Gradient Microstructure Induced by Surface Mechanical Attrition Treatment (SMAT) in Magnesium Studied Using Positron Annihilation Spectroscopy and Complementary Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. X-ray Diffraction Measurements
2.3. Optical Microscopy and Electron Backscatter Diffraction
2.4. Micro-Hardness Tests and Surface Characterization
2.5. Positron Annihilation Spectroscopy
2.6. Corrosion Tests
3. Results and Discussion
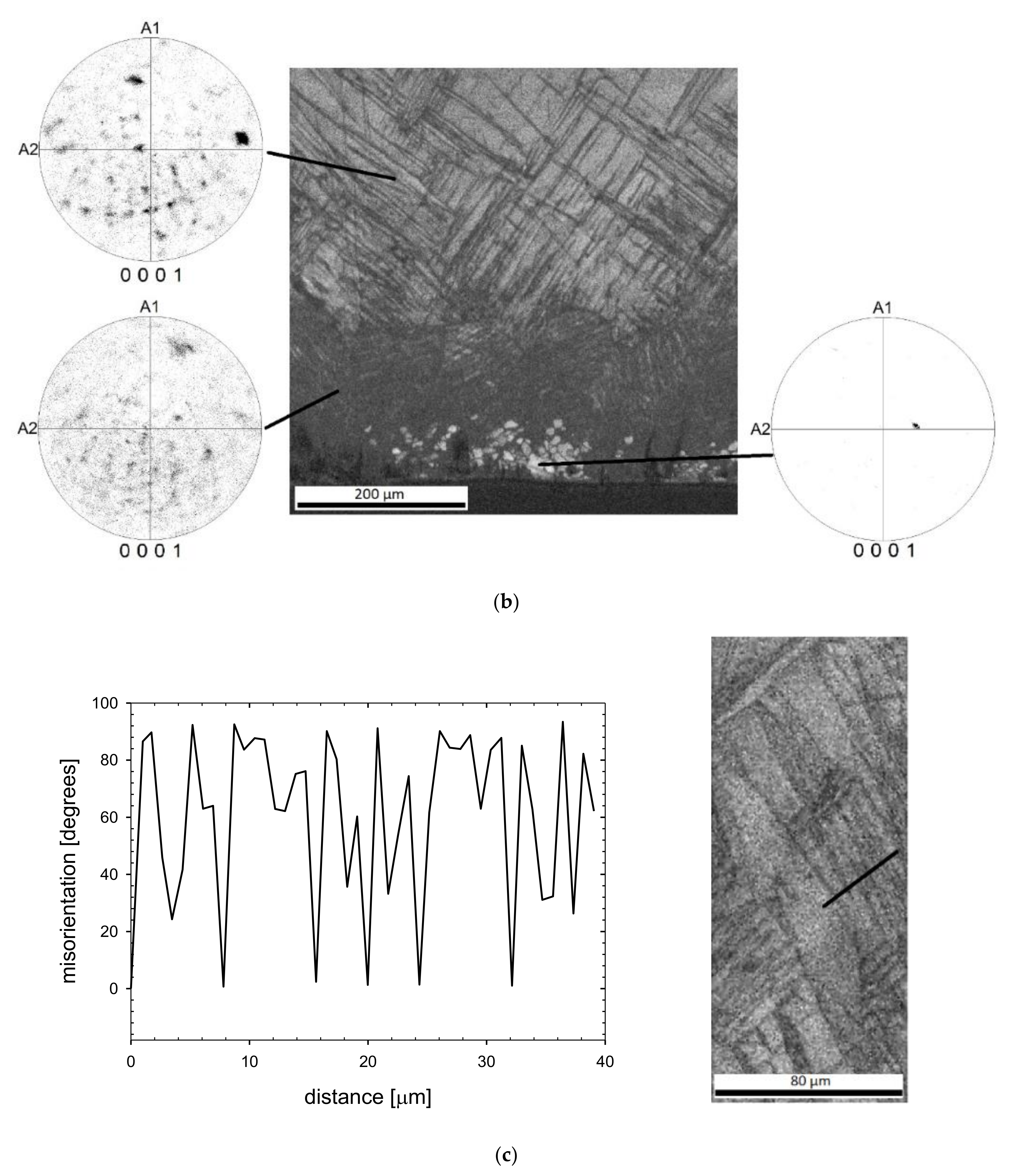
3.1. Surface Characterization
3.2. Microstructure
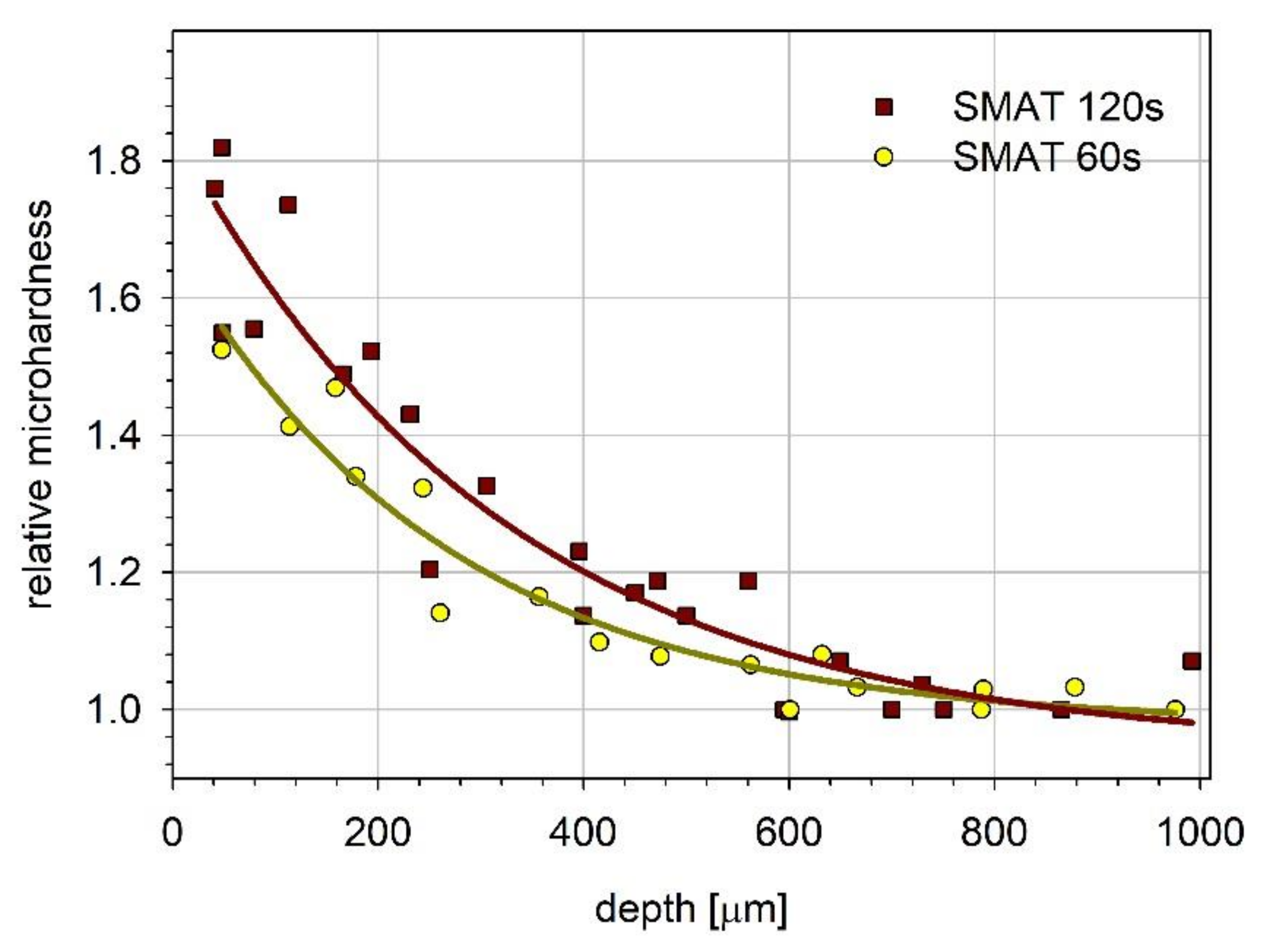
3.3. Microhardness
3.4. Residual Stress
3.5. Positron Lifetime Measurements
3.6. Variable Energy Positron Beam Measurements
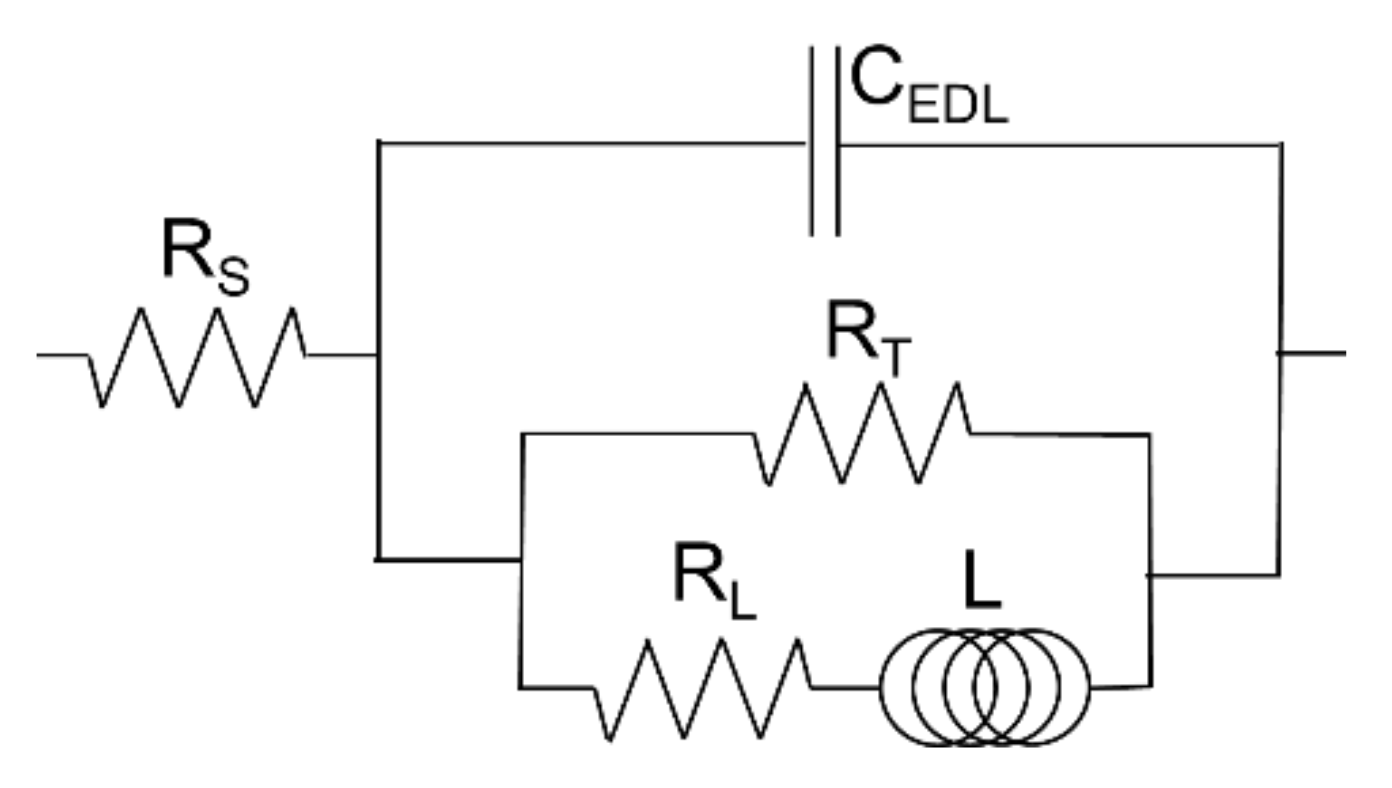
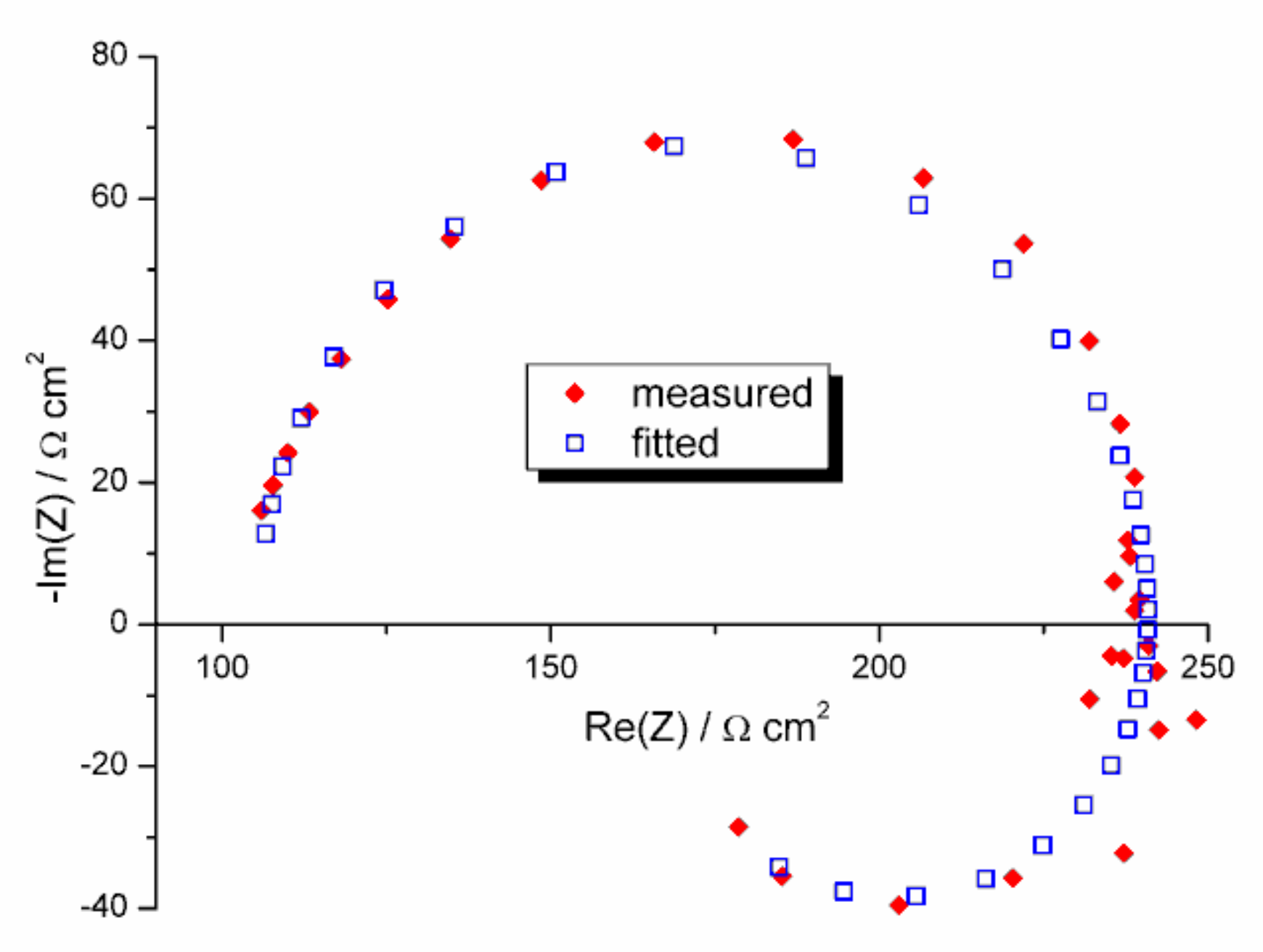
3.7. Corrosion Resistance
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Primary Magnesium Production Worldwide from 2010 to 2019 (in Thousand Metric Tons). Available online: https://www.statista.com/statistics/569515/primary-magnesium-production-worldwide/ (accessed on 14 May 2020).
- Magnesium Metal, Global Industry, Markets & Outlook. Available online: https://roskill.com/market-report/magnesium-metal/ (accessed on 14 May 2020).
- Cardarelli, F. Materials Handbook; Springer: London, UK, 2008. [Google Scholar]
- Powell, B.R.; Krajewski, P.E.; Luo, A.A. Magnesium alloys for lightweight powertrains and automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Mallick, P.K., Ed.; Woodhead Publishing Limited: Sawston, UK, 2010; pp. 114–173. [Google Scholar]
- Polmear, I. Light Alloys: From Traditional Alloys to Nanocrystals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Zeng, Z.; Nie, J.-F.; Xu, S.-W.; Davies, C.H.; Birbilis, N. Super-formable pure magnesium at room temperature. Nat. Commun. 2017, 8, 972. [Google Scholar] [CrossRef]
- Patzer, G. The magnesium industry today… the global perspective. In Essential readings in Magnesium Technology; Mathaudhu, S.N., Luo, A.A., Neelameggham, N.R., Nyberg, E.A., Sillekens, W.H., Eds.; Springer International Publishing: Heildeberg, Germany, 2010; pp. 13–18. [Google Scholar]
- Razavi, M.; Fathi, M.H.; Meratian, M. Microstructure, mechanical properties and biocorrosion evaluation of biodegradable AZ91-FA nanocomposites for biomedical applications. Mater. Sci. Eng. A 2010, 527, 6938–6944. [Google Scholar] [CrossRef]
- Feng, A.; Han, Y. The microstructure, mechanical and corrosion properties of calcium phosphate reinforced ZK60A magnesium alloy composites. J. Alloys Compd. 2010, 504, 585–593. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Brundavanam, S.; Fawcett, D. Biomedical Magnesium Alloys: A Review of Material Properties, Surface Modifications and Potential as a Biodegradable Orthopaedic Implant. Am. J. Biomed. Eng. 2012, 2, 218–240. [Google Scholar] [CrossRef]
- Chakraborty Banerjee, P.; Al-Saadi, S.; Choudhary, L.; Harandi, S.E.; Singh, R. Magnesium Implants: Prospects and Challenges. Materials 2019, 12, 136. [Google Scholar] [CrossRef]
- Hou, P.; Han, P.; Zhao, C.; Wu, H.; Ni, J.; Zhang, S.; Liu, J.; Zhang, Y.; Xu, H.; Cheng, P.; et al. Accelerating Corrosion of Pure Magnesium Co-implanted with Titanium In Vivo. Sci. Rep. 2017, 7, 41924. [Google Scholar] [CrossRef]
- Witte, F. Reprint of: The history of biodegradable magnesium implants: A review. Acta Biomater. 2015, 23, 28–40. [Google Scholar] [CrossRef]
- Li, C.-J.; Sun, H.-F.; Cheng, S.; Tan, H.-M.; He, T.-H.; Fang, W.-B. The corrosion behavior of cold drawn pure magnesium mini-tube for biomedical application. Mater. Res. Express 2019, 6, 026539. [Google Scholar] [CrossRef]
