Nanomaterials with Nanodispersion and their Application in Wearable Electronic Sensors. Nanomaterials 2020, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Cho, M.; Yu, K.J. Flexible and Stretchable Bio-Integrated Electronics Based on Carbon Nanotube and Graphene. Materials 2018, 11, 1163. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Zhu, D.; Wang, Y.; Jin, S. Electromechanical Behaviors of Graphene Reinforced Polymer Composites: A Review. Materials 2020, 13, 528. [Google Scholar] [CrossRef] [Green Version]
- Sohn, H.; Park, C.; Oh, J.M.; Kang, S.W.; Kim, M.J. Silver Nanowire Networks: Mechano-Electric Properties and Applications. Materials 2019, 12, 2526. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, J. Recent Advancements in Liquid Metal Flexible Printed Electronics: Properties, Technologies, and Applications. Micromachines 2016, 7, 206. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Y.; Liu, J. Preparations, Characteristics and Applications of the Functional Liquid Metal Materials. Adv. Eng. Mater. 2018, 20, 1700781. [Google Scholar] [CrossRef]
- Yu, Y.; Miyako, E. Recent Advances in Liquid Metal Manipulation toward Soft Robotics and Biotechnologies. Chemistry 2018, 24, 9456–9462. [Google Scholar] [CrossRef]
- Li, Y.; Nayak, S.; Luo, Y.; Liu, Y.; Salila Vijayalal Mohan, H.K.; Pan, J.; Liu, Z.; Heng, C.H.; Thean, A.V. A Soft Polydimethylsiloxane Liquid Metal Interdigitated Capacitor Sensor and Its Integration in a Flexible Hybrid System for On-Body Respiratory Sensing. Materials 2019, 12, 1458. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Li, Y.; Chen, Y.; Yang, J.; Zhu, T.; Zhu, D.; He, C.; Liu, Y.; Handschuh-Wang, S.; Zhou, X. Liquid metal sponges for mechanically durable, all-soft, electrical conductors. J. Mater. Chem. C 2017, 5, 1586–1590. [Google Scholar] [CrossRef]
- Peng, H.; Xin, Y.; Xu, J.; Liu, H.; Zhang, J. Ultra-stretchable hydrogels with reactive liquid metals as asymmetric force-sensors. Mater. Horiz. 2019, 6, 618–625. [Google Scholar] [CrossRef]
- Lu, H.; Tang, S.-Y.; Dong, Z.; Liu, D.; Zhang, Y.; Zhang, C.; Yun, G.; Zhao, Q.; Kalantar-Zadeh, K.; Qiao, R.; et al. Dynamic Temperature Control System for the Optimized Production of Liquid Metal Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 6905–6914. [Google Scholar] [CrossRef]
- Idrus-Saidi, S.A.; Tang, J.; Ghasemian, M.B.; Yang, J.; Han, J.; Syed, N.; Daeneke, T.; Abbasi, R.; Koshy, P.; O’Mullane, A.P.; et al. Liquid metal core–shell structures functionalised via mechanical agitation: The example of Field’s metal. J. Mater. Chem. A 2019, 7, 17876–17887. [Google Scholar] [CrossRef]
- Yan, J.; Malakooti, M.H.; Lu, Z.; Wang, Z.; Kazem, N.; Pan, C.; Bockstaller, M.R.; Majidi, C.; Matyjaszewski, K. Solution processable liquid metal nanodroplets by surface-initiated atom transfer radical polymerization. Nat. Nanotechnol. 2019, 14, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-Y.; Qiao, R.; Lin, Y.; Li, Y.; Zhao, Q.; Yuan, D.; Yun, G.; Guo, J.; Dickey, M.D.; Huang, T.J.; et al. Functional Liquid Metal Nanoparticles Produced by Liquid-Based Nebulization. Adv. Mater. Technol. 2019, 4, 1800420. