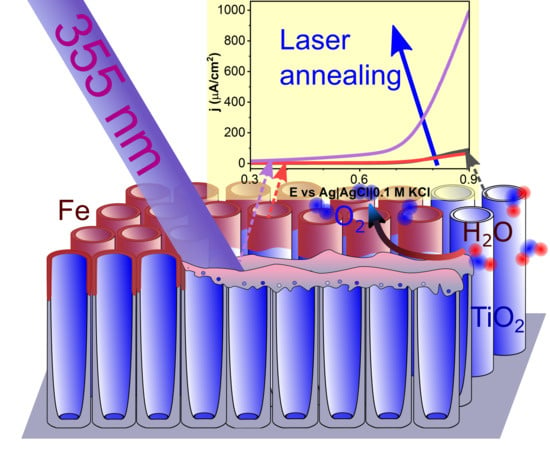
The Effect of Laser Re-Solidification on Microstructure and Photo-Electrochemical Properties of Fe-Decorated TiO2 Nanotubes
Abstract
:1. Introduction
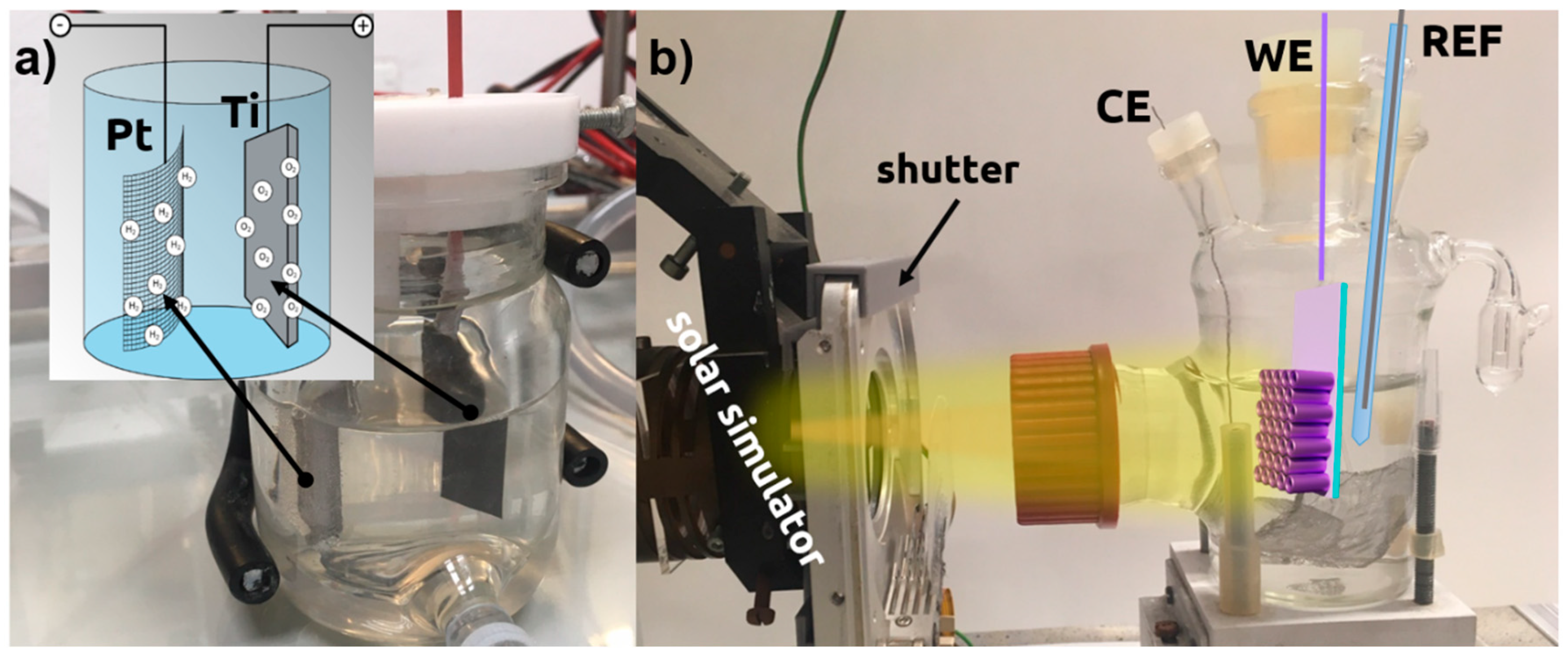
2. Materials and Methods
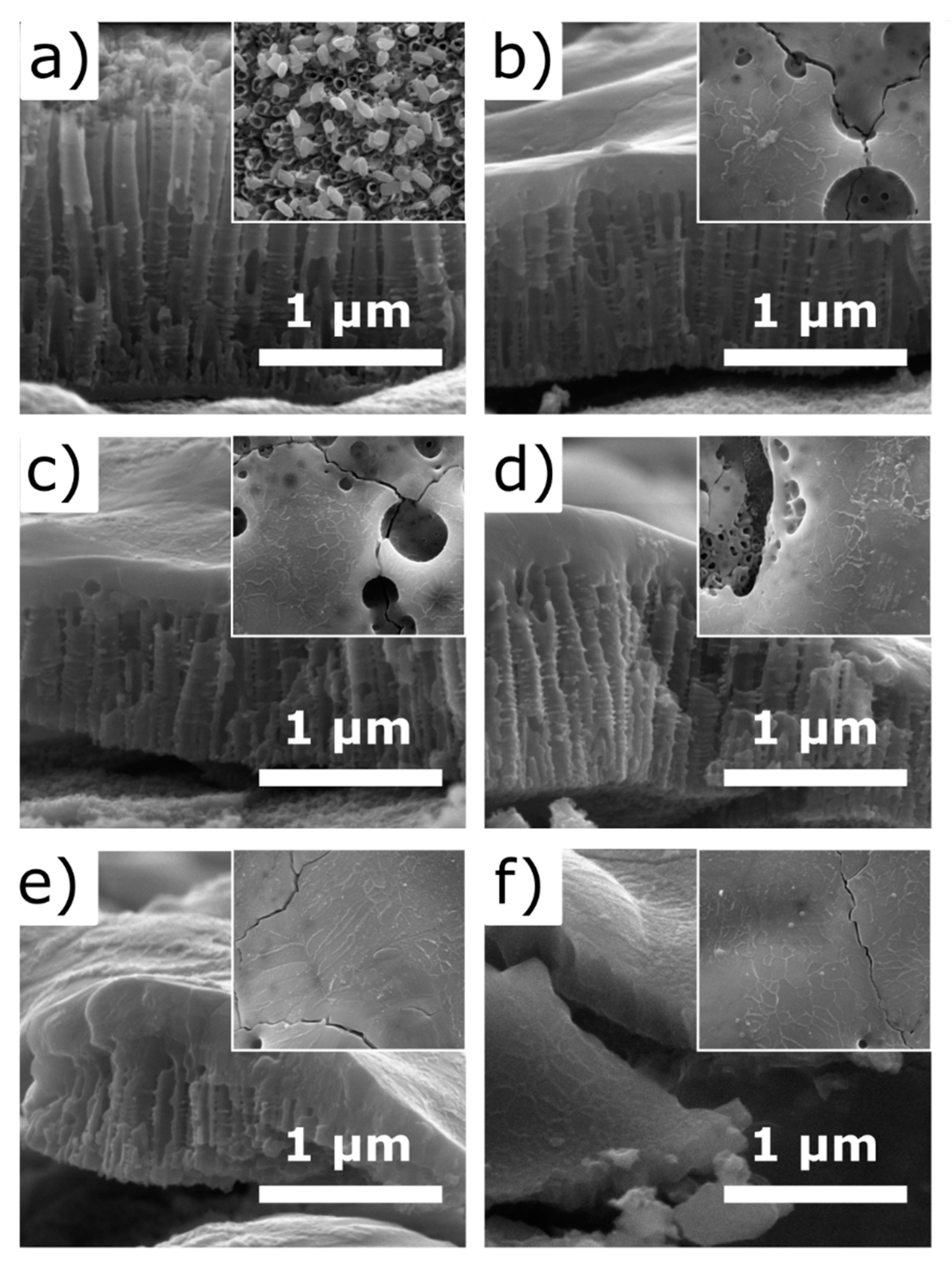
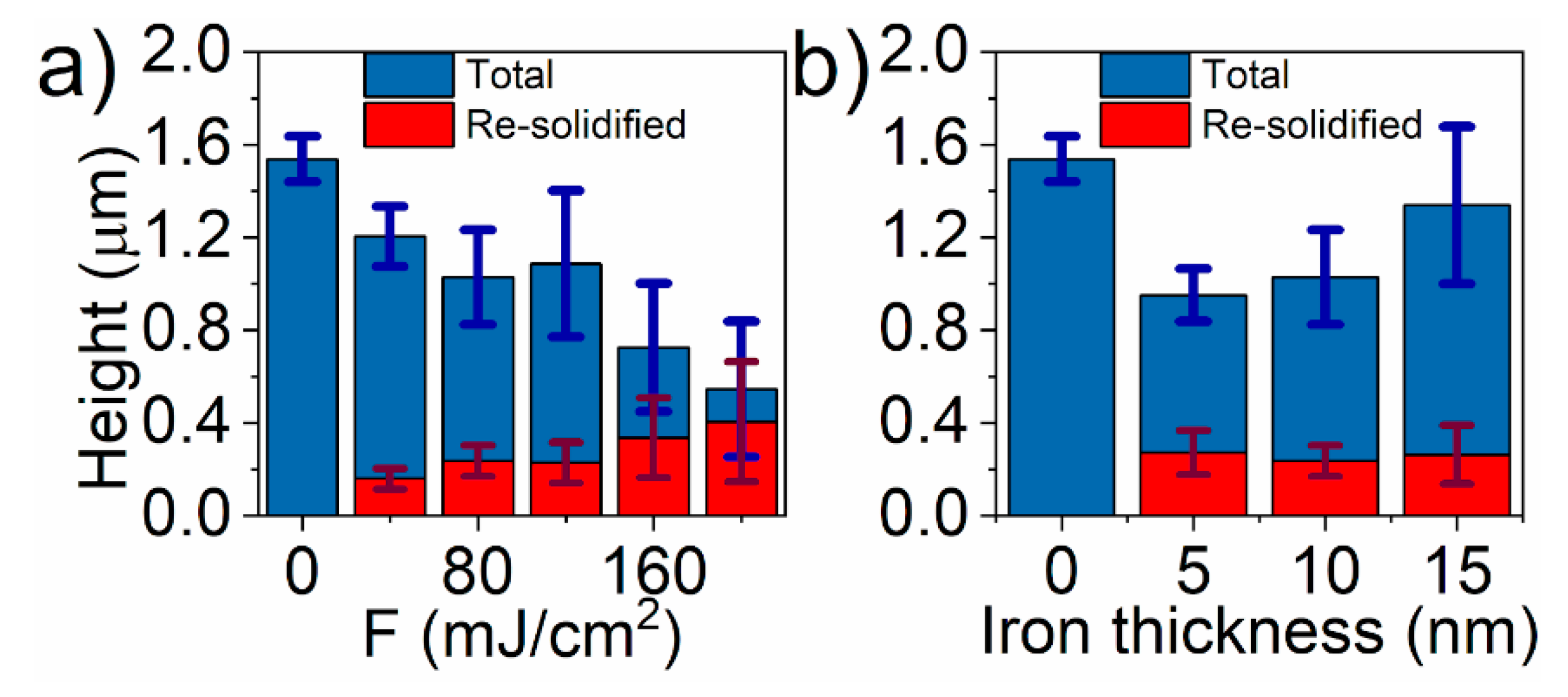
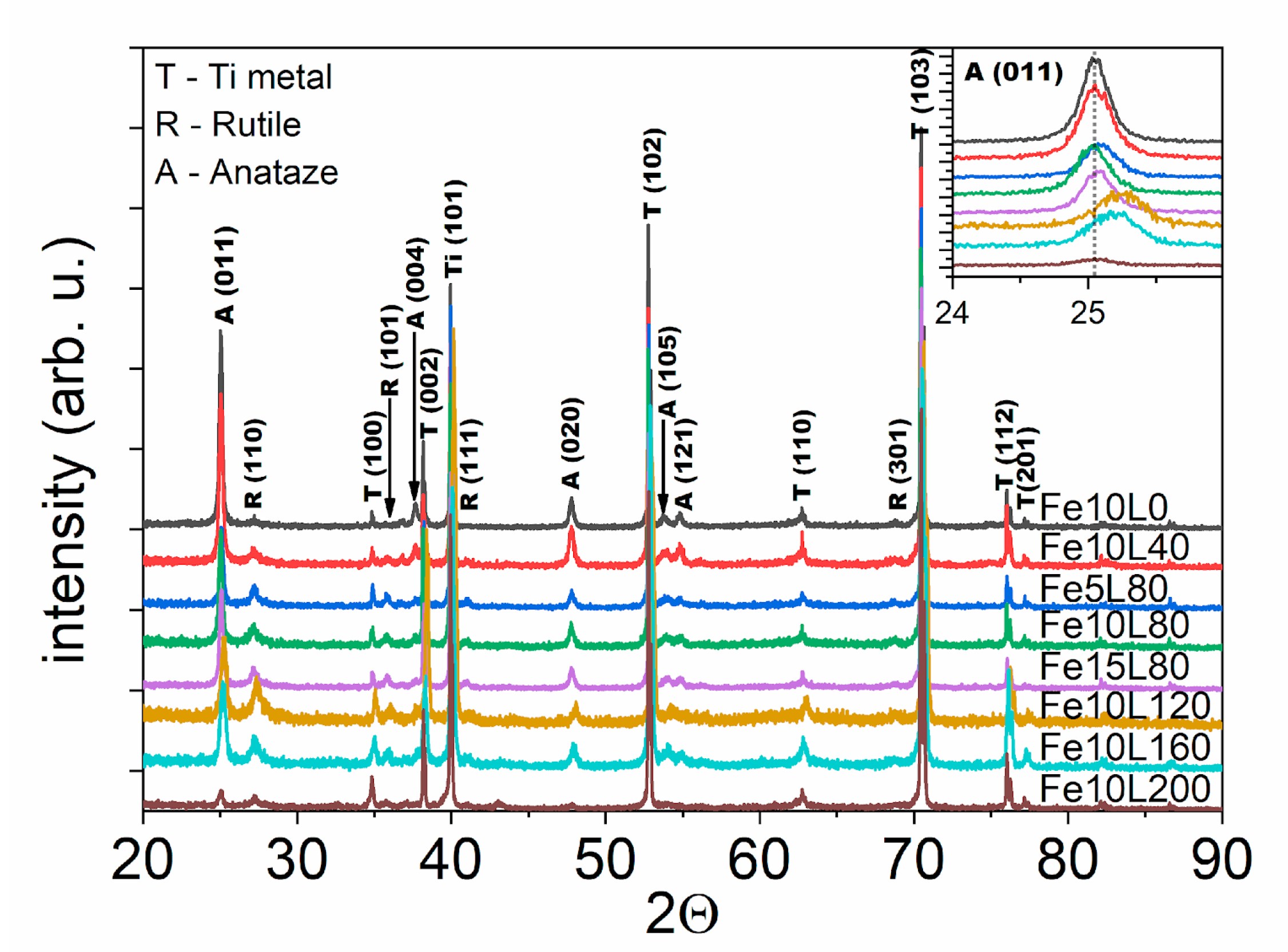
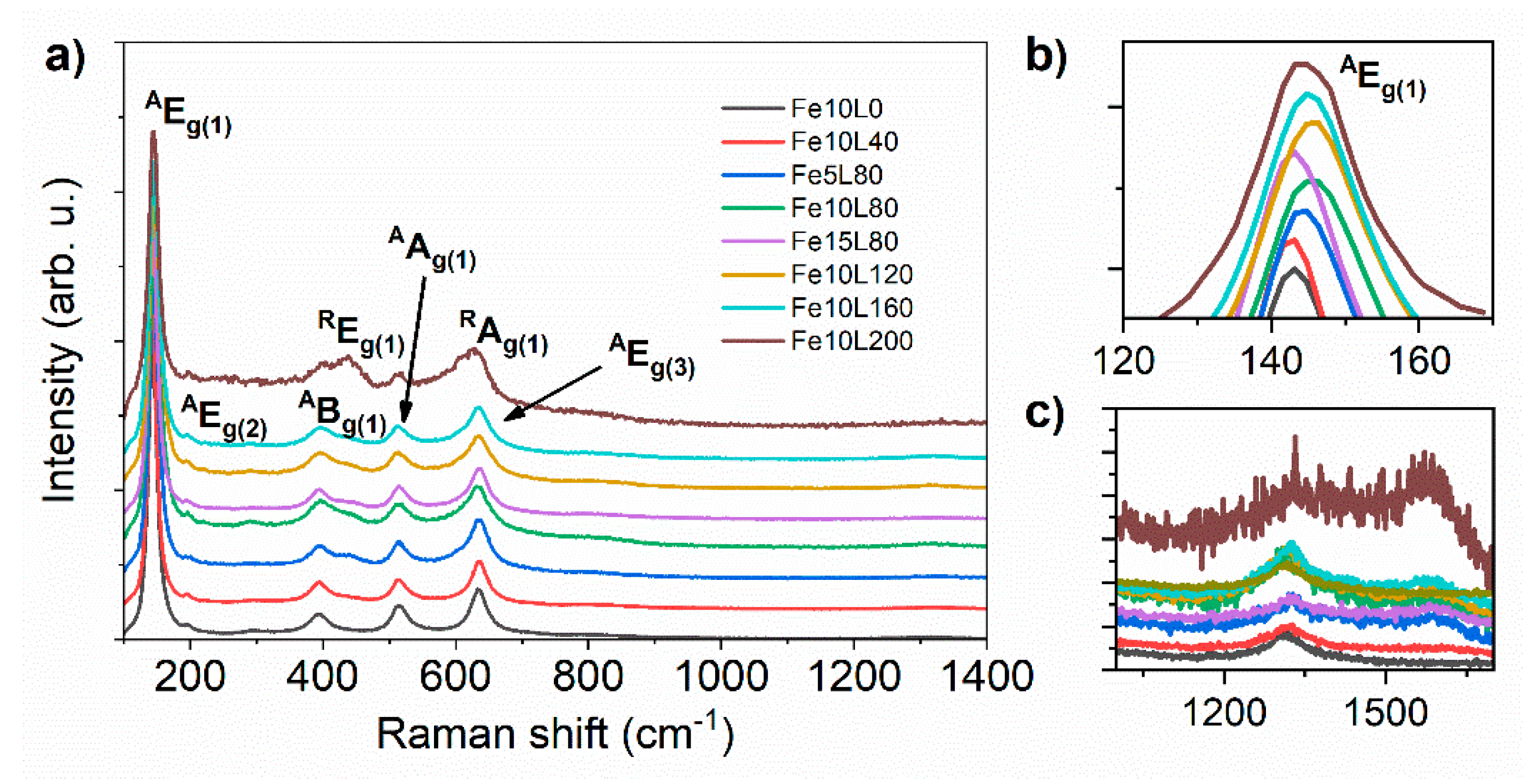
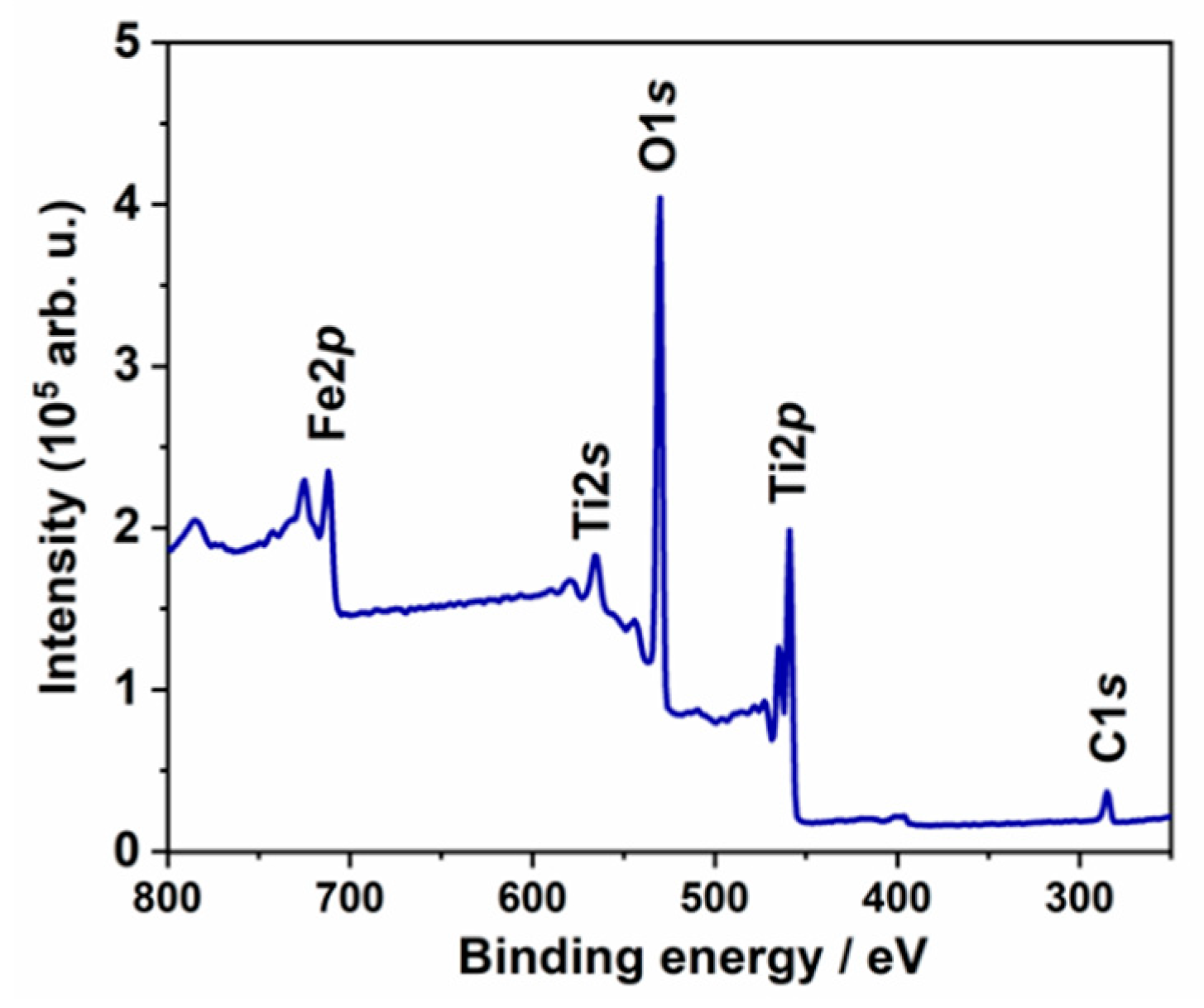
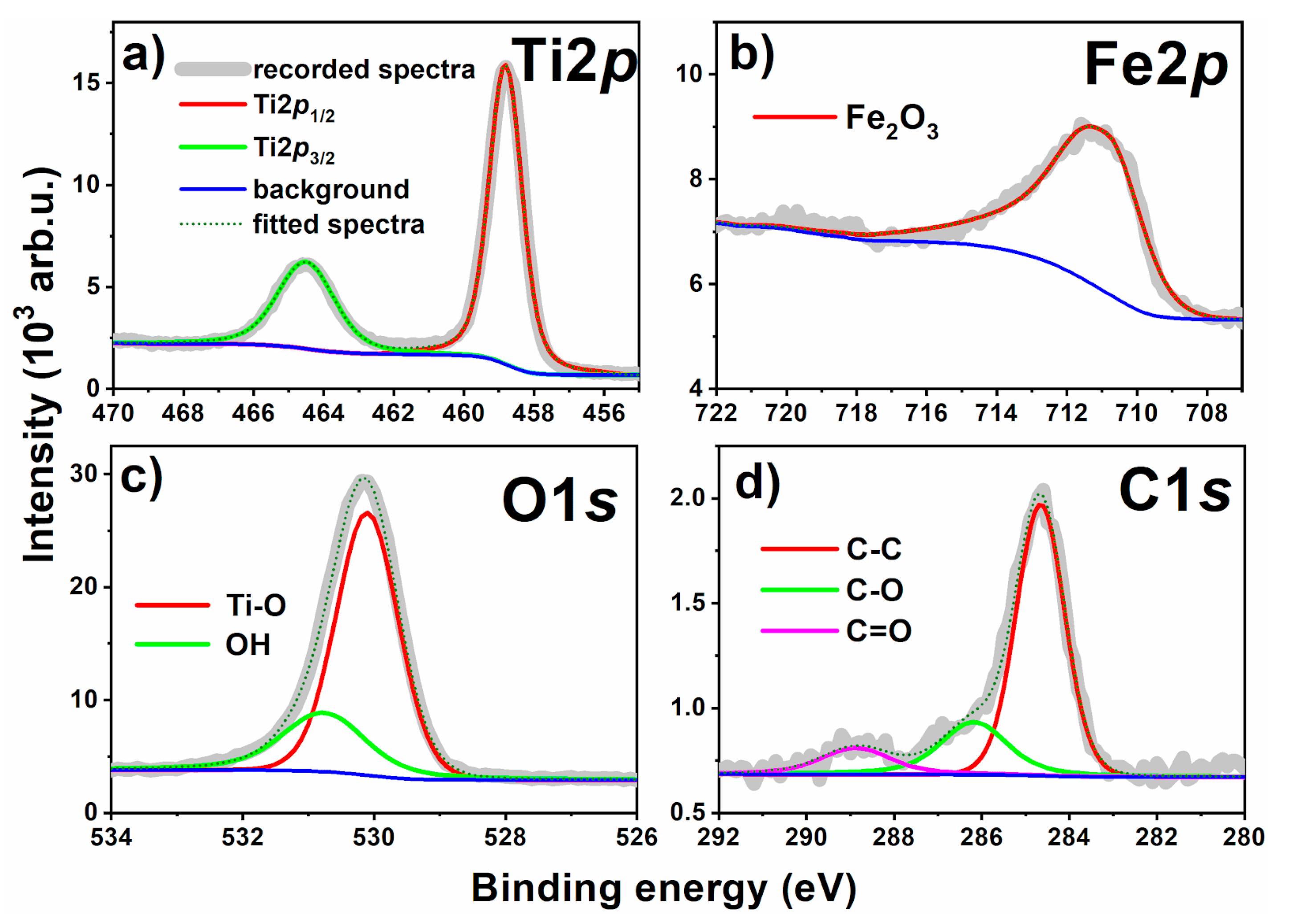
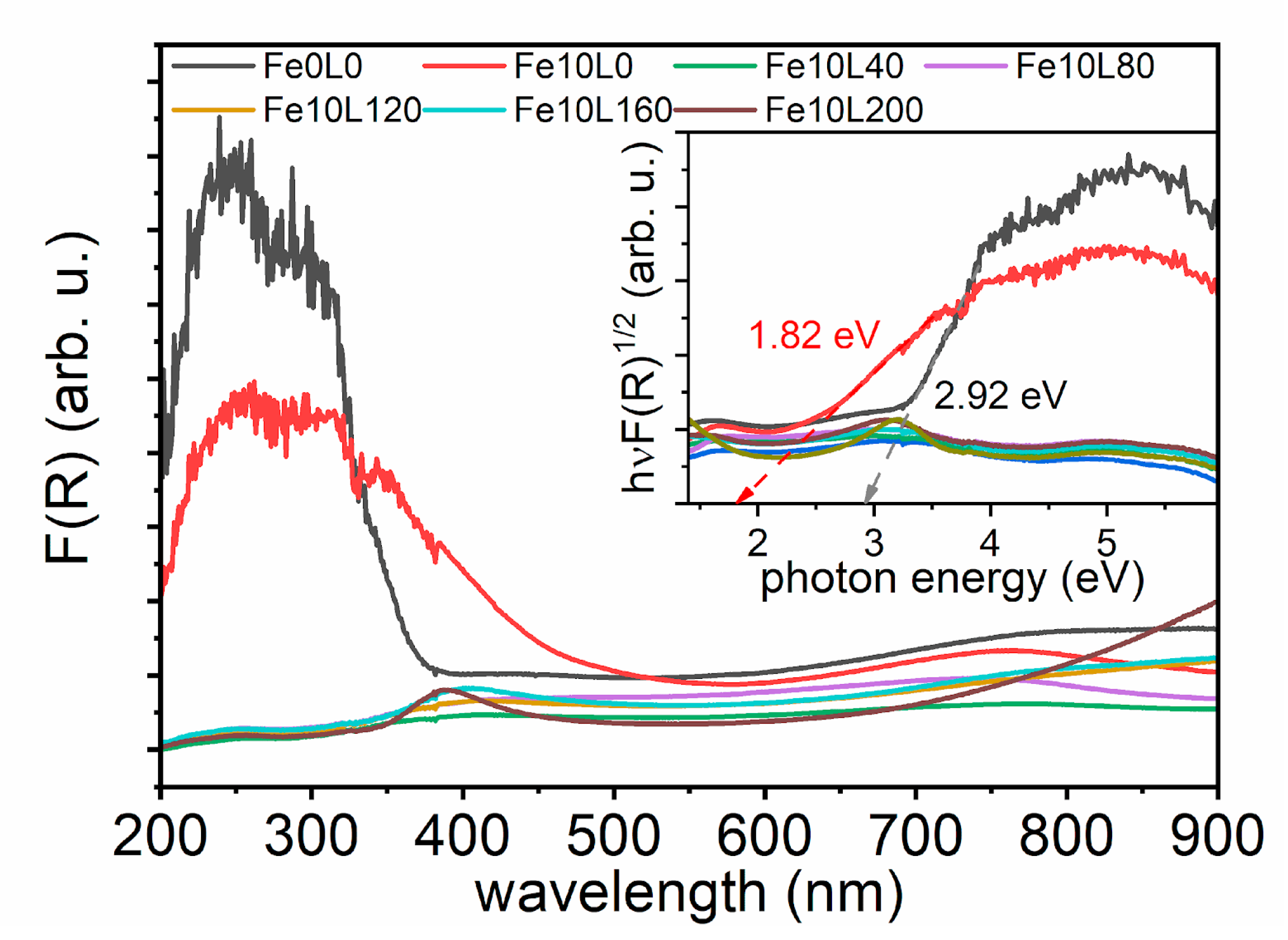
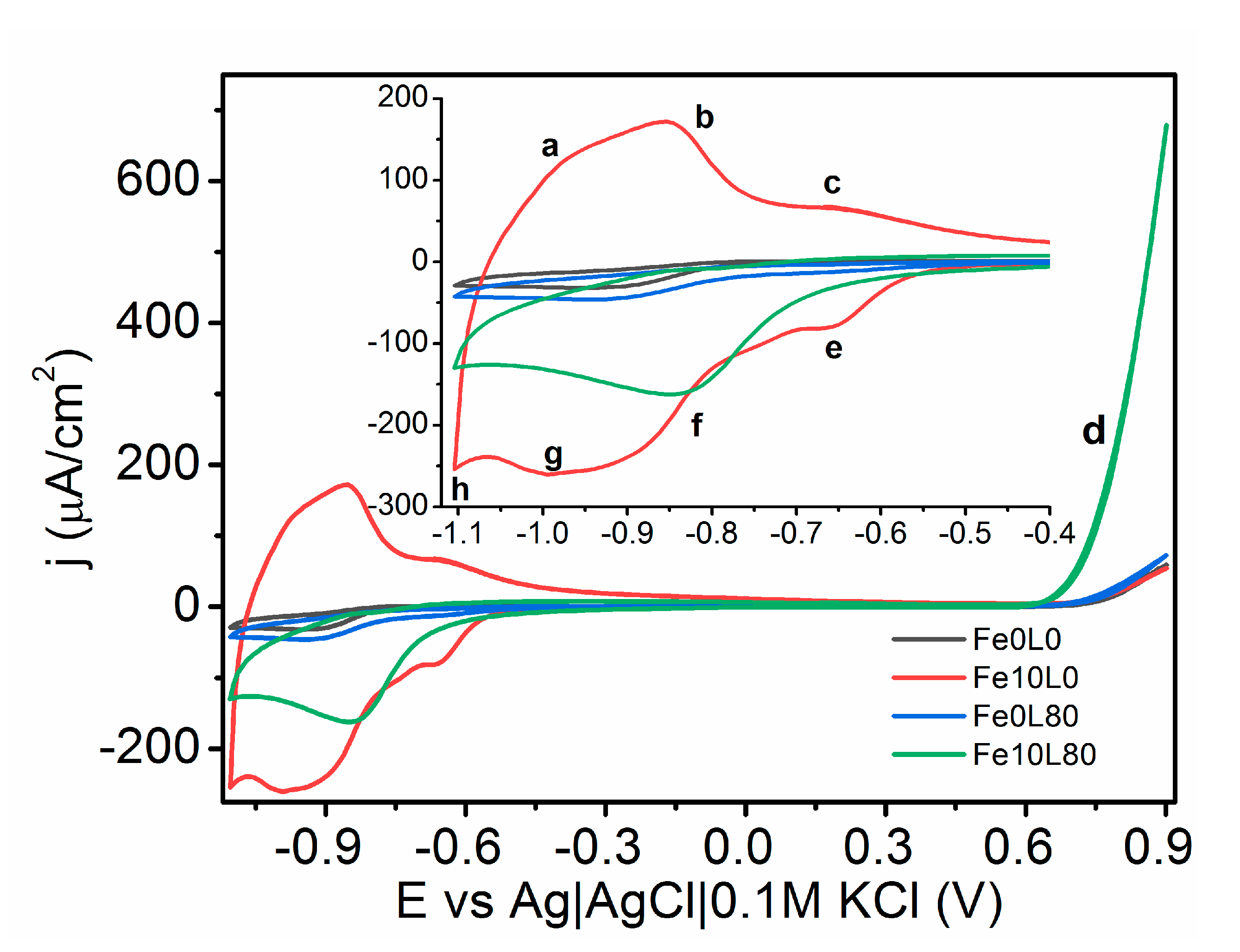
