Synthesis of Cryolite (Na3AlF6) from Secondary Aluminum Dross Generated in the Aluminum Recycling Process
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis Processes of Cryolite
2.2.1. Water-Washing Pretreatment of SAD
2.2.2. Extraction of Al Component via Pyro-Hydrometallurgical Process
2.2.3. Carbonation
2.3. Methods of Characterization
3. Results and Discussion
3.1. Characterization of As-Received SAD
3.2. Water-Washing Pretreatment of SAD
3.2.1. Hydrolysis of AlN in the SAD
3.2.2. Recovery of Salts in the SAD
3.3. Extraction of Al Component via Pyro-Hydrometallurgical Process
3.3.1. Effect of Different Factors on the Extraction Efficiency of Al
- Effect of alkali-to-dross mass ratio
- Effect of salt-to-dross mass ratio
- Effect of smelting time
- Effect of leaching temperature
- Effect of leaching time
3.3.2. The Removal of Si Impurity from NaAlO2 Solution
3.4. Synthesis of Cryolite (Na3AlF6) by Carbonation Method
3.4.1. Effect of Different Factors on the Quality of Synthetic Cryolite
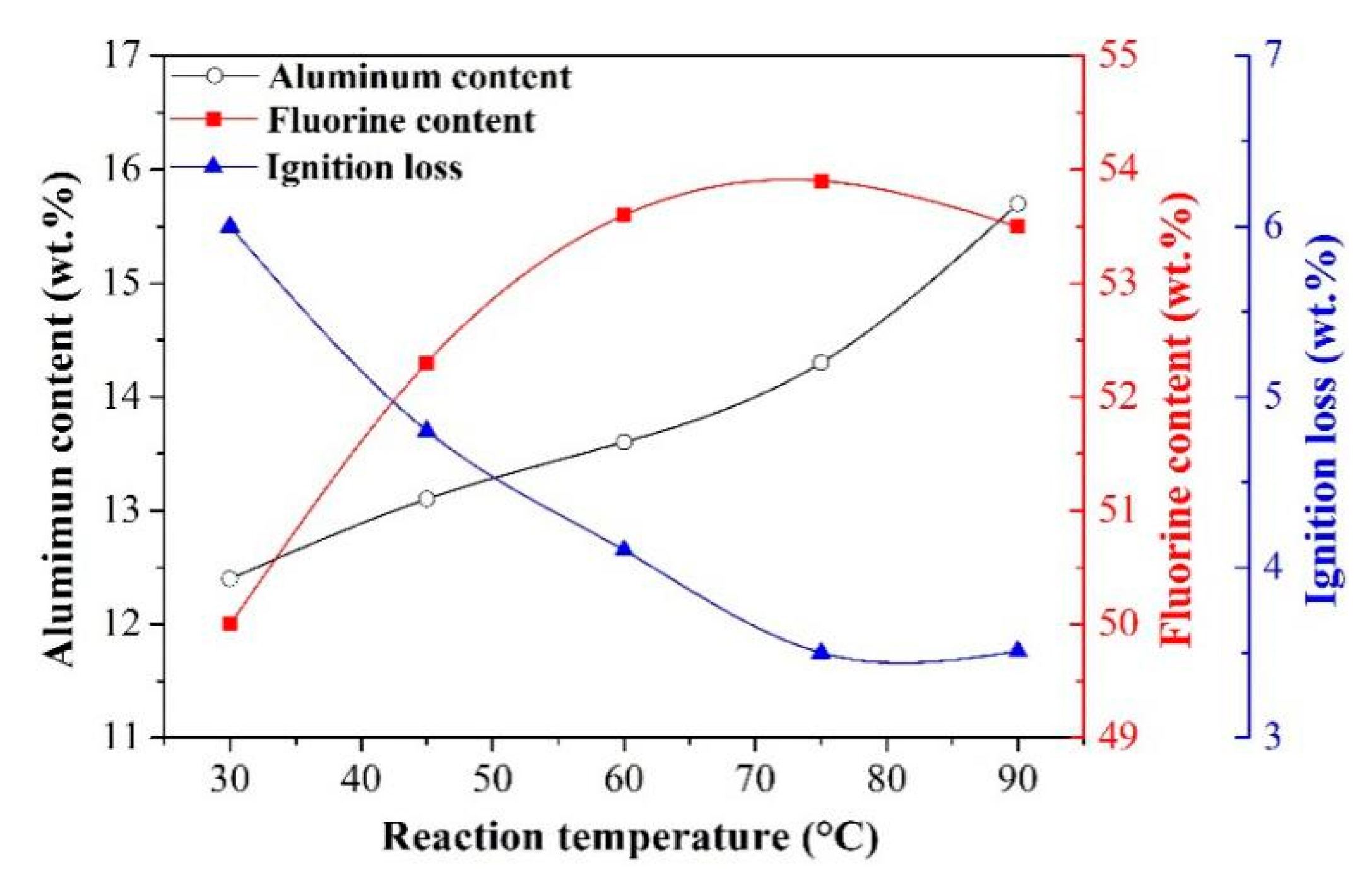
- Effect of reaction temperature
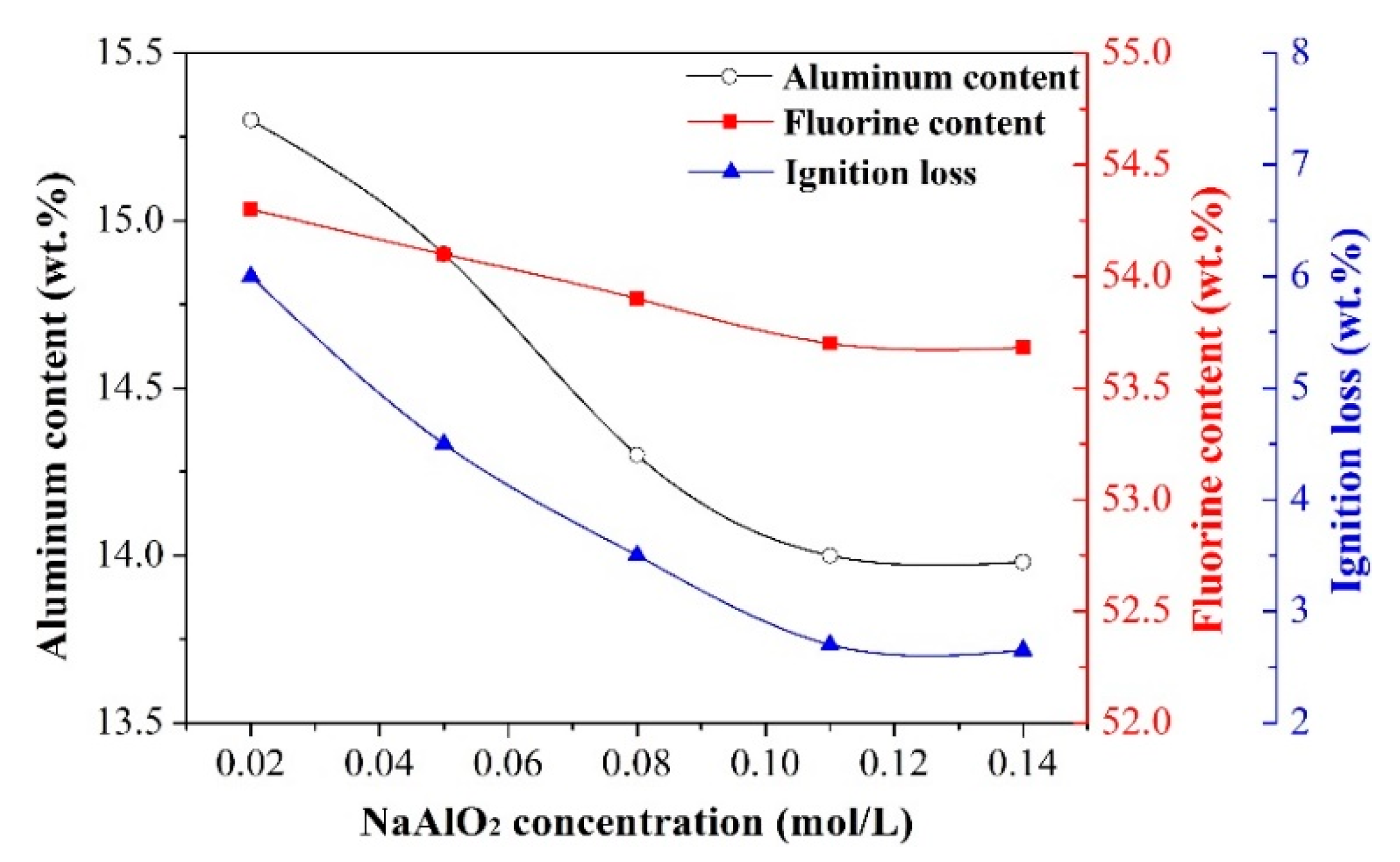
- Effect of NaAlO2 concentration