- Millenin, A.; Kustra, P.; Byrska-Wójcik, D.; Wróbel, M.; Paćko, M.; Sulej-Chojnacka, J.; Matuszyńska, S.; Płonka, B. The effect of in vitro corrosion on the mechanical properties of metallic high strength biodegradable surgical threads. Arch. Civ. Mech. Eng. 2020, 20, 60. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.-W.; Li, J.-G.; Sun, X.-D. Preparation and Characterizations of Bioglass Ceramic Cement/Ca−P Coating on Pure Magnesium for Biomedical Applications. ACS Appl. Mater. Interfaces 2014, 6, 513–525. [Google Scholar] [CrossRef]
- Zaludin, M.A.F.; Jamal, Z.A.Z.; Derman, M.N.; Kasmuin, M.Z. Fabrication of calcium phosphate coating on pure magnesium substrate via simple chemical conversion coating: Surface properties and corrosion performance evaluations. J. Mater. Res. Technol. 2019, 8, 981–987. [Google Scholar] [CrossRef]
- Zhou, S.J.; Li, J.F.; Long, J.P.; Du, H.Q. Corrosion Behavior of P-Coated Biomedical Pure Magnesium. Mater. Sci. Forum 2015, 814, 389–391. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N. Magnesium Biomaterials, Design, Testing and Best Practice; Springer International Publishing: Heildberg, Germany, 2014. [Google Scholar]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0.8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Y.D.; Li, Y.X.; Wu, Y.H.; Zhen, Y.F.; Han, Y. Effect of surface mechanical attrition treatment on biodegradable Mg–1Ca alloy. Mater. Sci. Eng. C 2014, 35, 314–321. [Google Scholar] [CrossRef]
- Gruzleski, J.E. Microstructure Development during Metal Casting; American Foundrymen’s Society, Inc.: Schaumburg, IL, USA, 2000. [Google Scholar]
- Kaibyshev, R. Dynamic recrystallization in magnesium alloys. In Advances in Wrought Magnesium Alloys—Fundamentals of Processing, Properties and Applications, 1st ed.; Bettles, C., Barnett, M., Eds.; Woodhead Publishing: Sawston, UK, 2012; pp. 186–225. [Google Scholar]
- Volkov, A.Y.; Kliukin, I.V. Improving the mechanical properties of pure magnesium through cold hydrostatic extrusion and low-temperature annealing. Mat. Sci. Eng. A 2015, 627, 56–60. [Google Scholar] [CrossRef]
- Mabuchi, M.; Iwasaki, H.; Higashi, K. Low temperature superplasticity of Magnesium Alloys Processed by ECAE. Mat. Sci. Forum 1997, 243, 547–552. [Google Scholar] [CrossRef]
- Lu, K. Making strong nanomaterials ductile with gradients. Science 2014, 345, 1455–1456. [Google Scholar] [CrossRef]
- Estrin, Y.; Vinogradov, A. Fatigue behaviour of light alloys with ultrafine grain structure produced by severe plastic deformation: An overview. Int. J. Fatigue 2010, 32, 898–907. [Google Scholar] [CrossRef]
- Fatemi-Varzaneh, S.M.; Zarei-Hanzaki, A.; Paul, H. Characterization of ultrafine and nano grained magnesium alloy processed by severe plastic deformation. Mater. Charact. 2014, 87, 27–35. [Google Scholar] [CrossRef]
- Birbilis, N.; Ralston, K.D.; Virtanen, S.; Fraser, H.L.; Davies, C.H.J. Grain character influences on corrosion of ECAPed pure magnesium. Corros. Eng. Sci. Technol. 2010, 45, 224–230. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, G.; Zhang, L.; Xu, T. A new approach of a gradient nanograined surface layer for Mg-3Al-1Zn alloy induced by SMRGT. Int. J. Adv. Manuf. Technol. 2018, 94, 2659–2665. [Google Scholar] [CrossRef]
- Chui, P.; Sun, K.; Sun, C.; Yang, X.; Shan, T. Effect of surface nanocrystallization induced by fast multiple rotation rolling on hardness and corrosion behavior of 316L stainless steel. Appl. Surf. Sci. 2011, 257, 6787–6791. [Google Scholar] [CrossRef]
