pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of ZnO QDs
2.3. Synthesis and Functionalization of MSCNs
2.3.1. Synthesis of MSCNs
2.3.2. Amino Functionalization of MSCNs
2.3.3. Carboxyl Functionalization of MSCNs
2.4. Biodegradation of MSCNs in PBS Solution and in Cells
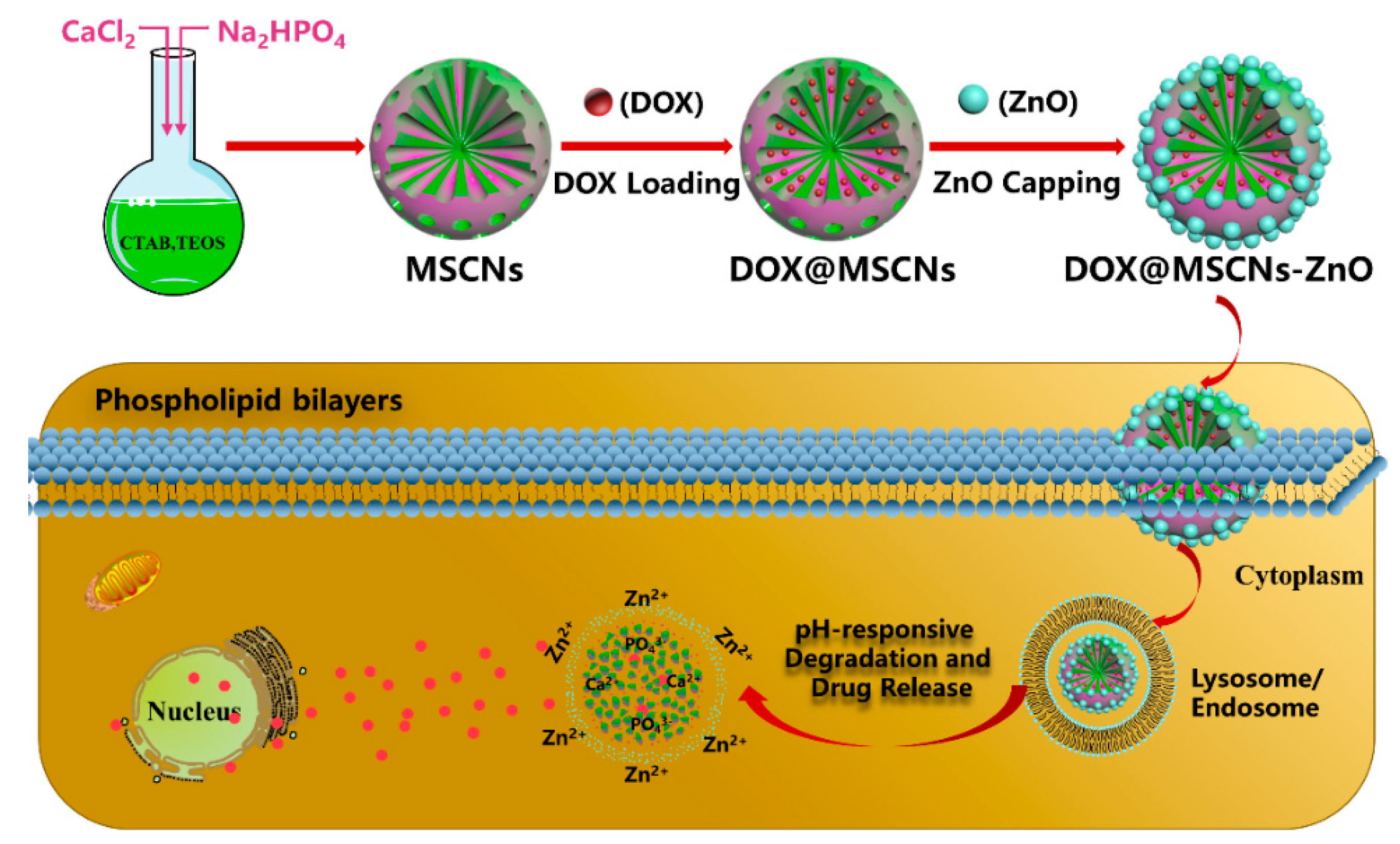
2.5. ZnO Capped DOX@MSCNs
2.5.1. Synthesis of DOX@MSCNs
2.5.2. ZnO-Capped DOX@MSCNs
2.6. In Vitro pH-Responsive Drug Release of DOX@MSCNs-ZnO
2.7. Cell Culture
2.8. Intracellular Endocytosis of DOX@MSCNs-ZnO
2.9. In Vitro Cytotoxicity Assays
2.10. Characterization of Nanoparticles
2.11. Statistical Analysis
3. Results and Discussions
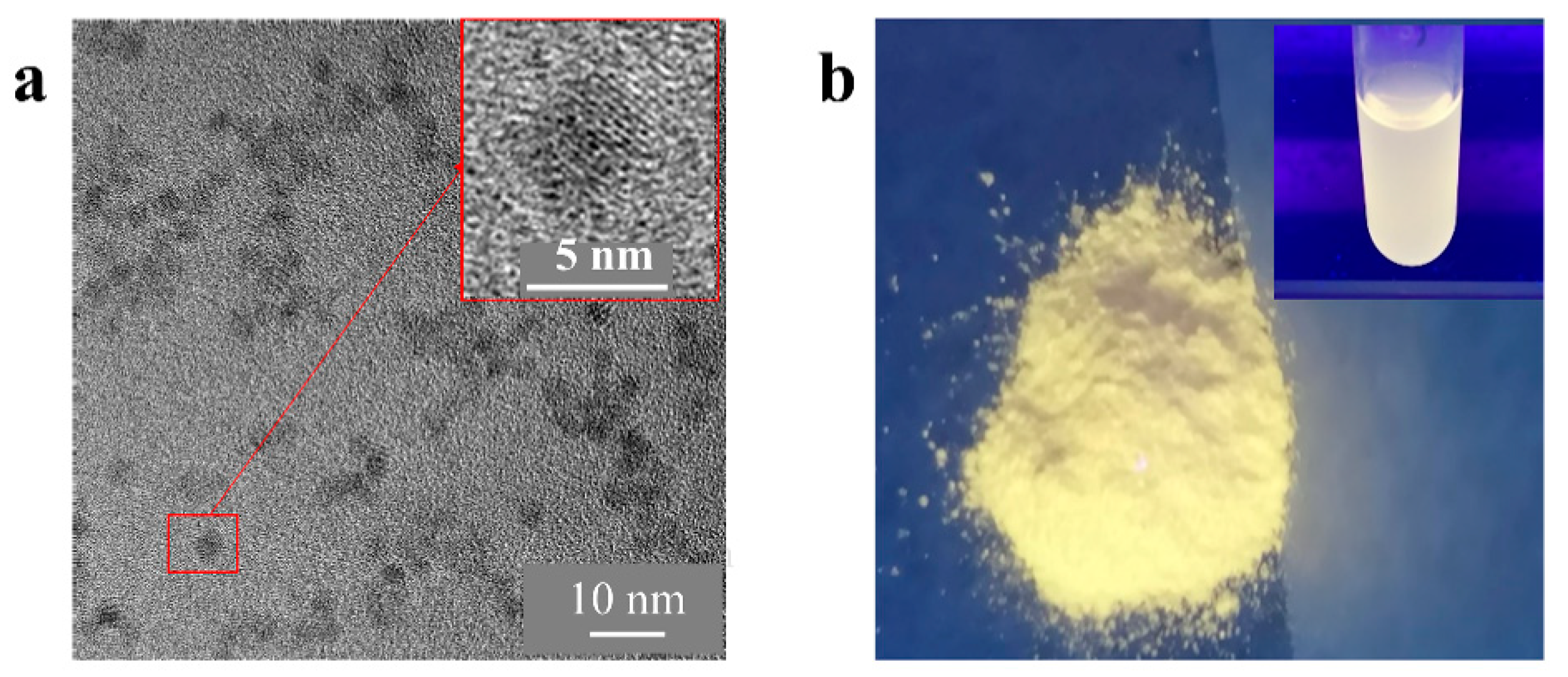
3.1. Synthesis and Characterization of ZnO QDs
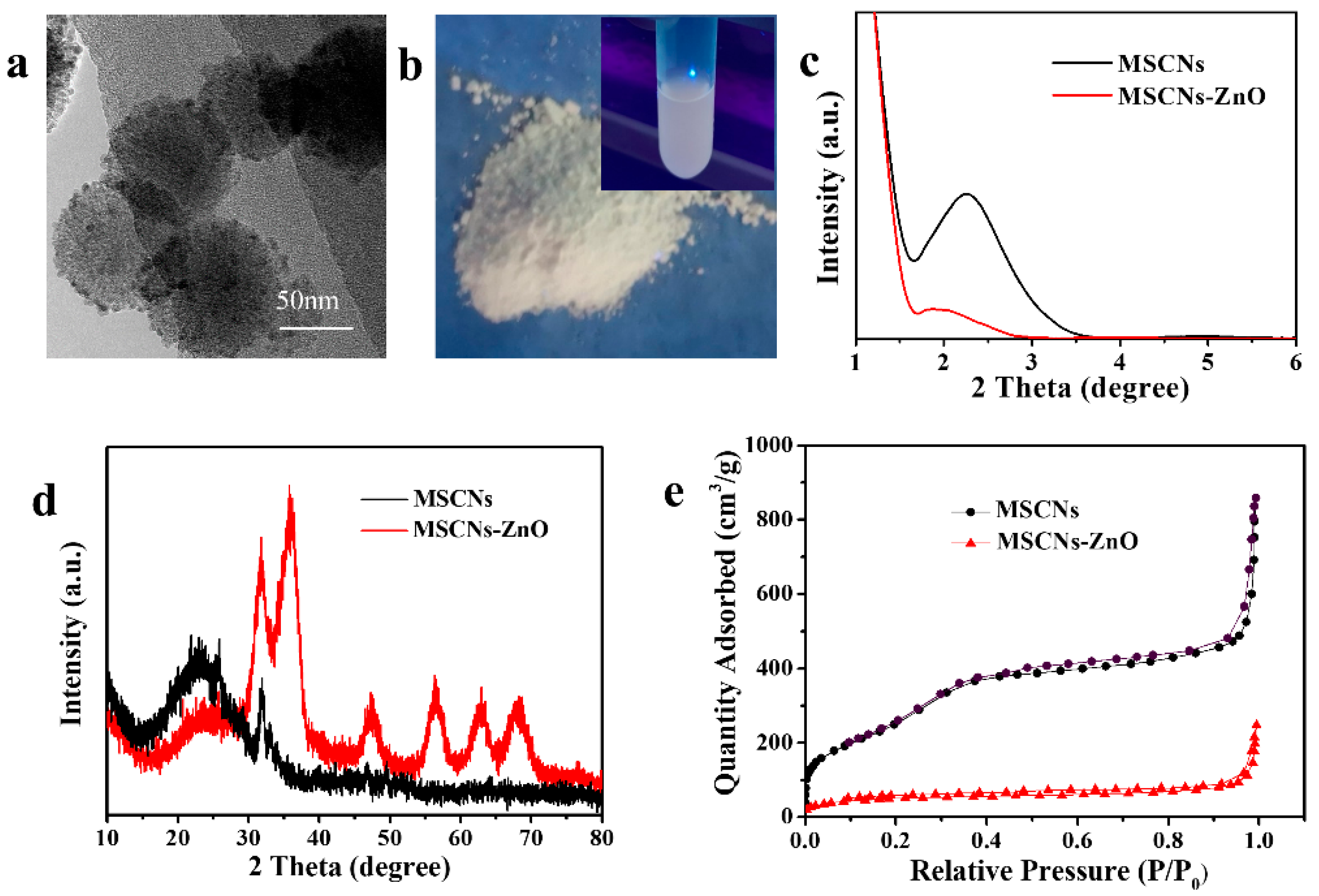
3.1.1. Morphology and Particle Size of ZnO QDs
3.1.2. Elemental and Structural Analysis of ZnO QDs
3.1.3. Acidic Degradation Behavior of ZnO QDs
3.2. Synthesis and Characterization of MSCNs
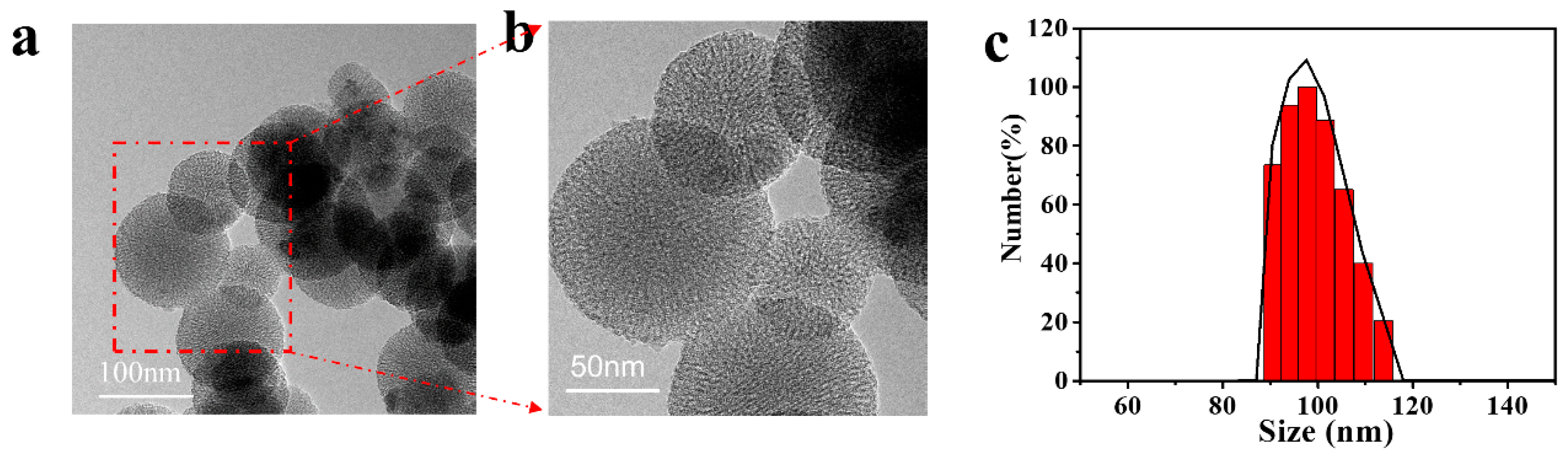
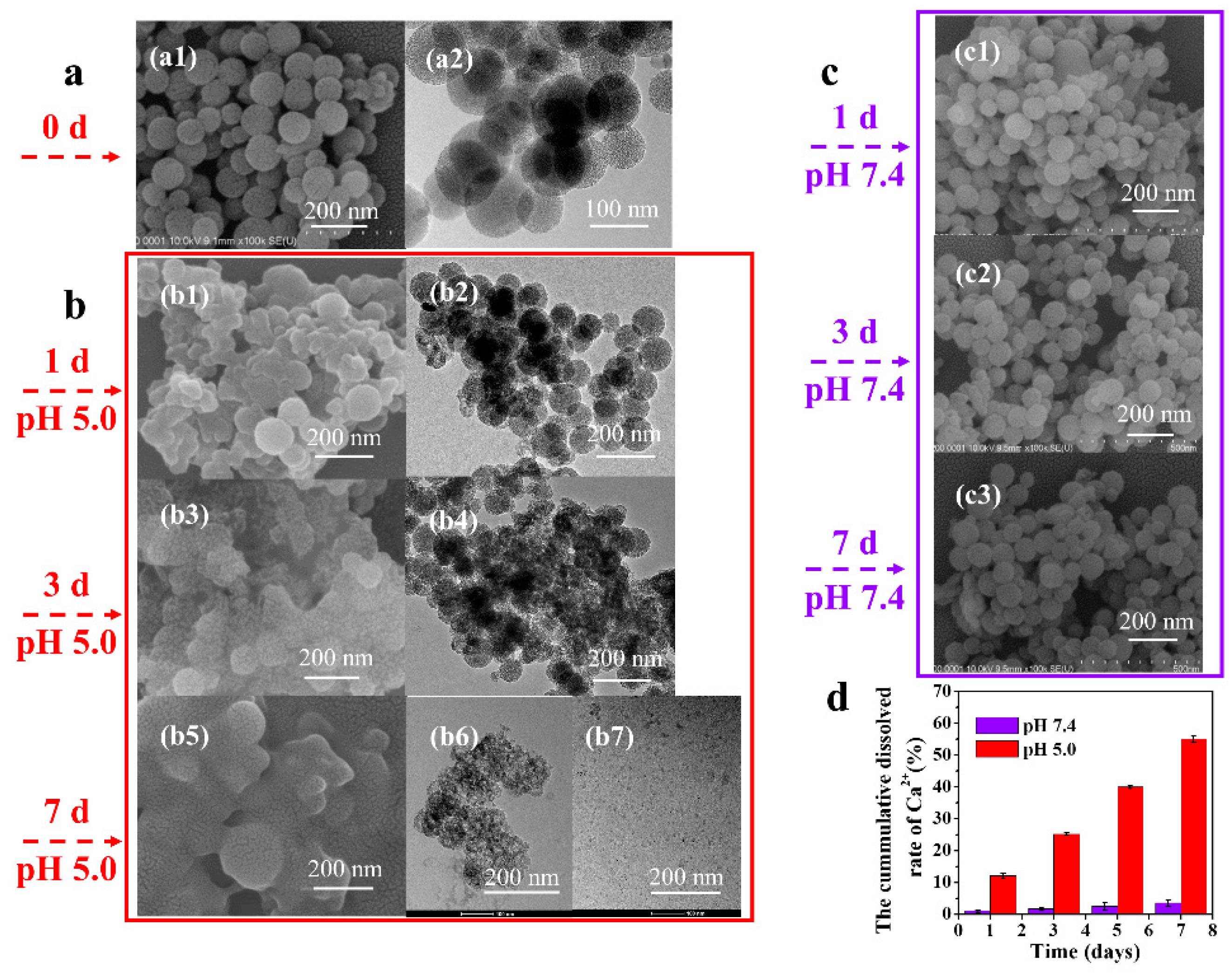
3.2.1. Morphology and Particle Size of MSCNs
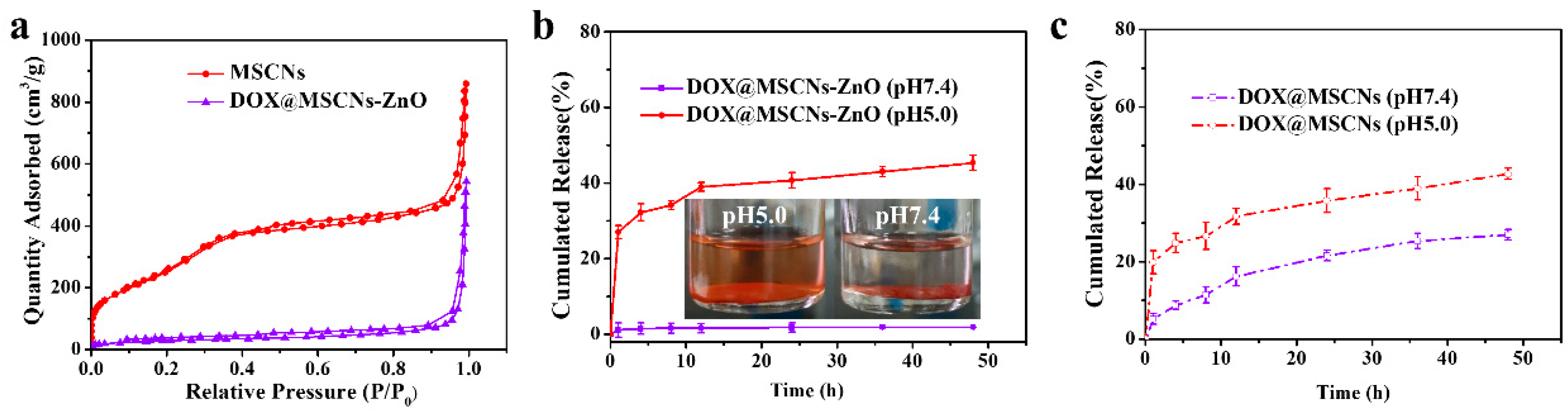
3.2.2. Structure, Specific Surface Area and Pore Diameter of MSCNs
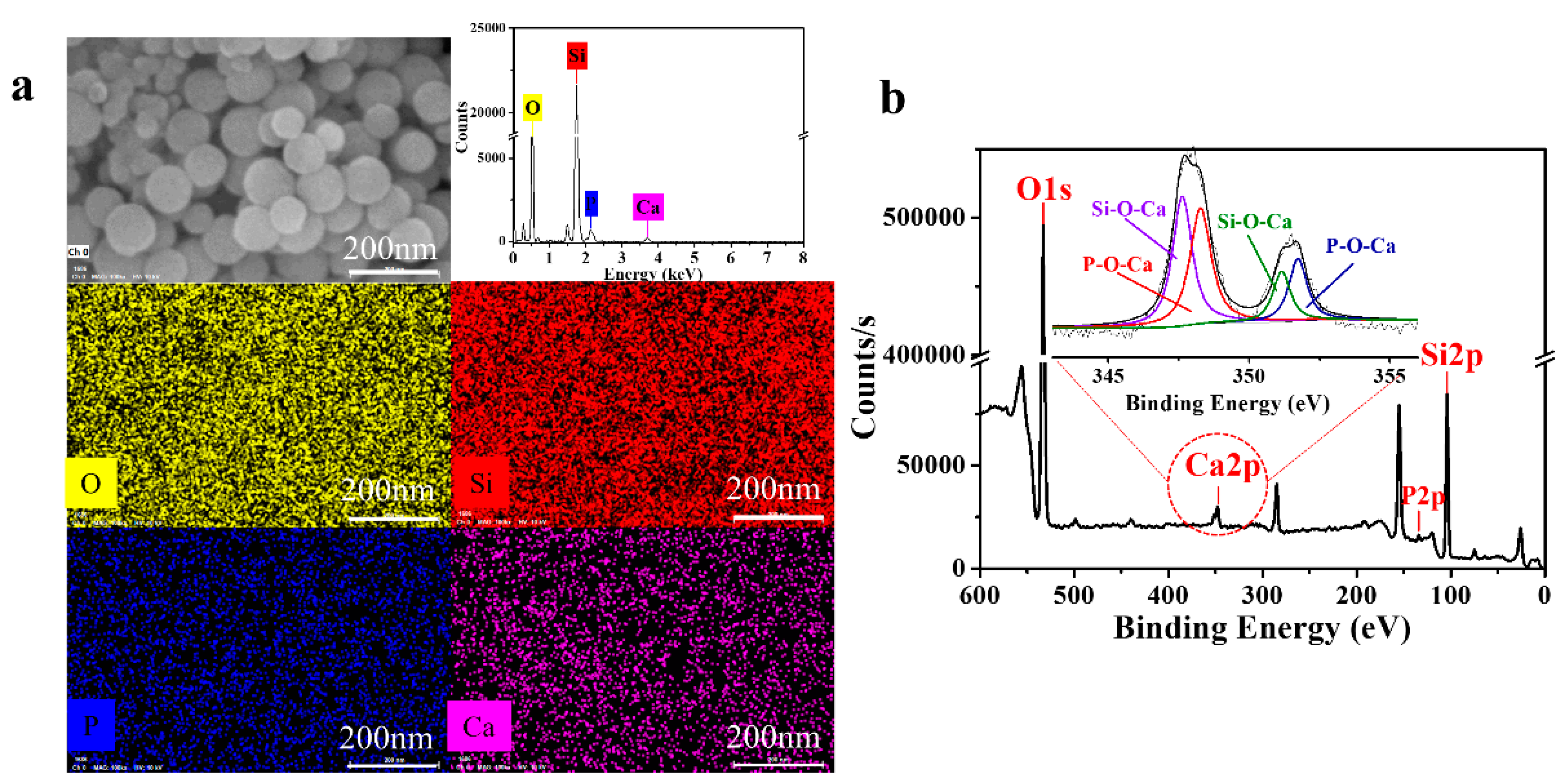
3.2.3. Elemental Analysis of MSCNs
3.3. The Biodegradation Behavior of MSCNs