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.Y.; Qiao, R.; Yan, S.; Yuan, D.; Zhao, Q.; Yun, G.; Davis, T.P.; Li, W. Microfluidic Mass Production of Stabilized and Stealthy Liquid Metal Nanoparticles. Small 2018, 14, 1800118. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Ding, Y.; Zhang, Q.; Wang, L.; Liu, J. Controllable dispersion and reunion of liquid metal droplets. Sci. China Mater. 2018, 62, 407–415. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tang, S.-Y.; Zhao, Q.; Yun, G.; Yuan, D.; Li, W. High-throughput production of uniformly sized liquid metal microdroplets using submerged electrodispersion. Appl. Phys. Lett. 2019, 114, 154101. [Google Scholar] [CrossRef]
- Mohammed, M.; Xenakis, A.; Dickey, M. Production of Liquid Metal Spheres by Molding. Metals 2014, 4, 465–476. [Google Scholar] [CrossRef]
- Lin, P.; Wei, Z.; Yan, Q.; Xie, J.; Fan, Y.; Wu, M.; Chen, Y.; Cheng, Z. Capillary-Based Microfluidic Fabrication of Liquid Metal Microspheres toward Functional Microelectrodes and Photothermal Medium. ACS Appl. Mater. Interfaces 2019, 11, 25295–25305. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, S.; Liu, H.; Wang, Z.; Shao, T. A Function Reconfigurable Antenna Based on Liquid Metal. Electronics 2020, 9, 873. [Google Scholar] [CrossRef]
- Bilodeau, R.A.; Zemlyanov, D.Y.; Kramer, R.K. Liquid Metal Switches for Environmentally Responsive Electronics. Adv. Mater. Interfaces 2017, 4, 1600913. [Google Scholar] [CrossRef]
- Teng, L.; Ye, S.; Handschuh-Wang, S.; Zhou, X.; Gan, T.; Zhou, X. Liquid Metal-Based Transient Circuits for Flexible and Recyclable Electronics. Adv. Funct. Mater. 2019, 29, 1808739. [Google Scholar] [CrossRef]
- Pan, C.; Markvicka, E.J.; Malakooti, M.H.; Yan, J.; Hu, L.; Matyjaszewski, K.; Majidi, C. A Liquid-Metal-Elastomer Nanocomposite for Stretchable Dielectric Materials. Adv. Mater. 2019, 31, 1900663. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.Y.; Ding, Y.J.; Li, H.P.; Chen, S.; Guo, R.; Liu, J. Multi-Substrate Liquid Metal Circuits Printing via Superhydrophobic Coating and Adhesive Patterning. Adv. Eng. Mater. 2019, 21, 1801363. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.; So, J.H.; Kim, K.; Koo, H.J. Cytotoxicity of Gallium-Indium Liquid Metal in an Aqueous Environment. ACS Appl. Mater. Interfaces 2018, 10, 17448–17454. [Google Scholar] [CrossRef]
- Castriciano, M.A. Functional Nanostructures for Sensors, Optoelectronic Devices, and Drug Delivery. Nanomaterials 2020, 10, 1195. [Google Scholar] [CrossRef]
- Xu, B.; Chang, G.; Li, R. A versatile approach for preparing stable and high concentration liquid metal nanoparticles on a large scale. J. Disper. Sci. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Zong, L.; Wu, X.; You, J.; Du, P.; Li, C. Liquid Metal Droplets Wrapped with Polysaccharide Microgel as Biocompatible Aqueous Ink for Flexible Conductive Devices. Adv. Funct. Mater. 2018, 28, 1804197. [Google Scholar] [CrossRef]