- Toth, L.S.; Gu, C. Ultrafine-grain metals by severe plastic deformation. Mater. Charact. 2014, 92, 1–14. [Google Scholar] [CrossRef]
- Samih, Y.; Beausir, B.; Bolle, B.; Grosdidier, T. In-depth quantitative analysis of the microstructures produced by Surface Mechanical Attrition Treatment (SMAT). Mater. Charact. 2013, 83, 129–138. [Google Scholar] [CrossRef]
- Lu, K.; Lu, J. Nanostructured surface layer on metallic materials induced by surface mechanical attrition treatment. Mat. Sci. Eng. A 2004, 375, 38–45. [Google Scholar] [CrossRef]
- Bernoulli, D.; Cao, S.C.; Lu, J.; Dao, M. Enhanced repeated frictional sliding properties in 304 stainless steel with a gradient nanostructured surface. Surf. Coat. Technol. 2018, 339, 14–19. [Google Scholar] [CrossRef]
- Sun, Z.; Retraint, D.; Baudin, T.; Helbert, A.L.; Brisset, F.; Chemkhi, M.; Zhou, J.; Kanouté, P. Experimental study of microstructure changes due to low cycle fatigue of a steel nanocrystallised by Surface Mechanical Attrition Treatment (SMAT). Mater. Charact. 2017, 124, 117–121. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, B.; Lu, J. Mechanical properties and thermal stability of nanocrystallized pure aluminum produced by surface mechanical attrition treatment. Mat. Sci. Eng. A Struct. A 2015, 636, 446–451. [Google Scholar] [CrossRef]
- Laleh, M.; Kargar, F. Effect of surface nanocrystallization on the microstructural and corrosion characteristics of AZ91D magnesium alloy. J. Alloys Compd. 2011, 509, 91509156. [Google Scholar] [CrossRef]
- Fabijanic, D.; Taylor, A.; Ralson, K.D.; Zhang, M.-X.; Birbilis, N. Influence of Surface Mechanical Attrition Treatment Attrition Media on the Surface Contamination and Corrosion of Magnesium. Corrosion 2013, 69, 527–535. [Google Scholar] [CrossRef]
- Yin, Z.; Yang, X.; Ma, X.; Moering, J.; Yang, J.; Gong, Y.; Zhu, Y.; Zhu, X. Strength and ductility of gradient structured copper obtained by surface mechanical attrition treatment. Mater. Design. 2016, 105, 89–95. [Google Scholar] [CrossRef]
- Jelliti, S.; Richard, C.; Retraint, D.; Roland, T.; Chemkhi, M.; Demangele, C. Effect of surface nanocrystallization on the corrosion behavior of Ti–6Al–4V titanium alloy. Surf. Coat. Technol. 2013, 224, 82–87. [Google Scholar] [CrossRef]
- Guo, K.W. A Review of Magnesium/Magnesium Alloys Corrosion and its Protection. Recent Pat. Corros. Sci. 2019, 2, 13–21. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Gu, X.; Jiang, B.; Liang, J. Effect of severe shot-peening on corrosion behavior of AZ31 and AZ91 magnesium alloys. J. Alloys Compd. 2019, 770, 500–506. [Google Scholar] [CrossRef]
- Song, G.; Pu, Z. Corrosion Resistance of Magnesium Alloy Article Surfaces. U.S. Patent Application US 2012/0067465A1, 22 March 2012. [Google Scholar]
- Pu, Z.; Song, G.-L.; Yang, S.; Outeiro, J.C.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Grain refined and basal textured surface produced by burnishing for improved corrosion performance of AZ31B Mg alloy. Corros. Sci. 2012, 57, 192–201. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, S.; Song, G.-L.; Dillon, O.W.; Puleo, D.A.; Jawahir, I.S. Ultrafine-grained surface layer on Mg–Al–Zn alloy produced by cryogenic burnishing for enhanced corrosion resistance. Scr. Mater 2011, 65, 520–523. [Google Scholar] [CrossRef]
- Hadzima, B.; Bukovina, M.; Dolezal, P. Shot peening influence on corrosion resistance of AE21 magnesium alloy. Mater. Eng. 2010, 17, 14–19. [Google Scholar]
