Chloride Distribution and Steel Corrosion in a Concrete Bridge after Long-Term Exposure to Natural Marine Environment
Abstract
:1. Introduction
2. Experimental Program
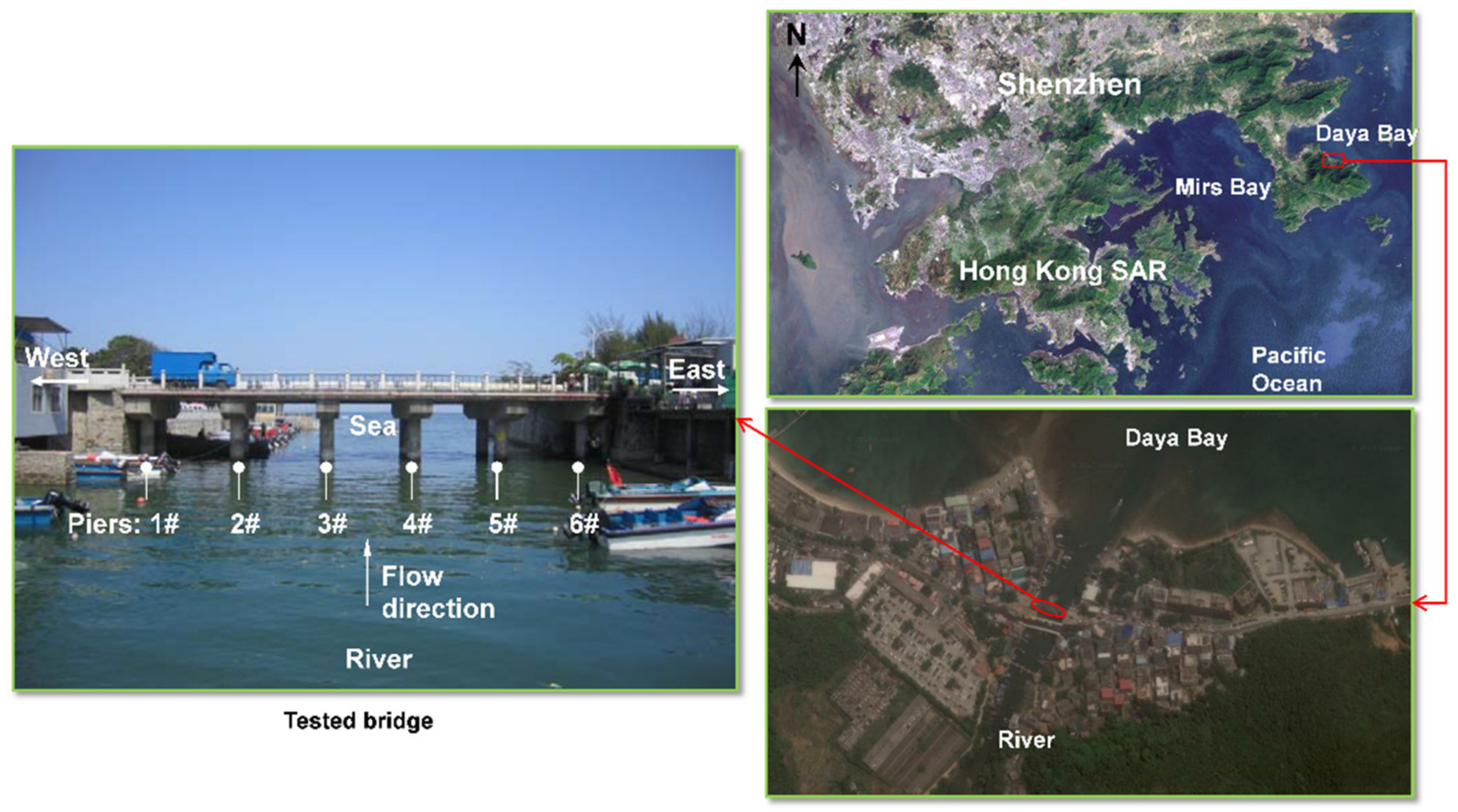
2.1. Description of the Tested Bridge
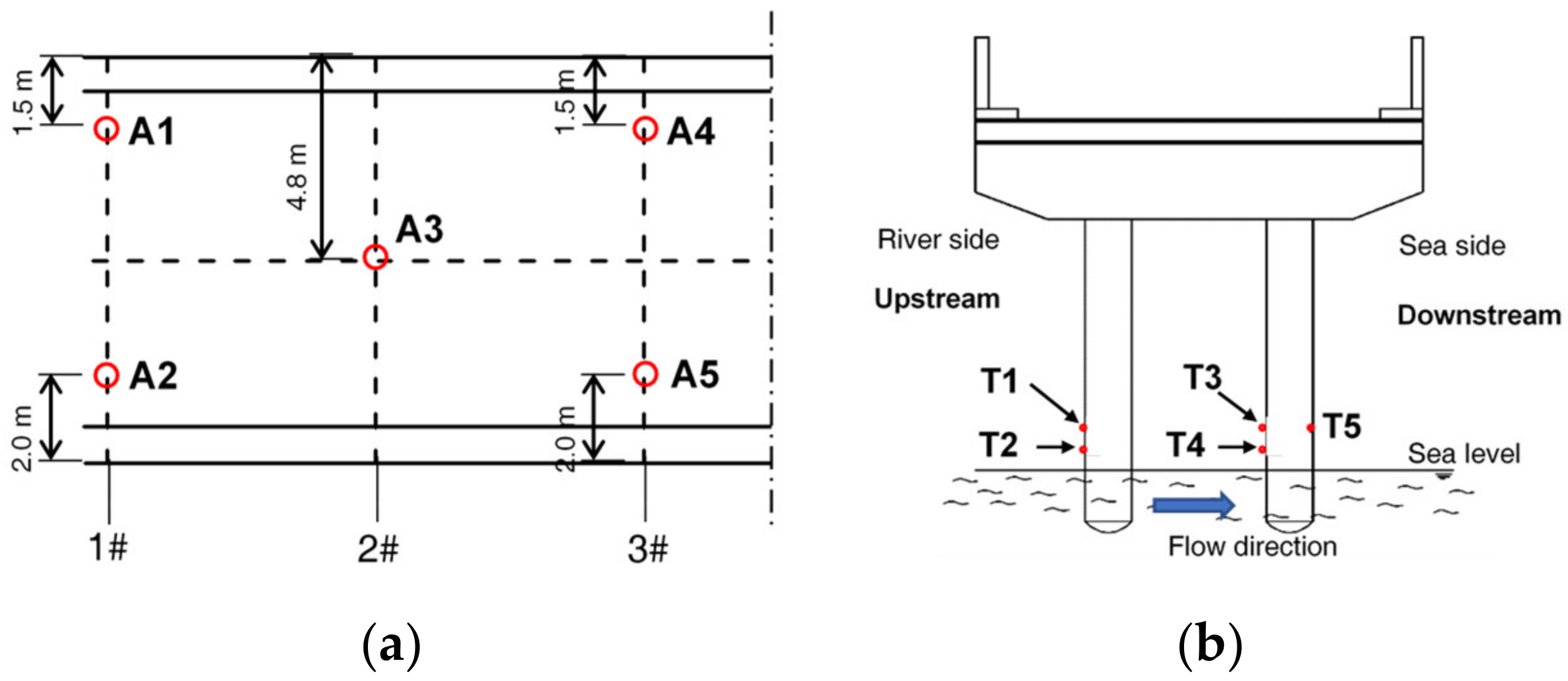
2.2. Concrete and Steel Samples
2.3. Measurement of Chloride Ion Content
2.4. Microscopy Tests
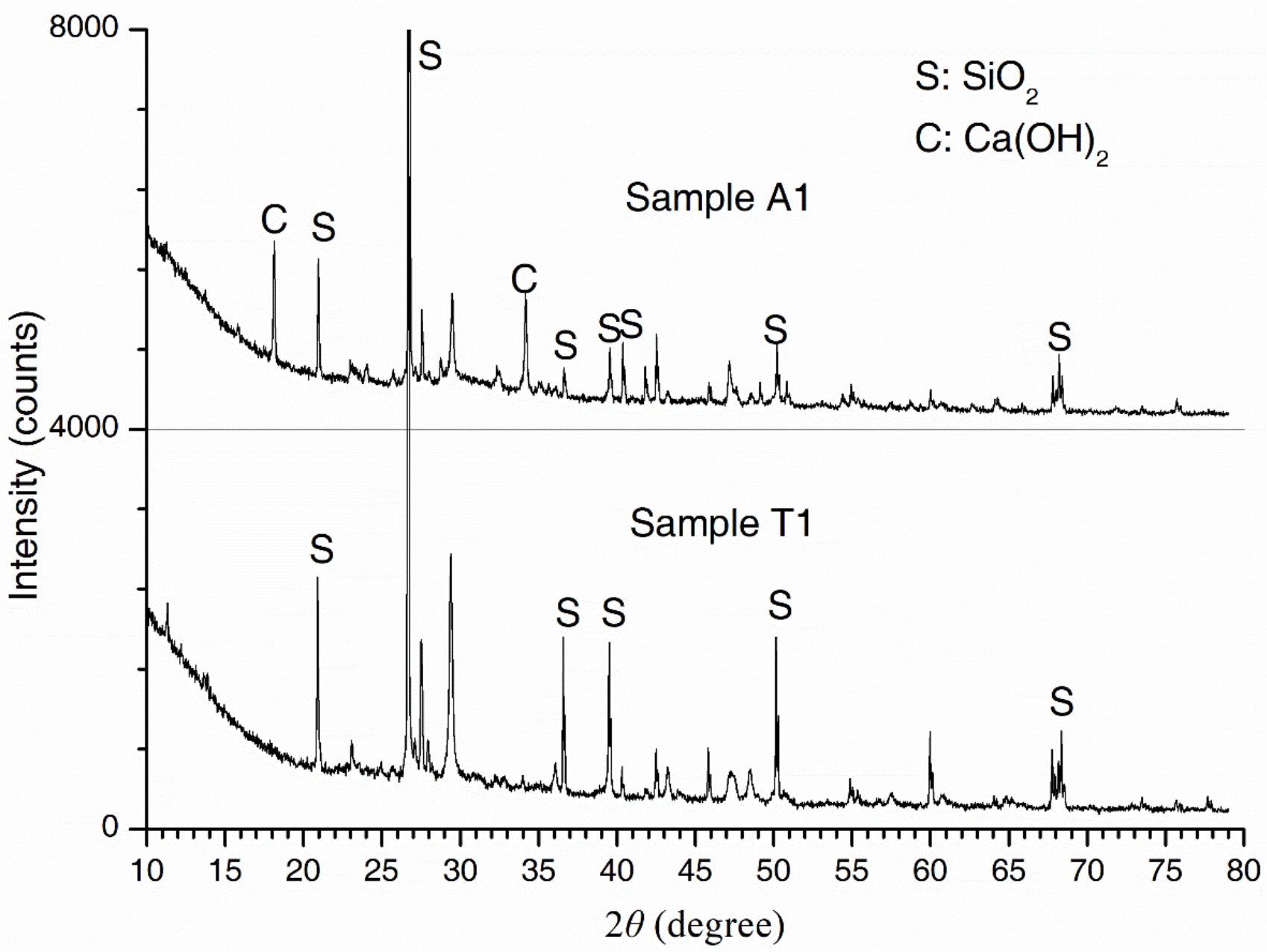
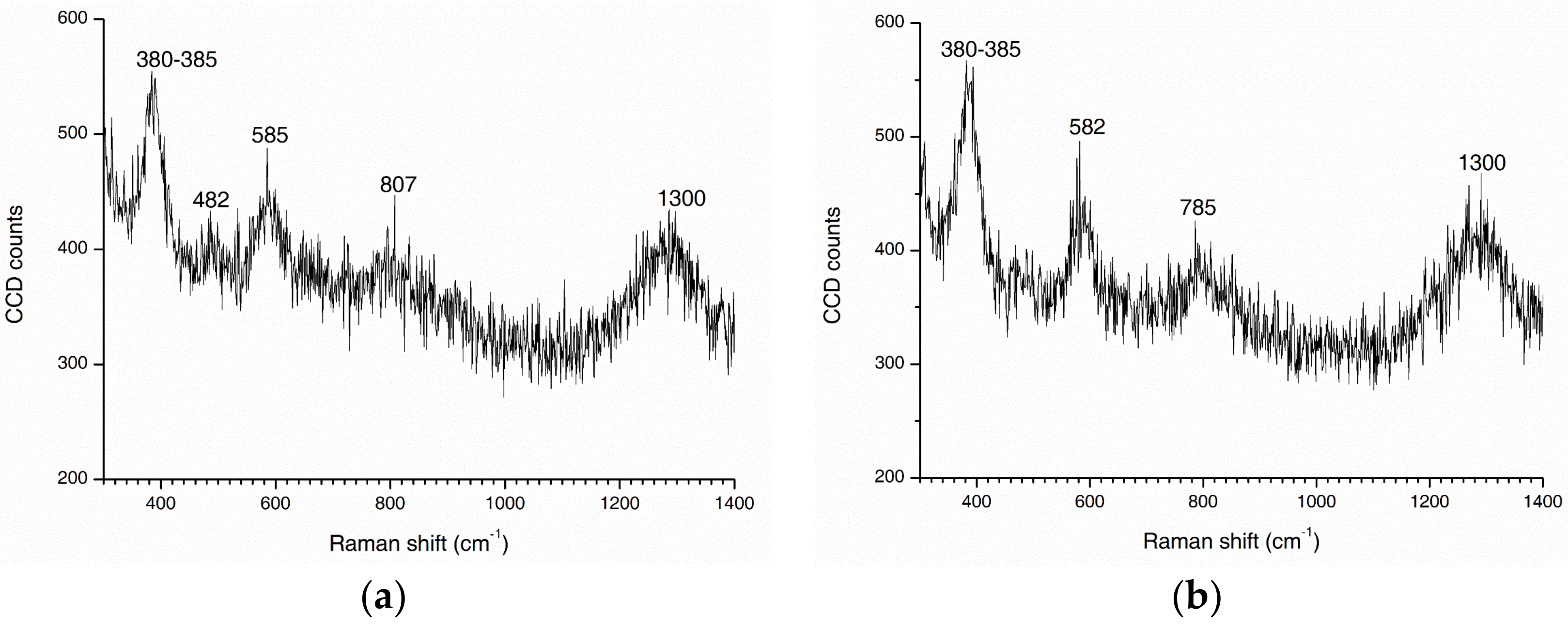
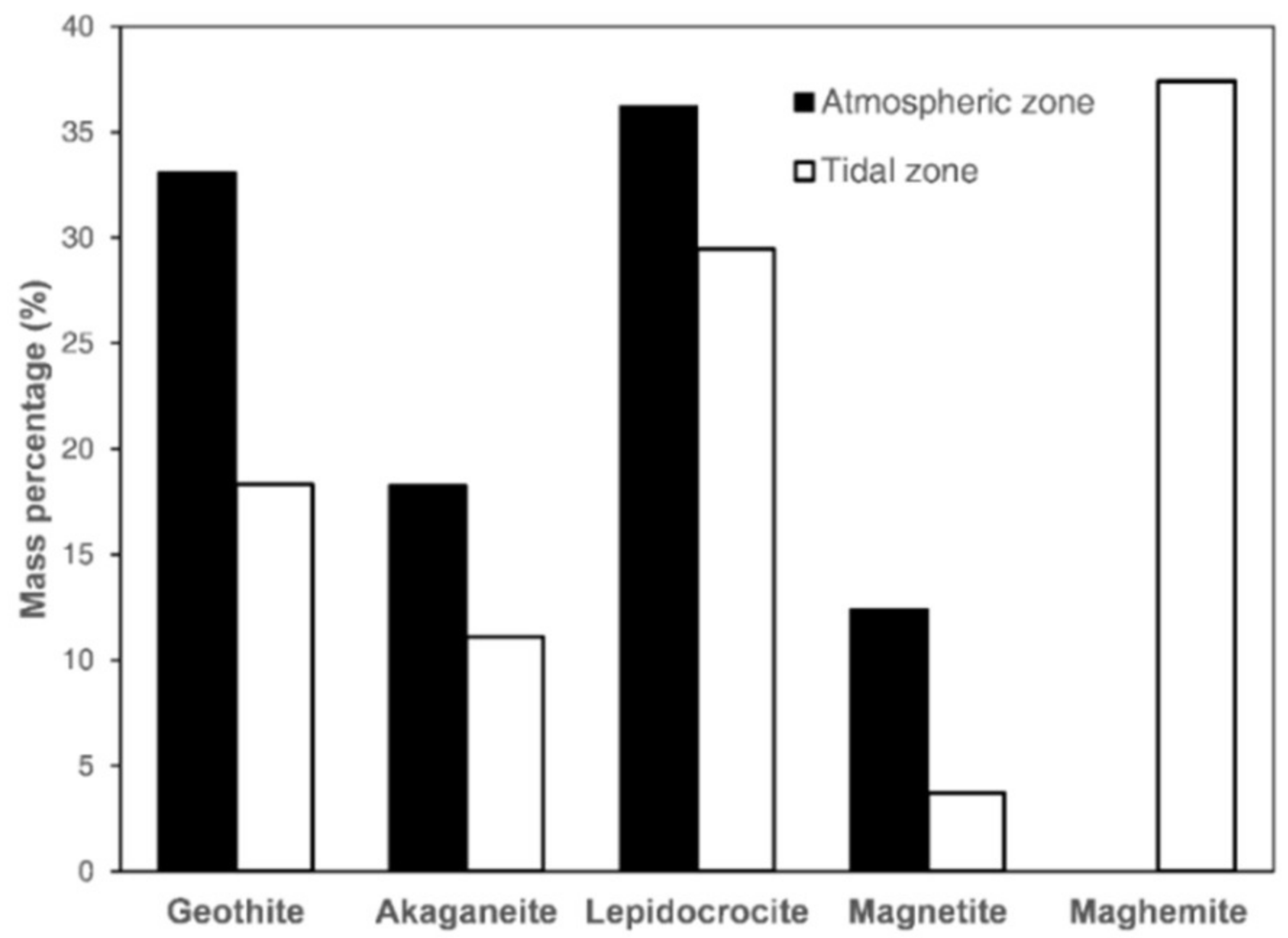
2.5. Chemical Phase Analysis
3. Results and Discussion
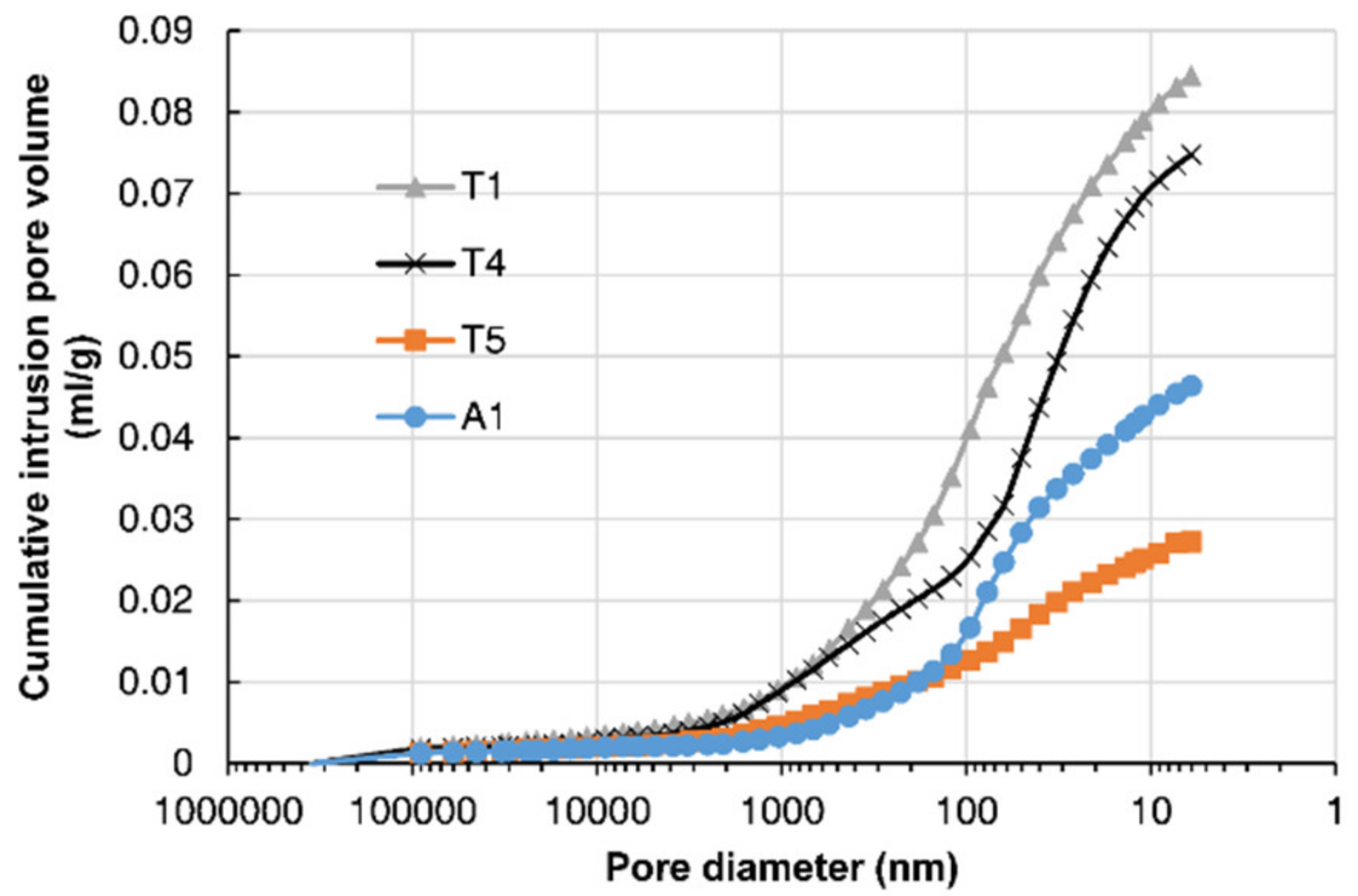
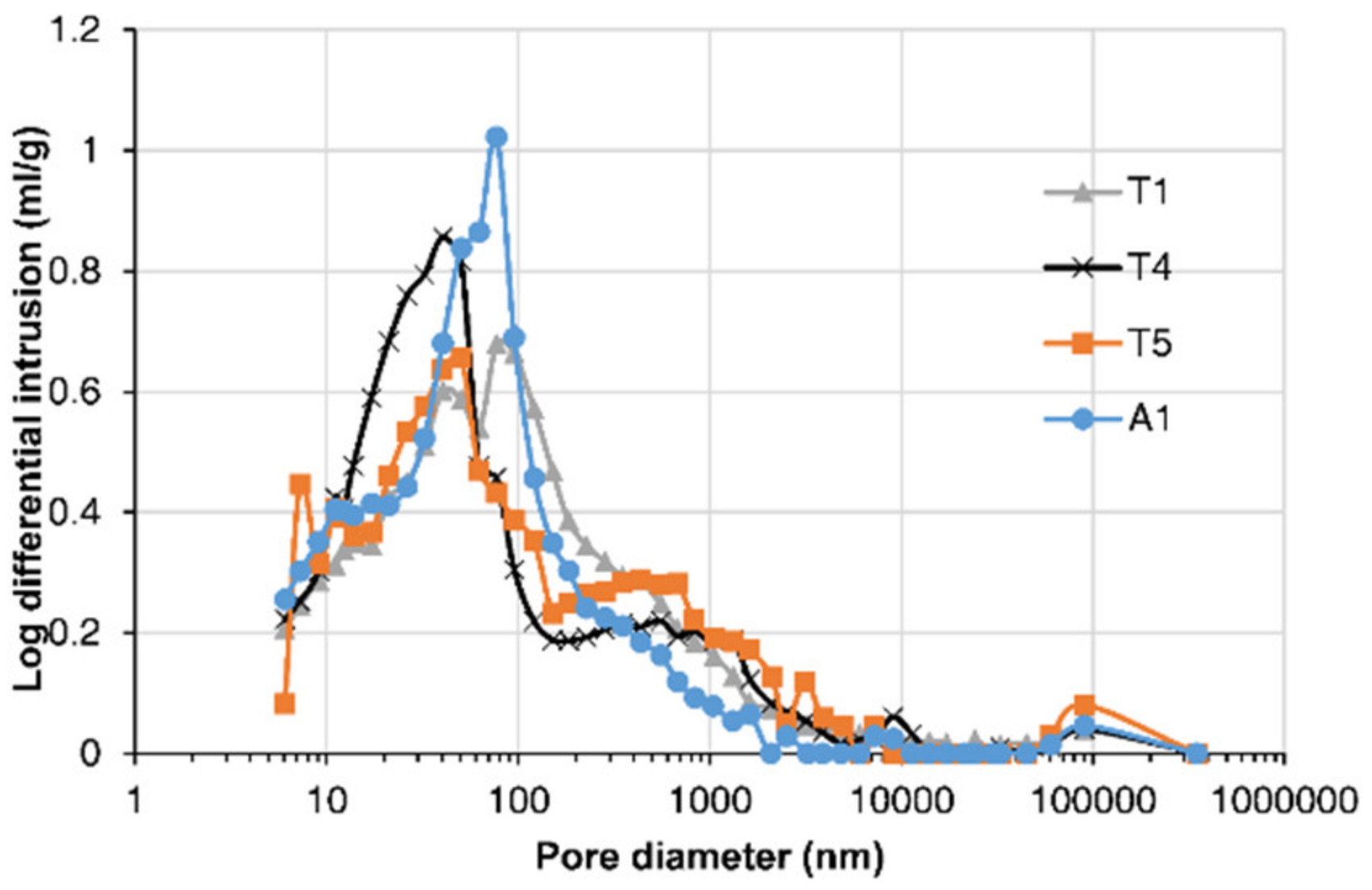
3.1. Concrete Microstructure
3.2. Chloride Distribution within the Concrete Cover
3.3. Evaluated Chloride Diffusivity and Surface Chloride Concentration
3.4. Steel Corrosion
4. Conclusions
- The porosity of concrete in a pier column facing upstream was greater due to the water impact and CH leaching. Concrete with a higher chloride content had lower porosity and a larger proportion of small pores.
- The convection zone depths of the chloride profiles were all in the range of 6–7 mm, except that the convection depths for the cracked concrete were 22–24 mm.
- The coefficients of variability of evaluated concrete chloride diffusivity for the bridge deck and the pier column were significantly different.
- The rust phases were primarily lepidocrocite and goethite in the atmospheric zone and lepidocrocite and maghemite in the tidal zone. This may imply that transformation of magnetite to maghemite occurred for a greater degree of corrosion.
Author Contributions
Funding
Conflicts of Interest
References