3.3.1. Degradation of MSCNs in PBS Solution
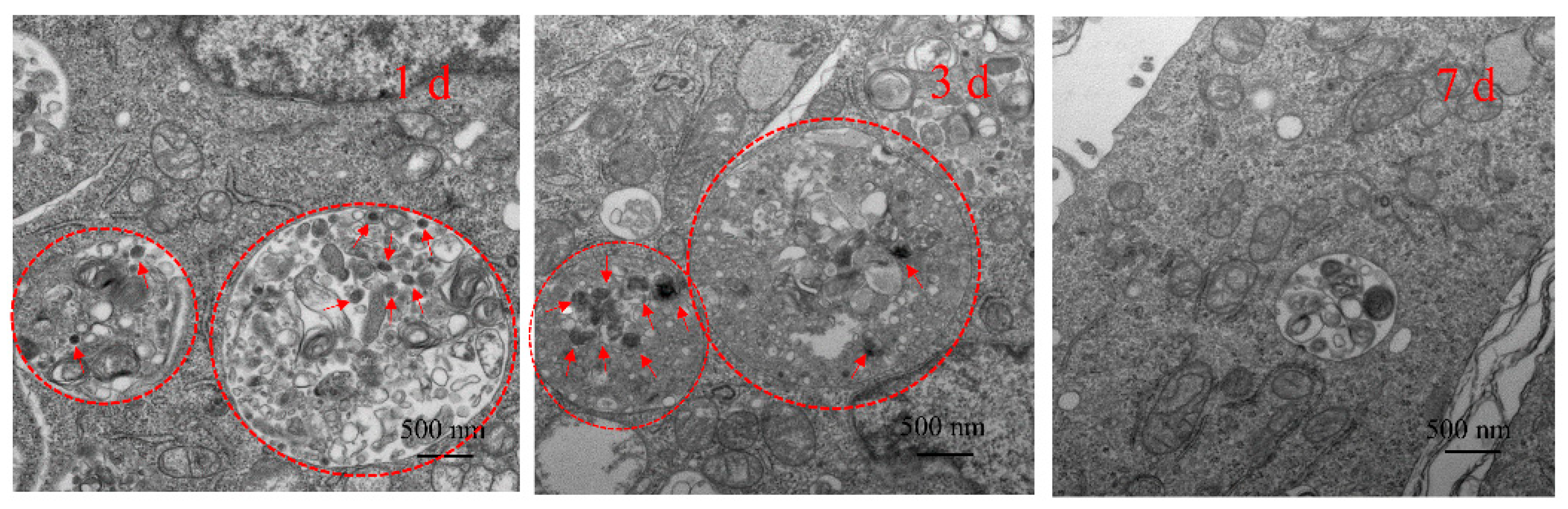
3.3.2. Degradation of MSCNs in 4T1 Cells
3.4. ZnO-Capped DOX@MSCNs
3.5. In Vitro pH-Responsive Drug Release of DOX@MSCNs-ZnO
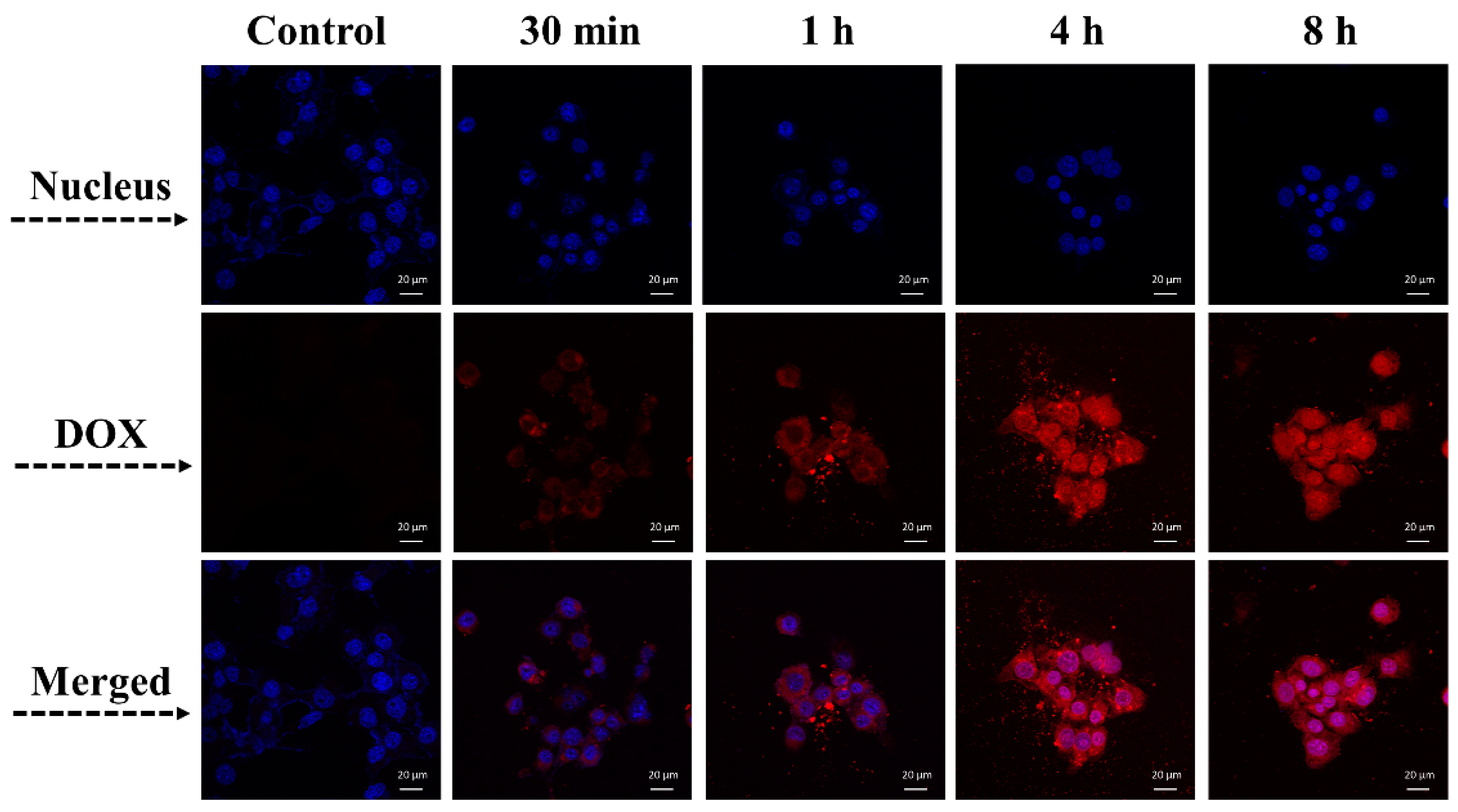
3.6. Endocytosis of DOX@MSCNs-ZnO
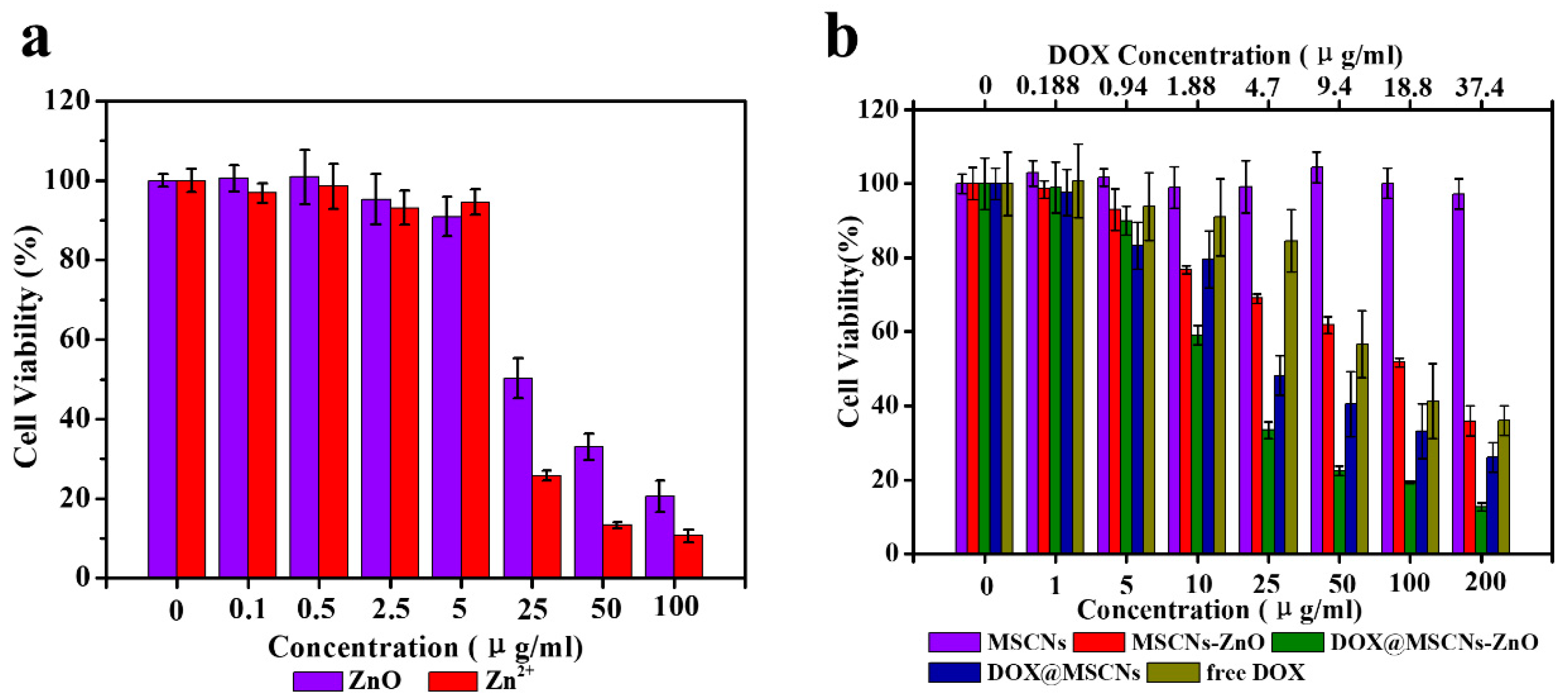
3.7. In Vitro Cell Cytotoxicity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, X.; Yao, X.; Wang, C.; Chen, L.; Chen, X. Mesoporous silica nanoparticles capped with fluorescence-conjugated cyclodextrin for pH-activated controlled drug delivery and imaging. Microporous Mesoporous Mater. 2015, 217, 46–53. [Google Scholar] [CrossRef]
- Rijt, S.H.V.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-Mediated Release of Chemotherapeutics from Mesoporous Silica Nanoparticles to ex Vivo Human and Mouse Lung Tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef]
- Li, T.; Shi, S.; Goel, S.; Shen, X.; Xie, X.; Chen, Z.; Zhang, H.; Li, S.; Qin, X.; Yang, H.; et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019, 89, 1–13. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hsu, B.Y.W.; Ren, C.; Li, X.; Wang, J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef]
- Castillo, R.R.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef]