- Fu, T.; Zhan, Z.; Zhang, L.; Yang, Y.; Liu, Z.; Liu, J.; Li, L.; Yu, X. Effect of surface mechanical attrition treatment on corrosion resistance of commercial pure titanium. Surf. Coat. Technol. 2015, 280, 129–135. [Google Scholar] [CrossRef]
- Sun, Q.; Han, Q.; Liu, X.; Xu, W.; Li, J. The effect of surface contamination on corrosion performance of ultrasonic shot peened 7150 Al alloy. Surf. Coat. Technol. 2017, 328, 469–479. [Google Scholar] [CrossRef]
- Chen, G.; Fu, Y.; Cui, Y.; Gao, J.; Guo, X.; Gao, H.; Wu, S.; Lu, J.; Lin, Q.; Shi, S. Effect of surface mechanical attrition treatment on corrosion fatigue behavior of AZ31B magnesium alloy. Int. J. Fatigue 2019, 127, 461–469. [Google Scholar] [CrossRef]
- Li, Q.; Yan, X.; Luo, L.; Xu, F.; Wu, G.; Liu, C.; Jing, Y.; Liu, Y.; Lu, J. Mechanical properties and corrosion behaviors of AZ31 alloy with dual-phase glass-crystal coating. Mater. Charact. 2019, 154, 200–211. [Google Scholar] [CrossRef]
- Song, D.; Ma, A.; Jiang, J.; Lin, P.; Yang, D.; Fan, J. Corrosion behavior of equal-channel-angular pressed pure magnesium in NaCl aqueous solution. Corros. Sci. 2010, 52, 481–490. [Google Scholar]
- Silva, C.L.P.; Soares, R.B.; Pereira, P.H.R.; Figueredo, R.B.; Lins, V.F.C.; Langdon, T.G. The effect of high-pressure torsion on microstructure, hardness and corrosion behavior for pure magnesium and different magnesium alloys. Adv. Eng. Mater. 2019, 21, 1801081. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Berndt, C.C.; Kapoor, A.; Wen, C. Development of Surface Nano-Crystallization in Alloys by Surface Mechanical Attrition Treatment (SMAT). Crit. Rev. Solid State 2014, 40, 164–181. [Google Scholar] [CrossRef]
- Wang, Z.B.; Tao, N.R.; Tong, W.P.; Lu, J.; Lu, K. Diffusion of Cr in Nanostructured Fe and Low Carbon Steel Produced by means of Surface Mechanical Attrition Treatment. Def. Diff. Forum 2006, 249, 147–154. [Google Scholar]
- Zaleski, R.; Zaleski, K.; Gorgol, M.; Wiertel, M. Positron annihilation study of aluminum, titanium, and iron alloys surface after shot peening. Appl. Phys. A 2015, 120, 551–559. [Google Scholar] [CrossRef]
- Dlubek, G.; Meyendorf, N. Positron Annihilation Spectroscopy (PAS). In Nondestructive Materials Characterization with Applications to Aerospace Materials; Meyendorf, N.G.H., Nagy, P.B., Rokhlin, S.I., Eds.; Springer: Berlin/Heidlberg, Germany; New York, NY, USA, 2004; pp. 374–416. [Google Scholar]
- Dryzek, J.; Wrobel, M.; Dryzek, E. Recrystallization in severely deformed Ag, Au, a nd Fe studied by positron-annihilation and XRD methods. Phys. Status Solidi B 2016, 253, 2031–2042. [Google Scholar] [CrossRef]
- Skowron, K.; Dryzek, E.; Wróbel, M.; Cieniek, Ł. Laser Peened Austenitic Stainless Steel Studied by Positron Annihilation Spectroscopy. Acta Phys. Pol. B 2020, 51, 317–321. [Google Scholar] [CrossRef]
- Horodek, P.; Kobets, A.G.; Meshkov, I.N.; Sidorin, A.A.; Orlov, O.S. Slow positron beam at the JINR, Dubna. Nukleonika 2015, 60, 725–728. [Google Scholar] [CrossRef]
- Badreddine, J.; Micoulaut, M.; Rouhaud, E.; Remy, S.; Retraint, D.; François, M. Effect of the confinement on the properties of ultrasonic vibrated granular gases. Granul. Matter 2013, 15, 367–376. [Google Scholar] [CrossRef]
- Marciszko, M.; Baczmański, A.; Wierzbanowski, K.; Wróbel, M.; Braham, C.; Chopart, J.-P.; Lodini, A.; Bonarski, J.; Tarkowski, L.; Zazi, N. Application of multireflection grazing incidence method for stress measurements in polished Al–Mg alloy and CrN coating. Appl. Surf. Sci. 2013, 266, 256–267. [Google Scholar] [CrossRef]