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Gao, X.J.; Wang, X.Y. Impacts of Global warming and sea level rise on service life of chloride-exposed concrete structure. Sustainability 2017, 9, 460. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Appleton, J. Case studies of concrete deterioration in a marine environment in Portugal. Cem. Concr. Compos. 2002, 24, 169–179. [Google Scholar] [CrossRef]
- Shafei, B.; Alipour, A.; Shinozuka, M. Prediction of corrosion initiation in reinforced concrete members subjected to environmental stressors: A finite-element framework. Cem. Concr. Res. 2012, 42, 365–376. [Google Scholar] [CrossRef]
- Al Mutlaq, F.M.; Page, C.L. Effects of electric arc furnace dust on susceptibility of steel to corrosion in chloride-contaminated concrete. Constr. Build. Mater. 2013, 39, 60–64. [Google Scholar] [CrossRef]
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. 2018, 51, 4. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Zeng, C.; Li, Y.; Li, L.; Li, P.; Li, D. The constitutive relationship for corroded steel bars: Model and analysis. Materials 2019, 12, 4058. [Google Scholar] [CrossRef] [Green Version]
- Bossio, A.; Imperatore, S.; Kioumarsi, M. Ultimate flexural capacity of reinforced concrete elements damaged by corrosion. Buildings 2019, 9, 160. [Google Scholar] [CrossRef] [Green Version]
- Tuutti, K. Corrosion of steel in concrete. Cem. Concr. Res. 1982, 4, 468–472. [Google Scholar]
- Zhang, Y.; Jin, W. Distribution of chloride accumulation in marine tidal zone along altitude. ACI Mater. J. 2011, 108, 467–475. [Google Scholar] [CrossRef]