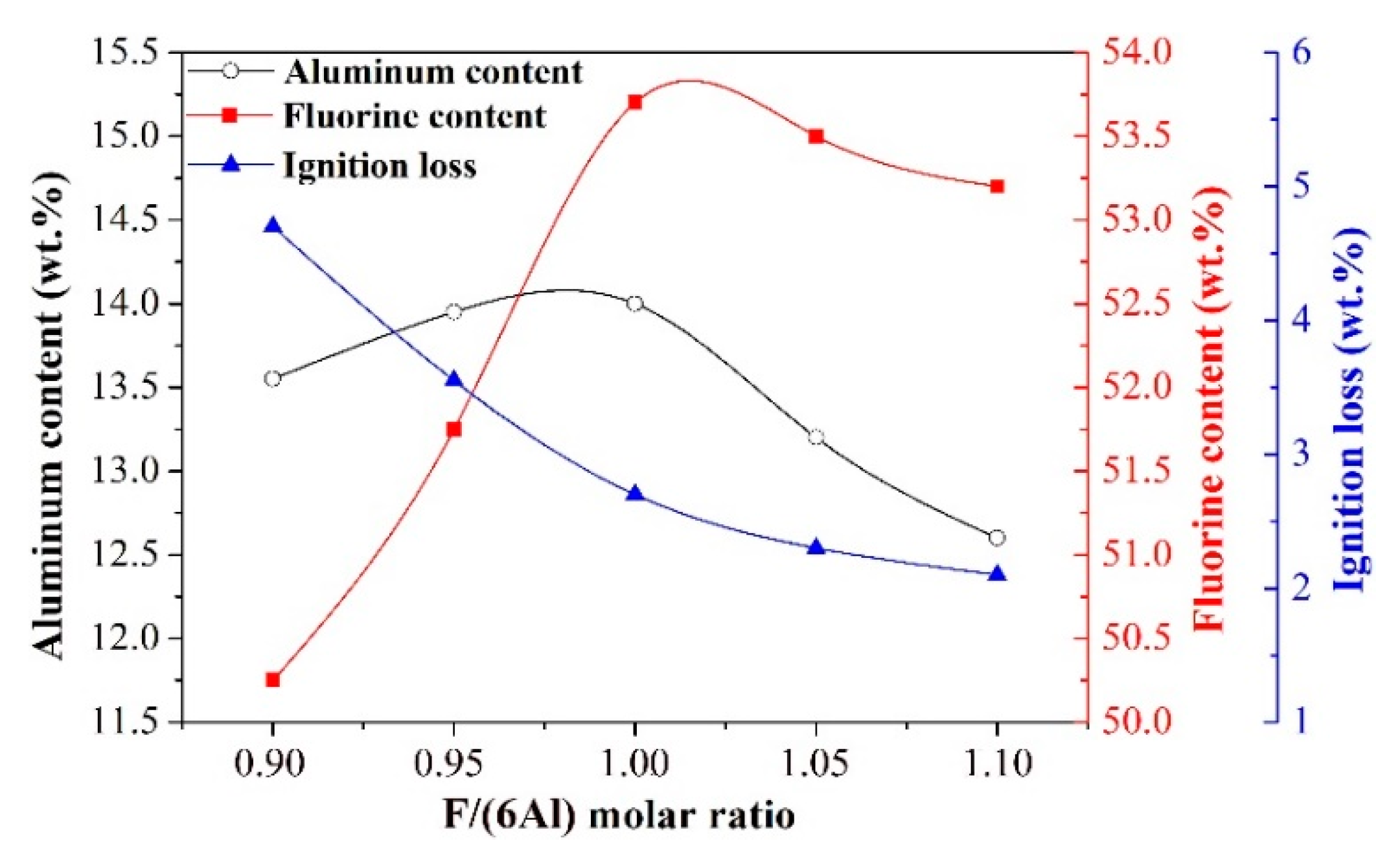
- Effect of F/(6Al) molar ratio
3.4.2. Characterization of As-Synthesized Cryolite
4. Conclusions
- (1)
- The maximum hydrolysis efficiency of AlN in the SAD, i.e., 68.3%, was achieved when the water-washing temperature, reaction time, stirring speed, and liquid-to-solid ratio were 90 °C, 2 h, 300 rpm, and 5 mL/g, respectively. Meanwhile, approximately 38 kg of NH3 can be produced after the water-washing pretreatment of one metric ton of the SAD. In addition, the water-soluble salts constituents such as NaCl and KCl were recovered by washing and evaporation.