- Marciszko, M.; Baczmanski, A.; Braham, C.; Wróbel, M.; Seiler, W.; Wronski, S.; Berent, K. Analysis of stresses and crystal structure in the surface layer of hexagonal polycrystalline materials: A new methodology based on grazing incidence diffraction. J. Appl. Cryst. 2016, 49, 85–102. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods Phys. Res. A 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Dryzek, E. Defect depth profiling after sphere indentation and blasting in aluminum and aluminum alloy detected by positron annihilation. J. Mater. Sci. 2003, 38, 3755–3763. [Google Scholar] [CrossRef]
- Dryzek, J.; Dryzek, E.; Suzuki, T.; Yu, R. Subsurface zone in pure magnesium studied by positron lifetime spectroscopy. Tribol. Let. 2005, 20, 91–97. [Google Scholar] [CrossRef]
- Puska, M.J.; Nieminen, R.M. Theory of positrons in solids and on solid surfaces. Rev. Mod. Phys. 1994, 66, 841–899. [Google Scholar] [CrossRef]
- Balusamy, T.; Kumar, S.; Sankara Narayanan, T.S.N. Effect of surface nanocrystallization on the corrosion behaviour of AISI 409 stainless steel. Corros. Sci. 2010, 52, 3826–3834. [Google Scholar] [CrossRef]
- Grosdidier, T.; Novelli, M. Recent Developments in the application of surface mechanical attrition treatments for improved gradient structures: Processing parameters and surface reactivity. Mater. Trans. 2019, 60, 1344–1355. [Google Scholar] [CrossRef]
- Novelli, M.; Bocher, P.; Grosdidier, T. Effect of cryogenic temperatures and processing parameters on gradient-structure of a stainless steel treated by ultrasonic surface mechanical attrition treatment. Mater. Charact. 2018, 139, 197–207. [Google Scholar] [CrossRef]
- Kargar, F.; Laleh, M.; Shahrabi, T.; Sabour Rouh Aghdam, A. Effect of treatment time on characterization and properties of nanocrystalline surface layer in copper induced by surface mechanical attrition treatment. Bull. Mater. Sci. 2014, 37, 1087–1094. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Zhou, H.; Moering, J.; Yin, Z.; Gong, Y.; Zhao, K. Mechanical Properties of Gradient Structure Mg Alloy. Met. Mater. Trans. A 2017, 48, 3961–3970. [Google Scholar] [CrossRef]
- Sun, H.Q.; Shi, Y.N.; Zhang, M.-X.; Lu, K. Surface alloying of an Mg alloy subjected to surface mechanical attrition treatment. Surf. Coat. Technol. 2008, 202, 3947–3953. [Google Scholar] [CrossRef]
- Wei, Y.H.; Liu, B.S.; Hou, L.-F.; Xu, B.-S.G.; Liu, G. Characterization and properties of nanocrystalline surface layer in Mg alloy induced by surface mechanical attrition treatment. J. Alloys Compd. 2008, 452, 336–342. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Vassel, A.; Brisset, F.; Lu, K.; Lu, J. Nanostructure formation mechanism of α-titanium using SMAT. Acta Mater. 2004, 52, 4101–4110. [Google Scholar] [CrossRef]
- Caceres, C.H.; Blake, P.L. Strain hardening due to {1012} twinning in pure magnesium. Philos. Mag. 2009, 88, 991–1003. [Google Scholar] [CrossRef]
- Ichikawa, R. Recrystallization temperature of high purity Magnesium (part 4). J. Jpn. Inst. Light Met. 1956, 20, 93–98. [Google Scholar] [CrossRef]
- Rouquette, S.; Rouhaud, E.; François, M.; Roos, A.; Chaboche, J.L. Coupled thermomechanical simulations of the shot peening process, effects of temperature on the residual stress field. J. Mater. Process. Technol. 2009, 209, 3879–3886. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena, 2nd ed.; Pergamon: Oxford, UK, 2004. [Google Scholar]
- Ungar, T.; Tichy, G.; Gubicza, J.; Hellmig, R.J. Correlation between subgrains and coherently scattering domains. Powder Diffr. 2005, 20, 366–375. [Google Scholar] [CrossRef]