- Liu, J.; Ou, G.; Qiu, Q.; Chen, X.; Hong, J.; Xing, F. Chloride transport and microstructure of concrete with/without fly ash under atmospheric chloride condition. Constr. Build. Mater. 2017, 146, 493–501. [Google Scholar] [CrossRef]
- McGee, R. Modelling of durability performance of Tasmanina bridges. In Applications of Statistics and Probability in Civil Engineering; Melchers, R.E., Stewart, M., Eds.; Balkema: Rotterdam, The Netherlands, 2000; pp. 297–306. [Google Scholar]
- Liu, J.; Tang, K.; Pan, D.; Lei, Z.; Wang, W.; Xing, F. Surface chloride concentration of concrete under shallow immersion conditions. Materials 2014, 7, 6620–6631. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, Q.; Chen, X.; Wang, X.; Xing, F.; Han, N.; He, Y. Degradation of fly ash concrete under the coupled effect of carbonation and chloride aerosol ingress. Corros. Sci. 2016, 112, 364–372. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, Q.; Chen, X.; Xing, F.; Han, N.; He, Y.; Ma, Y. Understanding the interacted mechanism between carbonation and chloride aerosol attack in ordinary Portland cement concrete. Cem. Concr. Res. 2017, 95, 217–225. [Google Scholar] [CrossRef]
- Küter, A. Management of Reinforcement Corrosion—A Thermodynamic Approach. Ph.D. Thesis, Technical University of Denmark, Copenhagen, Denmark, July 2009. [Google Scholar]
- Strehblow, H.H. Mechanisms of Pitting Corrosion. In Corrosion Mechanisms in Theory and Practice; Marcus, P., Ed.; Marcel Dekker Inc.: New York, NY, USA, 2002. [Google Scholar]