- (2)
- The optimal conditions of pyro-hydrometallurgical process were obtained as follows: alkali (NaOH)-to-dross mass ratio of 1.3, salt (NaNO3)-to-dross mass ratio of 0.3, smelting temperature of 500 °C, smelting time of 120 min, deionized water to smelting products ratio of 6 mL/g, leaching temperature of 80 °C, and leaching time of 40 min. Under these conditions, the extraction efficiency of Al reached up to 91.5%, which was 34% higher than that achieved in conventional alkaline leaching.
- (3)
- The synthetic cryolite of high quality was precipitated under the following optimal conditions of carbonation: synthesis temperature of 75 °C, NaAlO2 concentration of 0.11 mol/L, F/(6Al) molar ratio of 1.10, and 99.99% CO2 gas pressure and flow rate of 0.2 MPa and 0.5 L/min respectively. The XRD and SEM/EDX analyses suggested that, the as-synthesized cryolite mainly consisted of the Na3AlF6 phase, and despite the occurrence of agglomeration, its morphology still exhibited a polyhedral shape with ~1 μm size. Moreover, aluminum content (12.6 wt.%), fluorine content (53.2 wt.%), sodium content (32.18 wt.%), SiO2 impurity content (0.125 wt.%), and ignition loss (2.1 wt.%) of the as-synthesized cryolite with high molecular ratio complied well with the requirement of the Chinese national standard (GB/T 4291-2017). Besides, from the angle of aluminum source and fluorine source used to synthesize cryolite, this novel eco-friendly three-step process was of lower cost and higher efficiency compared to conventional synthetic methods, suggesting it had the potential to be applied to the cryolite industry.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahinroosta, M.; Allahverdi, A.; Dong, P.; Bassim, N. Green template-free synthesis and characterization of mesoporous alumina as a high value-added product in aluminum black dross recycling strategy. J. Alloy. Compd. 2019, 792, 161–169. [Google Scholar] [CrossRef]
- Bonet-Martínez, E.; Pérez-Villarejo, L.; Eliche-Quesada, D.; Castro, E. Manufacture of Sustainable Clay Bricks Using Waste from Secondary Aluminum Recycling as Raw Material. Materials 2018, 11, 2439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Recour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Sekunowo, O.I.; Taiwo, O.O.; Ayoola, W.A.; Machado, A. Physical and mechanical properties of aluminum dross. Adv. Mater. 2014, 3, 6–10. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Q.; Zhang, G.F.; Shi, Q. Investigations on the hydrolysis behavior of AlN in the leaching process of secondary aluminum dross. Hydrometallurgy 2018, 182, 121–127. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 2013, 261, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, Q.; Zhang, G.F.; Shi, Q.; Feng, H.G. Investigation of leaching kinetics of aluminum extraction from secondary aluminum dross with use of hydrochloric acid. Hydrometallurgy 2019, 187, 158–167. [Google Scholar] [CrossRef]
- Gil, A.; Arrieta, E.; Vicente, M.A.; Korili, S.A. Synthesis and CO2 adsorption properties of hydrotalcite-like compounds prepared from aluminum saline slag wastes. Chem. Eng. J. 2018, 334, 1341–1350. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.H.; Han, Z.Y.; Xiao, X.Y.; Peng, C. Feasibility of aluminum recovery and MgAl2O4 spinel synthesis from secondary aluminum dross. Int. J. Miner. Metall. Mater. 2019, 26, 309–318. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Enhanced alumina recovery from secondary aluminum dross for high purity nanostructured γ-alumina powder production: Kinetic study. J. Environ. Manag. 2018, 212, 278–291. [Google Scholar] [CrossRef]