- Attarilar, S.; Salehi, M.T.; Al-Fadhalah, K.J.; Djavanroodi, F.; Mozafari, M. Functionally graded titanium implants: Characteristic enhancement induced by combined severe plastic deformation. PLoS ONE 2019, 14, e0221491. [Google Scholar] [CrossRef]
- Kumar, S.A.; Kumar, P.S.; Raman, S.G.S. Narayanan, TSNS Influence of SMAT Parameters on Microstructural and Mechanical Properties of Al-Mg-Si Alloy AA 6061. J. Mater. Eng. Perform 2017, 26, 1947–1957. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, B.; Li, D.-J.; Zeng, X.-Q.; Lu, J. Wear behavior of nanocrystalline structured magnesium alloy induced by surface mechanical attrition treatment. Surf. Coat. Technol. 2015, 261, 219–226. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, Z.; Guo, P.; Liu, Z.; Li, Y.; Zhou, W.; Wu, Y. The effect of surface mechanical attrition treatment on texture evolution and mechanical properties of AZ31 magnesium alloy. Mater. Charact. 2019, 148, 26–34. [Google Scholar] [CrossRef]
- Wawszczak, R.; Baczmański, A.; Marciszko, M.; Wróbel, M.; Czeppe, T.; Sztwiertnia, K.; Braham, C.; Berent, K. Evolution of microstructure and residual stress during annealing of austenitic and ferritic steel. Mater. Charact. 2016, 112, 238–251. [Google Scholar] [CrossRef]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena; Elsevier Science Ltd.: Oxford, UK, 1995. [Google Scholar]
- Bagherifard, S.; Hickey, D.J.; Fintová, S.; Pastorek, F.; Fernandez-Pariente, I.; Bandini, M.; Webster, T.J.; Guagliano, M. Effects of Nanofeatures Induced by Severe Shot Peening (SSP) on Mechanical, Corrosion and Cytocompatibility Properties of Magnesium Alloy AZ31. Acta Biomater. 2018, 66, 93–108. [Google Scholar] [CrossRef]
- Fard, S.B.; Guagliano, M. Effects of surfaces nanocrystallization induced by shot peening on material properties: A Review. Frat. Ed Integrità Strutt. 2009, 7, 3–16. [Google Scholar] [CrossRef]
- Gallitelli, D.; Retraint, D.; Rouhaud, E. Effects of conventional shot peening (SP) and surface mechanical attrition treatment (SMAT) on a Titanium alloy. Adv. Mat. Res. 2014, 996, 964–968. [Google Scholar]
- Optasanu, V.; Kanjer, A.T.; Montesin, T. Influence of shot-peening on the high temperature oxidation of Zr and Ti plates, High Temperature Corrosion. In Proceedings of the 29th International Conference on Surface Modification Technologies, Kongens Lyngby, Denmark, 10–12 June 2015; Sudersham, T.S., Somers, M.A.J., Eds.; Curran Associates, Inc.: Morehouse, GA, USA, 2016; pp. 29–37. [Google Scholar]
- Raceanu, L.; Optasanu, V.; Montesin, T.; Montay, G.; François, M. Shot-Peening of Pre-Oxidized Plates of Zirconium: Influence of Residual Stress on Oxidation. Oxid. Met. 2013, 79, 135–145. [Google Scholar] [CrossRef][Green Version]
- Hautojärvi, P.; Johansson, J.; Vehanen, A.; Yli-Kauppila, J.; Hillairet, J.; Tzanétakis, P. Trapping of positrons at vacancies in magnesium. Appl. Phys. A 1982, 27, 49–56. [Google Scholar] [CrossRef]
- Río, J.; Gómez, C.; Ruano, M. Positron trapping mechanism in plastically deformed magnesium. Philos. Mag. 2012, 92, 535–549. [Google Scholar]
- Serra, A.; de Diego, N. Characterization of defects in deformed titanium. Phys. Status Solidi A 1988, 110, 409–414. [Google Scholar] [CrossRef]
- Folegati, P.; Dupasquier, A.; Ferragut, R.; Iglesias, M.M.; Makkonen, I.; Puska, M.J. Quantitative chemical analysis of vacancy-solute complexes in metallic solid solutions by coincidence Doppler broadening spectroscopy. Phys. Status Solidi C 2007, 4, 3493–3496. [Google Scholar] [CrossRef]