- Kayyali, O.A.; Haque, M.N. The C1−/OH− ratio in chloride-contaminated concrete—A most important criterion. Mag. Concr. Res. 1995, 47, 235–242. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Cao, Y.; Gehlen, C.; Angst, U.; Wang, L.; Wang, Z.; Yao, Y. Critical chloride content in reinforced concrete—An updated review considering Chinese experience. Cem. Concr. Res. 2019, 117, 58–68. [Google Scholar] [CrossRef]
- Kenny, A.; Katz, A. Steel-concrete interface influence on chloride threshold for corrosion—Empirical reinforcement to theory. Constr. Build. Mater. 2020, 224, 118376. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The mechanism of formation of iron oxide and oxyhydroxides in aqueous solutions at room temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Wang, G.; Wu, Q.; Li, X.Z.; Xu, J.; Xu, Y.; Shi, W.H.; Wang, S.L. Microscopic analysis of steel corrosion products in seawater and sea-sand concrete. Materials 2019, 12, 3330. [Google Scholar] [CrossRef] [Green Version]
- Criado, M.; Martínez-Ramirez, S.; Bastidas, J.M. A Raman spectroscopy study of steel corrosion products in activated fly ash mortar containing chlorides. Constr. Build. Mater. 2015, 96, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Poupard, O.; L’Hostis, V.; Catinaud, S.; Petre-Lazar, I. Corrosion damage diagnosis of a reinforced concrete beam after 40 years natural exposure in marine environment. Cem. Concr. Res. 2006, 36, 504–520. [Google Scholar] [CrossRef]
- Zhu, W.; François, R.; Fang, Q.; Zhang, D. Influence of long-term chloride diffusion in concrete and the resulting corrosion of reinforcement on the serviceability of RC beams. Cem. Concr. Compos. 2016, 71, 144–152. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, Y.; Zhang, L.; Bao, Z. Laboratory studies on influence of transverse cracking on chloride-induced corrosion rate in concrete. Cem. Concr. Compos. 