- Tripathy, A.K.; Mahalik, S.; Sarangi, C.K.; Tripathy, B.C.; Sanjay, K.; Bhattacharya, I.N. A pyro-hydrometallurgical process for the recovery of alumina from waste aluminium dross. Miner. Eng. 2019, 137, 181–186. [Google Scholar] [CrossRef]
- López-Delgado, A.; Robla, J.I.; Padilla, I.; López-Andrés, S.; Romero, M. Zero-waste process for the transformation of a hazardous aluminum waste into a raw material to obtain zeolites. J. Clean. Prod. 2020, 255, 120178. [Google Scholar] [CrossRef]
- López, F.A.; Martín, M.I.; Alguacil, F.J.; Sergio Ramírez, M.; González, J.R. Synthesis of Calcium Aluminates from Non-Saline Aluminum Dross. Materials 2019, 12, 1837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.S.; Wang, C.M.; Yu, Y.; Huang, X.W.; Long, Z.Q.; Hou, Y.K.; Cui, D.L. Recovery of fluorine from bastnasite as synthetic cryolite by-product. J. Hazard. Mater. 2012, 209, 77–83. [Google Scholar] [CrossRef]
- Murayama, N.; Maekawa, I.; Ushiro, H.; Miyoshi, T.; Shibata, J.; Valix, M. Synthesis of various layered double hydroxides using aluminum dross generated in aluminum recycling process. Int. J. Miner. Process. 2012, 110, 46–52. [Google Scholar] [CrossRef]
- Meshram, A.; Jain, A.; Gautam, D.; Singh, K.K. Synthesis and characterization of tamarugite from aluminium dross: Part, I. J. Environ. Manag. 2019, 232, 978–984. [Google Scholar] [CrossRef]
- Das, B.R.; Dash, B.; Tripathy, B.C.; Bhattacharya, I.N.; Das, S.C. Production of η-alumina from waste aluminium dross. Miner. Eng. 2007, 20, 252–258. [Google Scholar] [CrossRef]
- Dash, B.; Das, B.R.; Tripathy, B.C.; Bhattacharya, I.N.; Das, S.C. Acid dissolution of alumina from waste luminium dross. Hydrometallurgy 2008, 92, 48–53. [Google Scholar] [CrossRef]
- Amer, A.M. Aluminium extraction from aluminum industrial wastes. J. Miner. Met. Mater. Soc. 2010, 62, 60–63. [Google Scholar] [CrossRef]
- Sakiridis, P.E.; Oustadakis, P.; Agatzini-Leonardou, S. Aluminium recovery during black dross hydrothermal treatment. J. Environ. Chem. Eng. 2013, 1, 23–32. [Google Scholar] [CrossRef]
- Guo, X.Y.; Li, F.; Tian, Q.H.; Ji, K. Recovery of aluminium from secondary aluminum dross using low-temperature alkaline smelting. J. Cent. South Univ. (Sci. Technol.) 2012, 43, 809–814. [Google Scholar]
- Guo, X.Y.; Liu, J.X. Optimization of low-temperature alkaline smelting process of crushed metal enrichment originated from waste printed circuit boards. J. Cent. South Univ. 2015, 22, 1643–1650. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakamura, T. Process of Preparing High Purity Cryolite from Fluoride Solution Containing Dissolved Silica. U.S. Patent Application 64,363, 17 February 1979. [Google Scholar]
- Barzgar, S.; Lothenbach, B.; Tarik, M.; Di Giacomo, A.; Ludwig, C. The Effect of Sodium Hydroxide on Al uptake by Calcium Silicate Hydrates (C-S-H). J. Colloid Interface Sci. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Martín-Garrido, M.; Martínez-Ramírez, S. CO2 adsorption on calcium silicate hydrate gel synthesized by double decomposition method. J. Therm. Anal. Calorim. 2020, in press. [Google Scholar]
- Methods for Chemical Analysis of Bauxite–Part 3: Determination of Silicon Dioxide Content–Molybdenum Blue Photometric Method; YS/T 575.3–2007; National Development and Reform Commission: Beijing, China, 2007.
- Chemical Analysis Methods and Physical Properties of Cryolite–Part 2: Determination of Ignition Loss; YS/T 273.2–2006; National Development and Reform Commission: Beijing, China, 2006.