- Moulari, B.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Nanoparticle Targeting to Inflamed Tissues of the Gastrointestinal Tract. Curr. Drug Deliv. 2013, 10, 9–17. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomedicine 2016, 12, 317–332. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
- Rahmani, S.; Bouchmella, K.; Budimir, J.; Raehm, L.; Cardoso, M.B.; Trens, P.; Durand, J.O.; Charnay, C. Degradable Hollow Organosilica Nanoparticles for Antibacterial. ACS Omega 2019, 4, 1479–1486. [Google Scholar] [CrossRef] [Green Version]
- Shao, D.; Li, M.; Wang, Z.; Zheng, X.; Lao, Y.H.; Chang, Z.; Zhang, F.; Lu, M.; Yue, J.; Hu, H.; et al. Bioinspired Diselenide-Bridged Mesoporous Silica Nanoparticles for Dual-Responsive Protein Delivery. Adv. Mater. 2018, 30, 1801198. [Google Scholar] [CrossRef]
- Peng, Y.K.; Tseng, Y.J.; Liu, C.L.; Chou, S.W.; Chen, Y.W.; Tsang, S.C.E.; Chou, P.T. One-step synthesis of degradable T1-FeOOH functionalized hollow mesoporous silica nanocomposites from mesoporous silica spheres. Nanoscale 2015, 7, 2676–2687. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Tsuji, N.M.; Ohno, T. Biodegradable Metal Ion-Doped Mesoporous Silica Nanospheres Stimulate Anticancer Th1 Immune Response in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 43538–43544. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Li, Y.; Gong, Y.; Shi, H.; Huo, Q.; Liu, Y.; Xu, Q. Stimuli responsive delivery vehicles based on mesoporous silica nanoparticles recent advances and challenges. J. Mater. Chem. B 2017, 5, 1339–1352. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, C.; Zhao, N.; Xu, F.J. Redox-Responsive and Drug-Embedded Silica Nanoparticles with Unique Self-Destruction Features for Efficient Gene/Drug Codelivery. Adv. Funct. Mater. 2017, 27, 1606229. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Luo, G.F.; Zhu, J.Y.; Xu, X.D.; Zeng, X.; Cheng, D.B.; Li, Y.M.; Wu, Y.; Zhang, X.Z.; Zhuo, R.X.; et al. Enzyme-induced and tumor-targeted drug delivery system based on multifunctional mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 9078–9087. [Google Scholar] [CrossRef]
- Huang, P.; Chen, Y.; Lin, H.; Yu, L.; Zhang, L.; Wang, L.; Zhu, Y.; Shi, J. Molecularly organic_inorganic hybrid hollow mesoporous organosilica nanocapsules with tumor-specific biodegradability and enhanced chemotherapeutic functionality. Biomaterials 2017, 125, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Lin, H.; Du, W.; Chen, H.; Shi, J. Ultrasmall mesoporous organosilica nanoparticles: Morphology modulations and redox-responsive biodegradability for tumor-specific drug delivery. Biomaterials 2018, 161, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Fatieiev, Y.; Croissant, J.G.; Julfakyan, K.; Deng, L.; Anjum, D.H.; Gurinov, A.; Khashab, N.M. Enzymatically degradable hybrid organic-inorganic bridged silsesquioxane nanoparticles for in vitro imaging. Nanoscale 2015, 7, 15046–15050. [Google Scholar] [CrossRef] [Green Version]
- Bass, J.D.; Grosso, D.; Boissiere, C.; Belamie, E.; Coradin, T.; Sanchez, C. Stability of Mesoporous Oxide and Mixed Metal Oxide Materials under Biologically Relevant Conditions. Chem. Mater. 2007, 19, 4349–4356. [Google Scholar] [CrossRef]
- Colilla, M.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Boissiére, C.; Sanchez, C. Advanced Drug Delivery Vectors with Tailored Surface Properties Made of Mesoporous Binary Oxides Submicronic Spheres. Chem. Mater. 2010, 22, 1821–1830. [Google Scholar] [CrossRef]
- Mitchell, K.K.; Sandoval, S.; Cortes-Mateos, M.J.; Alfaro, J.G.; Kummel, A.C.; Trogler, W.C. Self-assembled Targeting of Cancer Cells by Iron(III)-doped, Silica Nanoparticles. J. Mater. Chem. B 2014, 2, 8017–8025. [Google Scholar] [CrossRef]
- Liu, C.G.; Han, Y.H.; Zhang, J.T.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Shen, B.; He, Q.; Cui, Z.; Liu, Y.; Zheng, X.; Zhao, K.; Shi, J. Intelligent MnO2 Nanosheets Anchored with Upconversion Nanoprobes for Concurrent pH-/H2O2-Responsive UCL Imaging and Oxygen-Elevated Synergetic Therapy. Adv. Mater. 