2016, 69, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Laurens, S.; Hénocq, P.; Rouleau, N.; Deby, F.; Samson, E.; Marchand, J.; Bissonnette, B. Steady-state polarization response of chloride-induced macrocell corrosion systems in steel reinforced concrete—Numerical and experimental investigations. Cem. Concr. Res. 2016, 79, 272–290. [Google Scholar] [CrossRef]
- Duffó, G.S.; Morris, W.; Raspini, I.; Saragovi, C. A study of steel rebars embedded in concrete during 65 years. Corros. Sci. 2004, 46, 2143–2157. [Google Scholar] [CrossRef]
- Duffó, G.S.; Reinoso, M.; Ramos, C.P.; Farina, S.B. Characterization of steel rebars embedded in a 70-year old concrete structure. Cem. Concr. Res. 2012, 42, 111–117. [Google Scholar] [CrossRef]
- Gartner, N.; Kosec, T.; Legat, A. Monitoring the corrosion of steel in concrete exposed to a marine environment. Materials 2020, 13, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattah, A.A.E.; Al-Duais, I.; Riding, K.; Thomas, M. Al-Dulaijan, S.; Al-Zahrani, M. Field validation of concrete transport property measurement methods. Materials 2020, 13, 1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otieno, M.; Beushausen, H.; Alexander, M. Chloride-induced corrosion of steel in cracked concrete—Part I: Experimental studies under accelerated and natural marine environments. Cem. Concr. Res. 2016, 79, 373–385. [Google Scholar] [CrossRef]
- Younis, A.; Ebead, U.; Suraneni, P.; Nanni, A. Fresh and hardened properties of seawater-mixed concrete. Constr. Build. Mater. 2018, 190, 276–286. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Wang, F. Corrosion of low carbon steel in atmospheric environments of different chloride content. Corros. Sci. 2009, 112, 997–1006. [Google Scholar] [CrossRef]