- Čížek, J.; Procházka, I.; Smola, B.; Stuliková, I.; Vlach, M.; Islamgaliev, R.K.; Kulyasova, O. Microstructure development and precipitation effects in ultrafine grained Mg-3Tb-2Nd alloy prepared by high pressure torsion. Mater. Sci. Forum 2008, 584–586, 591–596. [Google Scholar]
- Sun, H.Q.; Shi, Y.-N.; Zhang, M.-X.; Lu, K. Plastic strain-induced grain refinement in the nanometer scale in a Mg alloy. Acta Mater. 2007, 55, 975–982. [Google Scholar] [CrossRef]
- Meng, X.; Duan, M.; Luo, L.; Zhan, D.; Jin, B.; Jin, Y.; Rao, X.; Liu, Y.; Lu, J. The deformation behavior of AZ31 Mg alloy with surface mechanical attrition treatment. Mater. Sci. Eng. A 2017, 707, 636–646. [Google Scholar] [CrossRef]
- Okhubo, H.; Tang, Z.; Nagai, Y.; Hasegawa, M.; Tanawara, T.; Kiritani, M. Positron annihilation study of vacancy-type defects in high-speed deformed Ni, Cu and Fe. Mater. Sci. Eng. A 2003, 350, 95–101. [Google Scholar]
- Dryzek, J. Positron annihilation studies of recrystallization in the subsurface zone induced by friction in magnesium—Effect of the inhomogeneity on measured positron annihilation characteristics. Appl. Phys. A 2014, 114, 465–475. [Google Scholar] [CrossRef][Green Version]
- Dryzek, J. Detection of tribolayer in different metals using positron lifetime spectroscopy. Tribol. Int. 2019, 131, 268–276. [Google Scholar] [CrossRef]
- Dryzek, J.; Horodek, P. GEANT4 Simulation of Slow Positron Beam Implantation Profiles. Nucl. Instrum. Methods Phys. Res. B 2008, 266, 4000–4009. [Google Scholar] [CrossRef]
- Hebert, K.R.; Gessmann, T.; Lynn, K.G.; Asoka-Kumar, P. Positron annihilation spectroscopy study of interfacial defects formed by anodic oxidation of aluminum. J. Electrochem. Soc. 2004, 151, B22–B26. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.C.; Zhai, T.; Coleman, P.G. A Positron annihilation study of corrosion of aluminum and aluminum alloy by NaOH. Metall. Mater. Trans. A 2011, 43, 2823–2831. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Z.J.; Wang, J.J.; Wu, Y.C.; Zhai, T.; Song, G.-L. Slow positron beam study of corrosion behavior of AM60B magnesium alloy in NaCl solution. Corros. Sci. 2016, 106, 271–280. [Google Scholar] [CrossRef]
- Dryzek, J.; Schut, H.; Dryzek, E. Subsurface zones in magnesium detected by variable energy positron beam. Phys. Status Solidi C 2007, 4, 3522–3525. [Google Scholar] [CrossRef]
- Van Veen, A.; Schut, H.; Clement, M.; Kruseman, A.; Ijpma, M.R.; De Nijs, J.M.M. VEPFIT applied to depth profiling problems. Appl. Surf. Sci. 1995, 85, 216–224. [Google Scholar] [CrossRef]
- Eijt, S.W.H.; Kind, R.; Singh, S.; Schut, H.; Legerstee, W.J.; Hendrikx, R.W.A.; Svetchnikov, V.L.; Westerwaal, R.J.; Dam, B. Positron depth profiling of the structural and electronic structure transformations of hydrogenated Mg-based thin films. J. Appl. Phys. 2009, 105, 043514. [Google Scholar] [CrossRef]
- Hruška, P.; Čížek, J.; Anwand, W.; Bulíř, J.; Lančok, J.; Stráská, J.; Melikhova, O.; Procházka, I. Structural studies of thin Mg films. J. Phys. Conf. Ser. 2014, 505, 012024. [Google Scholar]
- Clement, M.; de Nijs, J.M.M.; Balk, P.; Schut, H.; van Veen, A. Analysis of positron beam a by the combined use of the shape- and wing-parameters. J. Appl. Phys. 1996, 79, 9029–9036. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, Z.; Hort, N.; Abidin, N.I.Z.; Atrens, A. Corrosion behaviour of a nominally high purity Mg ingot produced by permanent mould direct chill casting. Corros. Sci. 2012, 61, 185–207. [Google Scholar] [CrossRef]