- Yoldi, M.; Fuentes-Ordoñez, E.G.; Korili, S.A.; Gil, A. Efficient recovery of aluminum from saline slag wastes. Miner. Eng. 2019, 140, 105884. [Google Scholar] [CrossRef]
- Ma, J.Y.; Li, Z.B.; Zhang, Y.; Demopoulos, G.P. Desilication of sodium aluminate solution by Friedel′s salt (FS: 3CaO·A12O3·CaCl2·10H2O). Hydrometallurgy 2009, 99, 225–230. [Google Scholar] [CrossRef]
- Sun, H.L.; Wang, B.; Zhang, J.X.; Zong, S.F.; Liu, J.J. Secondary reaction mechanism of leaching process of calcium aluminate slag. Trans. Nonferr. Met. Soc. China 2015, 25, 1334–1340. [Google Scholar] [CrossRef]
- Synthetic Cryolite; GB/T 4291–2017; General Administration of Quality Supervision, Inspection & Quarantine of the People’s Republic of China (AQSIQ), Standardization Administration of the People’s Republic of China (SAC): Beijing, China, 2017.
- Bénézeth, P.; Palmer, D.A.; Anovitz, L.M.; Horita, J. Dawsonite synthesis and reevaluation of its thermodynamic properties from solubility measurements: Implications for mineral trapping of CO2. Geochim. Cosmochim. Acta 2007, 71, 4438–4455. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, C.W.; Lin, P.H.; Li, C.W.; Liang, Y.M.; Liu, J.C.; Chen, S.S. Fluoride recovery from spent fluoride etching solution through crystallization of Na3AlF6 (synthetic cryolite). Sep. Purif. Technol. 2014, 137, 53–58. [Google Scholar] [CrossRef]
- Wan, B.B.; Li, W.F.; Liu, F.F.; Lu, T.W.; Jin, S.X.; Wang, K.; Yi, A.H.; Tian, J.; Chen, W.P. Determination of fluoride component in the multifunctional refining flux used for recycling aluminum scrap. J. Mater. Res. Technol. 2020, 9, 3447–3459. [Google Scholar] [CrossRef]
- Veryasov, G.; Matsumoto, K.; Hagiwara, R. The discrete AlF52− fluoroaluminate anion in the structure of [Tetraethylammonium]2[AlF5](H2O)2. Eur. J. Inorg. Chem. 2015, 32, 5306–5310. [Google Scholar] [CrossRef] [Green Version]












| Element | O | Al | Si | Na | Mg | F | Cl | K | Ca |
| Content | 31.68 | 42.87 | 3.54 | 5.71 | 1.68 | 2.06 | 7.56 | 2.39 | 1.22 |
| Element | Fe | Ti | Cu | Mn | S | Sr | Zn | Cr | Hg |
| Content | 0.19 | 0.41 | 0.35 | 0.17 | 0.09 | 0.02 | 0.02 | 0.03 | 0.01 |
| Stages | Major Components (g/L) | ||
|---|---|---|---|
| Al2O3 | Na2O | SiO2 | |
| Before the purification | 48.16 | 73.84 | 2.41 |
| After the purification | 43.52 | 71.59 | 0.097 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, B.; Li, W.; Sun, W.; Liu, F.; Chen, B.; Xu, S.; Chen, W.; Yi, A. Synthesis of Cryolite (Na3AlF6) from Secondary Aluminum Dross Generated in the Aluminum Recycling Process. Materials 2020, 13, 3871. https://doi.org/10.3390/ma13173871
Wan B, Li W, Sun W, Liu F, Chen B, Xu S, Chen W, Yi A. Synthesis of Cryolite (Na3AlF6) from Secondary Aluminum Dross Generated in the Aluminum Recycling Process. Materials. 2020; 13(17):3871. https://doi.org/10.3390/ma13173871
Chicago/Turabian StyleWan, Bingbing, Wenfang Li, Wanting Sun, Fangfang Liu, Bin Chen, Shiyao Xu, Weiping Chen, and Aihua Yi. 2020. "Synthesis of Cryolite (Na3AlF6) from Secondary Aluminum Dross Generated in the Aluminum Recycling Process" Materials 13, no. 17: 3871. https://doi.org/10.3390/ma13173871