- American Association of State Highway and Transportation Officials. Standard Method of Test for Sampling and Testing for Chloride Ion in Concrete and Concrete Raw Materials: T206-297 AASHTO; American Association of State Highway and Transportation Officials: Washington, DC, USA, 2009. [Google Scholar]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Dubois, F.; Mendibide, C.; Pagnier, T.; Perrard, F.; Duret, C. Raman mapping of corrosion products formed onto spring steels during salt spray experiments. A correlation between the scale composition and the corrosion resistance. Corros. Sci. 2008, 50, 3401–3409. [Google Scholar] [CrossRef]
- Liu, J.; Tang, K.; Qiu, Q.; Pan, D.; Lei, Z.; Xing, F. Experimental investigation on pore structure characterization of concrete exposed to water and chlorides. Materials 2014, 7, 6646–6659. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.; Jin, N.; Jin, X.; Fu, C. Model of chloride penetration into cracked concrete subject to drying-wetting cycles. Constr. Build. Mater. 2012, 36, 259–269. [Google Scholar] [CrossRef]
- Han, S.H. Influence of diffusion coefficient on chloride ion penetration of concrete structure. Constr. Build. Mater. 2007, 21, 370–378. [Google Scholar] [CrossRef]
- Yuan, Q. Fundamental Studies on Test Methods for the Transport of Chloride Ions in Cementitious Materials. Ph.D. Thesis, Central South University, Changsha, China, June 2009. [Google Scholar]