2015, 27, 4155–4161. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Dong, X.; Liang, J.; Shi, J. Preparation of mesoporous calcium doped silica spheres with narrow size dispersion and their drug loading and degradation behavior. Microporous Mesoporous Mater. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- Hao, X.; Hu, X.; Zhang, C.; Chen, S.; Li, Z.; Yang, X.; Liu, H.; Jia, G.; Liu, D.; Ge, K.; et al. Hybrid Mesoporous Silica-Based Drug Carrier Nanostructures with Improved Degradability by Hydroxyapatite. ACS Nano 2015, 9, 9614–9625. [Google Scholar] [CrossRef]
- He, Y.; Zeng, B.; Liang, S.; Long, M.; Xu, H. Synthesis of pH-Responsive Biodegradable Mesoporous Silica− Calcium Phosphate Hybrid Nanoparticles as a High Potential Drug Carrier. ACS Appl. Mater. Interfaces 2017, 9, 44402–44409. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Yang, Y.W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015, 44, 3474–3504. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated Materials for On-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chu, X.; Hou, Y. Stimuli-responsive cancer therapy based on nanoparticles. Chem. Commun. (Camb.) 2014, 50, 11614–11630. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9, 1230. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered Controlled Drug Release from Mesoporous Silica Nanoparticles via Intracelluar Dissolution of ZnO Nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, D.; Li, M.F.; Feng, J. Multifunctional Mesoporous Silica Nanoparticles Based on Charge-Reversal Plug-Gate Nanovalves and Acid-Decomposable ZnO Quantum Dots for Intracellular Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 26666–26673. [Google Scholar] [CrossRef]
- Zhao, Q.G.; Wang, J.; Zhang, Y.P.; Zhang, J.; Tang, A.N.; Kong, D.M. A ZnO-gated porphyrinic metal–organic framework-based drug delivery system for targeted bimodal cancer therapy. J. Mater. Chem. B 2018, 6, 7898–7907. [Google Scholar] [CrossRef]
- Wu, J.; Chi, Y.H.; Zhuang, J. Synthesis and Fluorescence Properties of Water-Solube ZnO/Aminopropyl-Siloxane Quantum Dots. Chin. J. Inorg. Chem. 2010, 26, 1199–1206. [Google Scholar]
- Stöber, W.; Fink, A. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ren, H.; Chen, S.; Jin, Y.; Zhang, C.; Yang, X.; Ge, K.; Liang, X.; Li, Z.; Zhang, J. A traceable and bone-targeted nanoassembly based on defect-related luminescent mesoporous silica for enhanced osteogenic differentiation. J. Mater. Chem. B 2017, 5, 1585–1593. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Yang, J.; Li, X.; Zhou, L.; Zhang, R.; Li, W.; Chen, L.; Wang, R.; Zhang, F.; Zhao, D. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014, 14, 923–932. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Mädler, L.; et al. Use of a Rapid Cytotoxicity Screening Approach To Engineer a Safer Zinc Oxide Nanoparticle through Iron Doping. ACS Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Luan, Q.; Chen, W.; Wang, Y.; Wu, M.; Zhang, H.; Jiao, Z. Nanosized zinc oxide particles induce neural stem cell apoptosis. Nanotechnology 2009, 20, 115101. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Hu, J.; Bian, C.; Zhu, C.; Chen, C.; Guo, Z.; Zhang, Z.; Agyekum, G.A.; Zhang, Z.; Cao, X. pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery. Materials 2020, 13, 3950. https://doi.org/10.3390/ma13183950
Chen M, Hu J, Bian C, Zhu C, Chen C, Guo Z, Zhang Z, Agyekum GA, Zhang Z, Cao X. pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery. Materials. 2020; 13(18):3950. https://doi.org/10.3390/ma13183950
Chicago/Turabian StyleChen, Minmin, Jinxia Hu, Cancan Bian, Chenghao Zhu, Chen Chen, Zhijun Guo, Zhimin Zhang, Godfred Amfo Agyekum, Zhuoqi Zhang, and Xichuan Cao. 2020. "pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery" Materials 13, no. 18: 3950. https://doi.org/10.3390/ma13183950