- Amoabeng, D.; Velankar, S.S. A review of conductive polymer composites filled with low melting point metal alloys. Polym. Eng. Sci. 2018, 58, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Dickey, M.D. Emerging applications of liquid metals featuring surface oxides. ACS Appl. Mater. Interfaces 2014, 6, 18369–18379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Kim, T.; Kang, S.; Jin, H.; Lee, K.; Yoon, H.J. Ga-Based Liquid Metal Micro/Nanoparticles: Recent Advances and Applications. Small 2020, 16, 1903391. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Genzer, J.; Li, W.; Qiao, R.; Dickey, M.D.; Tang, S.Y. Sonication-enabled rapid production of stable liquid metal nanoparticles grafted with poly(1-octadecene-alt-maleic anhydride) in aqueous solutions. Nanoscale 2018, 10, 19871–19878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschuh-Wang, S.; Chen, Y.; Zhu, L.; Gan, T.; Zhou, X. Electric Actuation of Liquid Metal Droplets in Acidified Aqueous Electrolyte. Langmuir 2018, 35, 372–381. [Google Scholar] [CrossRef]
- Finkenauer, L.R.; Lu, Q.; Hakem, I.F.; Majidi, C.; Bockstaller, M.R. Analysis of the Efficiency of Surfactant-Mediated Stabilization Reactions of EGaIn Nanodroplets. Langmuir 2017, 33, 9703–9710. [Google Scholar] [CrossRef]
- Zhang, W.; Naidu, B.S.; Ou, J.Z.; O’Mullane, A.P.; Chrimes, A.F.; Carey, B.J.; Wang, Y.; Tang, S.Y.; Sivan, V.; Mitchell, A.; et al. Liquid metal/metal oxide frameworks with incorporated Ga2O3 for photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 1943–1948. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-transformable liquid metal nanoparticles in aqueous solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, J. Nano liquid metal as an emerging functional material in energy management, conversion and storage. Nano Energy 2013, 2, 863–872. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, M.; Wang, R.; Deng, Z.; Gui, L. Stretchable Pressure Sensor with Leakage-Free Liquid-Metal Electrodes. Sensors 2019, 19, 1316. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, Q.; Guo, R.; Zhang, M.; Wang, S.; Lu, C.; Xue, M.; Fan, J.; He, Z.; Rao, W. Self-assembled ultrathin film of CNC/PVA–liquid metal composite as a multifunctional Janus material. Mater. Horiz. 2019, 6, 1643–1653. [Google Scholar] [CrossRef]
- Chang, H.; Guo, R.; Sun, Z.; Wang, H.; Hou, Y.; Wang, Q.; Rao, W.; Liu, J. Direct Writing and Repairable Paper Flexible Electronics Using Nickel-Liquid Metal Ink. Adv. Mater. Interfaces 2018, 5, 1800571. [Google Scholar] [CrossRef]
- Lin, Y.; Cooper, C.; Wang, M.; Adams, J.J.; Genzer, J.; Dickey, M.D. Handwritten, Soft Circuit Boards and Antennas Using Liquid Metal Nanoparticles. Small 2015, 11, 6397–6403. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Ye, F.; Chang, G.; Li, R. A Simple and Cost-Effective Method for Producing Stable Surfactant-Coated EGaIn Liquid Metal Nanodroplets. Materials 2020, 13, 3753. https://doi.org/10.3390/ma13173753
Xu B, Ye F, Chang G, Li R. A Simple and Cost-Effective Method for Producing Stable Surfactant-Coated EGaIn Liquid Metal Nanodroplets. Materials. 2020; 13(17):3753. https://doi.org/10.3390/ma13173753
Chicago/Turabian StyleXu, Bingbing, Feng Ye, Guangtao Chang, and Ruoxin Li. 2020. "A Simple and Cost-Effective Method for Producing Stable Surfactant-Coated EGaIn Liquid Metal Nanodroplets" Materials 13, no. 17: 3753. https://doi.org/10.3390/ma13173753
APA StyleXu, B., Ye, F., Chang, G., & Li, R. (2020). A Simple and Cost-Effective Method for Producing Stable Surfactant-Coated EGaIn Liquid Metal Nanodroplets. Materials, 13(17), 3753. https://doi.org/10.3390/ma13173753