- Collepard, M.; Marcialis, A.; Turriziani, R. Penetration of chloride ions into cement pastes and concretes. J. Am. Ceram. Soc. 1972, 55, 534–535. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1979. [Google Scholar]
- Zhang, H.; Zhang, W.; Gu, X.; Jin, X.; Jin, N. Chloride penetration in concrete under marine atmospheric environment-analysis of the influencing factors. Struct. Infrastruct. Eng. 2016, 12, 1428–1438. [Google Scholar] [CrossRef]
- Yang, C.C. On the relationship between pore structure and chloride diffusivity from accelerated chloride migration test in cement-based materials. Cem. Concr. Res. 2006, 36, 1304–1311. [Google Scholar] [CrossRef]
- Salta, M.; Melo, A.; Ricardo, J.; Povoa, A. Chloride profiles in a coastal bridge. Struct. Infrastruct. Eng. 2012, 8, 583–594. [Google Scholar] [CrossRef]
- Razvan, A.; Raman, A. Morphology of rust phases formed on naturally weathered weathering steels in bridge spans. Metallography 1986, 23, 223–236. [Google Scholar]
- De la Fuente, D.; Alcántara, J.; Chico, B.; Díaz, I.; Jiménez, J.A.; Morcillo, M. Characterisation of rust surfaces formed on mild steel exposed to marine atmospheres using XRD and SEM/Micro-Raman techniques. Corros. Sci. 2016, 110, 253–264. [Google Scholar] [CrossRef]
- Morcillo, M.; Alcantara, J.; Diaz, I.; Chico, B.; Simancas, J.; Fuente, D. Marine atmospheric corrosion of carbon steels. Rev. Metal. 2015, 51, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Ren, H.; Dai, H.; Jin, W. Composition and expansion coefficient of rust based on X-ray diffraction and thermal analysis. Corros. Sci. 2011, 53, 1646–1658. [Google Scholar] [CrossRef]







| Condition | Value |
|---|---|
| Seawater chlorinity | 12.93‰ |
| Chloride content in atmosphere | 0.05–0.12 mg/m3 |