- Shi, Z.; Cao, F.; Song, G.; Atrens, A. Low apparent valence of Mg during corrosion. Corros. Sci. 2014, 88, 434–443. [Google Scholar] [CrossRef]
- Mosiałek, M.; Mordarski, G.; Nowak, P.; Simka, W.; Nawrat, G.; Hanke, M.; Socha, R.P.; Michalska, J. Phosphate–permanganate conversion coatings on the AZ81 magnesium alloy: SEM, EIS and XPS studies. Surf. Coat. Technol. 2011, 206, 51–62. [Google Scholar]
- Nowak, P.; Mosiałek, M.; Nawrat, G. Corrosion of magnesium and its alloys - new observations and ideas. Ochr. Przed Korozją (Corros. Prot.) 2015, 58, 371–377. [Google Scholar] [CrossRef]
- op’t Hoog, C.; Birbilis, N.; Estrin, Y. Corrosion of Pure Mg as a Function of Grain Size and Processing Route. Adv. Eng. Mater. 2008, 10, 579–581. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scr. Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Nye, J.F. Some geometrical relations in dislocated crystals. Acta Metall. 1953, 153–162. [Google Scholar] [CrossRef]
- Jiang, P.; Wei, Q.; Hong, Y.S.; Lu, J.; Wu, X.L. In situ synthesis of nanocrystalline intermetallic layer during surface plastic deformation of zirconium. Surf. Coat. Technol. 2007, 202, 583–589. [Google Scholar] [CrossRef]






| Sample | Crystallite Size [nm] |
|---|---|
| Reference | 205 ± 63 |
| SMAT 60 s SMAT 120 s | 38 ± 3 37 ± 3 |
| Thickness Reduction 10% Thickness Reduction 40% | 48 ± 6 35 ± 5 |
| Sample | L+layer [nm] | d [nm] |
|---|---|---|
| SMAT 60 s | 74 ± 4 | 148 ± 9 |
| SMAT 120 s | 74 ± 2 | 370 ± 32 |
| reference | 21 ± 10 | 80 ± 43 |
| SMAT 60 s & etched | 6 ± 2 | 320 ± 2 |
| SMAT 120 s & etched | 12 ± 1 | 708 ± 78 |
| Compressed & Etched | 6 ± 1 | 266 ± 18 |
| Time [s] | OCP [V] | Ecorr [V] | Ecorr–OCP [V] | Rp,LSV [Ωcm2] | jcorr [Acm−2] | Rp×jcorr [V] | Rp,EIS [Ωcm2] |
|---|---|---|---|---|---|---|---|
| 0 | −1.601 | −1.528 | 0.073 | 173 | 1.32 × 10−4 | 0.0228 | 59 |
| 60 | −1.586 | −1.448 | 0.138 | 636 | 5.00 × 10−5 | 0.0318 | 46 |
| 120 | −1.576 | −1.354 | 0.222 | 1612 | 2.03 × 10−5 | 0.0327 | 34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, K.; Dryzek, E.; Wróbel, M.; Nowak, P.; Marciszko-Wiąckowska, M.; Le Joncour, L.; François, M.; Panicaud, B.; Baczmański, A. Gradient Microstructure Induced by Surface Mechanical Attrition Treatment (SMAT) in Magnesium Studied Using Positron Annihilation Spectroscopy and Complementary Methods. Materials 2020, 13, 4002. https://doi.org/10.3390/ma13184002
Skowron K, Dryzek E, Wróbel M, Nowak P, Marciszko-Wiąckowska M, Le Joncour L, François M, Panicaud B, Baczmański A. Gradient Microstructure Induced by Surface Mechanical Attrition Treatment (SMAT) in Magnesium Studied Using Positron Annihilation Spectroscopy and Complementary Methods. Materials. 2020; 13(18):4002. https://doi.org/10.3390/ma13184002
Chicago/Turabian StyleSkowron, Konrad, Ewa Dryzek, Mirosław Wróbel, Paweł Nowak, Marianna Marciszko-Wiąckowska, Léa Le Joncour, Manuel François, Benoit Panicaud, and Andrzej Baczmański. 2020. "Gradient Microstructure Induced by Surface Mechanical Attrition Treatment (SMAT) in Magnesium Studied Using Positron Annihilation Spectroscopy and Complementary Methods" Materials 13, no. 18: 4002. https://doi.org/10.3390/ma13184002
APA StyleSkowron, K., Dryzek, E., Wróbel, M., Nowak, P., Marciszko-Wiąckowska, M., Le Joncour, L., François, M., Panicaud, B., & Baczmański, A. (2020). Gradient Microstructure Induced by Surface Mechanical Attrition Treatment (SMAT) in Magnesium Studied Using Positron Annihilation Spectroscopy and Complementary Methods. Materials, 13(18), 4002. https://doi.org/10.3390/ma13184002