| Temperature | Annual average: 22.5 °C Range: 8.7–36.8 °C |
| Annual average relative humidity | 77% Range: 55–80% |
| Annual average rainfall | 1846 mm Range: 913–2662 mm |
| Prevailing wind direction | Southeast (SE) |
| Annual average wind speed | 3.0 m/s Range: 2.5–3.3 m/s |
| Specimen Number | Chloride Diffusivity (×10−12 m2/s) | Surface Chloride Concentration (%) | Coefficients of Determination R2 |
|---|---|---|---|
| A1 | 4.15 | 0.086 | 0.880 |
| A2 | 4.10 | 0.068 | 0.904 |
| A3 | 1.24 | 0.094 | 0.956 |
| A4 | 4.26 | 0.052 | 0.895 |
| A5 | 7.59 | 0.080 | 0.978 |
| T1 | 8.93 | 0.706 | 0.990 |
| T2 | 2.69 | 0.650 | 0.866 |
| T3 | 1.77 | 1.100 | 0.939 |
| T4 | 1.81 | 1.094 | 0.904 |
| T5 | 3.82 | 0.892 | 0.848 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Jiang, Z.; Zhao, Y.; Zhou, H.; Wang, X.; Zhou, H.; Xing, F.; Li, S.; Zhu, J.; Liu, W. Chloride Distribution and Steel Corrosion in a Concrete Bridge after Long-Term Exposure to Natural Marine Environment. Materials 2020, 13, 3900. https://doi.org/10.3390/ma13173900
Liu J, Jiang Z, Zhao Y, Zhou H, Wang X, Zhou H, Xing F, Li S, Zhu J, Liu W. Chloride Distribution and Steel Corrosion in a Concrete Bridge after Long-Term Exposure to Natural Marine Environment. Materials. 2020; 13(17):3900. https://doi.org/10.3390/ma13173900
Chicago/Turabian StyleLiu, Jun, Zhilu Jiang, Yulong Zhao, Hao Zhou, Xiaodong Wang, Haijun Zhou, Feng Xing, Shanglin Li, Jihua Zhu, and Wei Liu. 2020. "Chloride Distribution and Steel Corrosion in a Concrete Bridge after Long-Term Exposure to Natural Marine Environment" Materials 13, no. 17: 3900. https://doi.org/10.3390/ma13173900