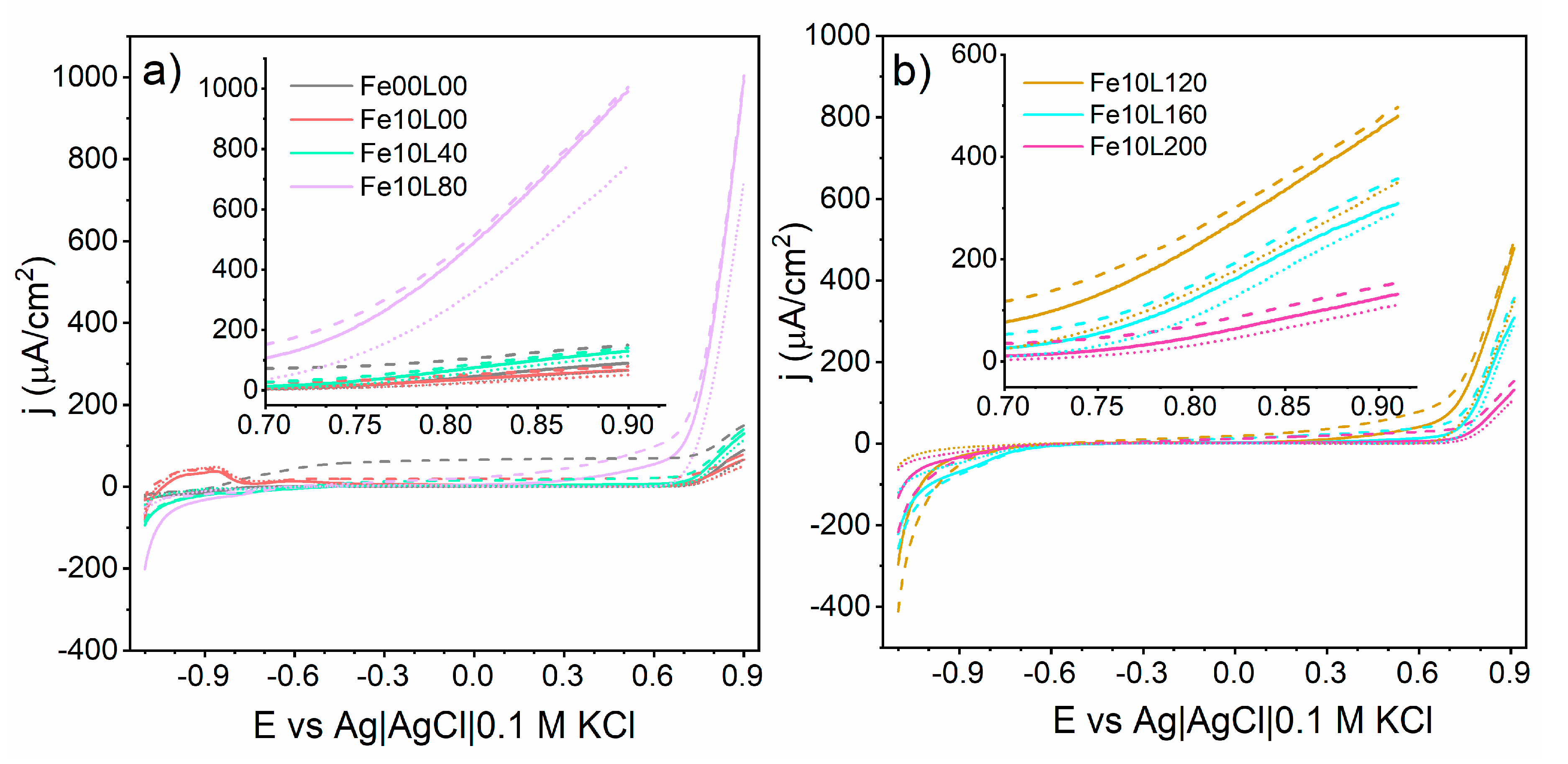
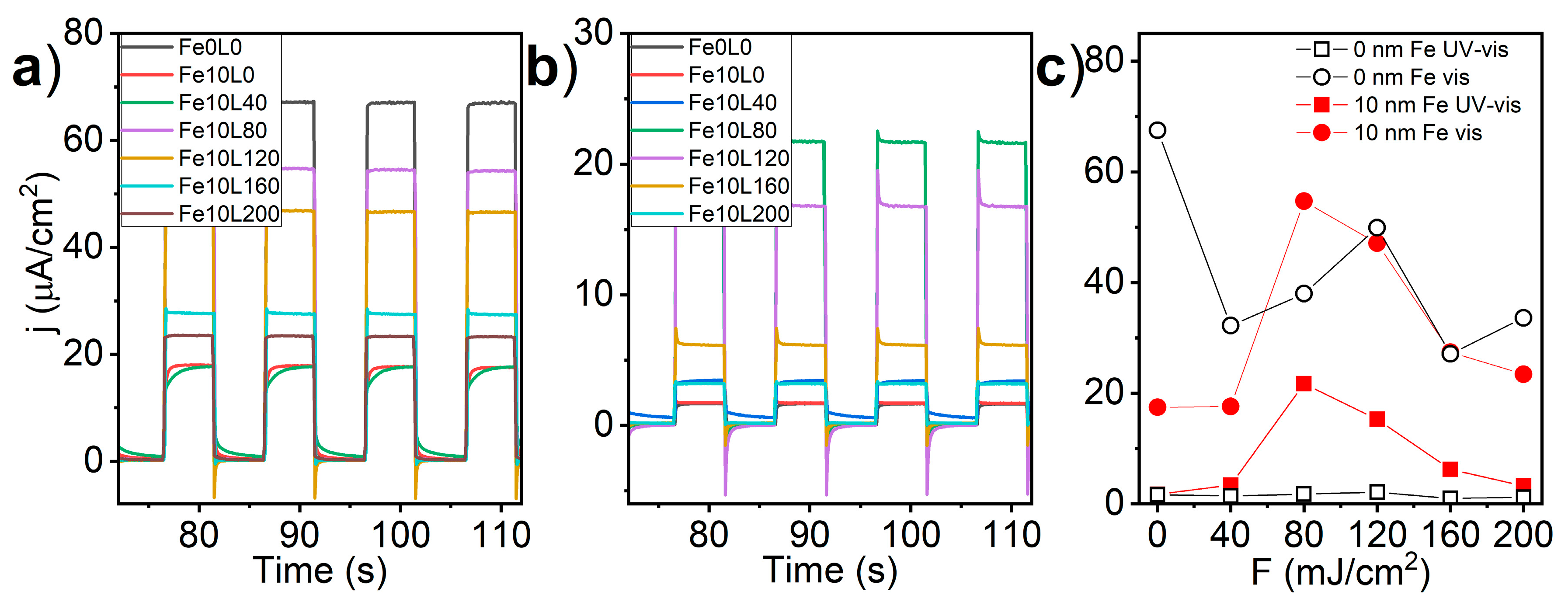
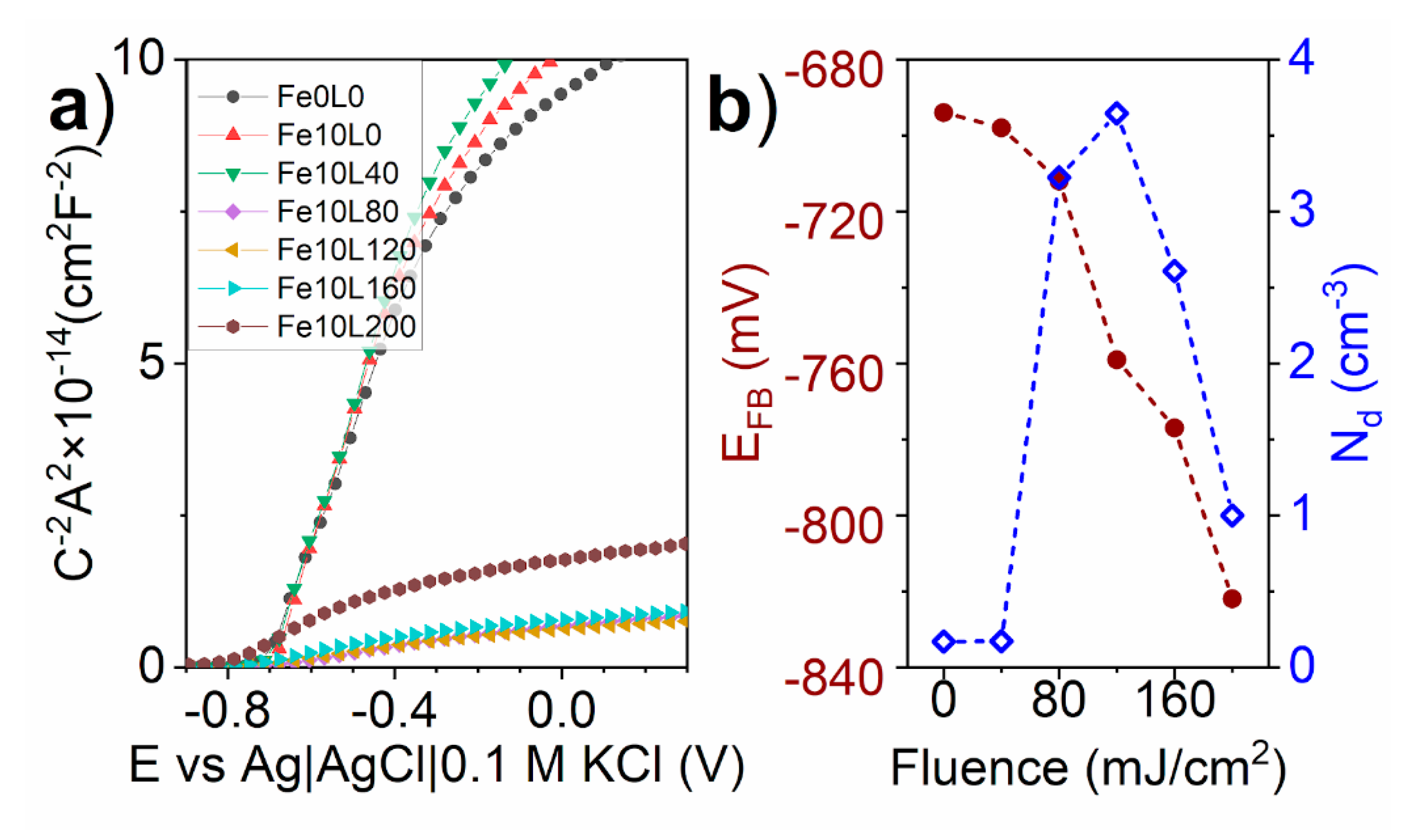
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Energy Outlook 2019—Analysis. Available online: https://www.iea.org/reports/world-energy-outlook-2019 (accessed on 4 May 2020).
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F. Hydrogen energy. Philos. Trans. R. Soc. A 2007, 365, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar Energy Supply and Storage for the Legacy and Nonlegacy Worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shih, I.; Wang, D.; Mi, Z. Roadmap on solar water splitting: Current status and future prospects. Nano Futures 2017, 1, 022001. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef] [PubMed]
- Van Benthem, K.; Elsässer, C.; French, R.H. Bulk electronic structure of SrTiO3: Experiment and theory. J. Appl. Phys. 2001, 90, 6156–6164. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Syama, S.; Reshma, S.C.; Sreekannth, P.J.; Varma, H.K.; Mohanan, P.V. Effect of zinc oxide nanoparticles on cellular oxidative stress and antioxidant defence mechanisms in mouse liver. Environ. Toxicol. Chem. 2013, 95, 495–503. [Google Scholar] [CrossRef]
- Makumire, S.; Chakravadhanula, V.S.K.; Kollisch, G.; Redel, E.; Shonhai, A. Immunomodulatory activity of zinc peroxide (ZnO2) and titanium dioxide (TiO2) nanoparticles and their effects on DNA and protein integrity. Toxicol. Lett. 2014, 227, 56–64. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, V.; Aucouturier, M.; Darque-Ceretti, E. Anodic oxidation of titanium and TA6V alloy in chromic media. An electrochemical approach. Electrochim. Acta 1999, 45, 921–929. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Melia, M.A.; Tsui, L.; Fitz-Gerald, J.M.; Zangari, G. Laser-Induced Surface Modification at Anatase TiO2 Nanotube Array Photoanodes for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2017, 121, 17121–17128. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Sawczak, M.; Karczewski, J.; Ryl, J.; Cenian, A. Ordered titania nanotubes layer selectively annealed by laser beam for high contrast electrochromic switching. Thin Solid Films 2018, 659, 48–56. [Google Scholar] [CrossRef]
- Liang, Z.; Hou, H.; Fang, Z.; Gao, F.; Wang, L.; Chen, D.; Yang, W. Hydrogenated TiO2 Nanorod Arrays Decorated with Carbon Quantum Dots toward Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 19167–19175. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2 -production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Zu, G.; Li, H.; Liu, S.; Li, D.; Wang, J.; Zhao, J. Highly efficient mass determination of TiO2 nanotube arrays and its application in lithium-ion batteries. Sustain. Mater. Technol. 2018, 18, e00079. [Google Scholar] [CrossRef]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Optimized Spacing between TiO2 Nanotubes for Enhanced Light Harvesting and Charge Transfer. ChemElectroChem 2018, 5, 3183–3190. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Cai, J.; Huang, P.; Su, Y.; Lin, C. A facile hydrothermal deposition of ZnFe2O4 nanoparticles on TiO2 nanotube arrays for enhanced visible light photocatalytic activity. J. Mater. Chem. A 2013, 1, 12082. [Google Scholar] [CrossRef]
- Kuang, S.; Yang, L.; Luo, S.; Cai, Q. Fabrication, characterization and photoelectrochemical properties of Fe2O3 modified TiO2 nanotube arrays. Appl. Surf. Sci. 2009, 255, 7385–7388. [Google Scholar] [CrossRef]
- Litter, M.I.; Navío, J.A. Comparison of the photocatalytic efficiency of TiO2, iron oxides and mixed Ti(IV) Fe(III) oxides: Photodegradation of oligocarboxylic acids. J. Photochem. Photobiol. A Chem. 1994, 84, 183–193. [Google Scholar] [CrossRef]
- Molenda, Z.; Grochowska, K.; Karczewski, J.; Ryl, J.; Darowicki, K.; Rysz, J.; Cenian, A.; Siuzdak, K. The influence of the Cu2O deposition method on the structure, morphology and photoresponse of the ordered TiO2NTs/Cu2O heterojunction. Mater. Res. Express 2020, 6, 1250b6. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Sun, L.; Mao, Y.; Wang, M.; Lin, C. An ultrasound-assisted deposition of NiO nanoparticles on TiO2 nanotube arrays for enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8223. [Google Scholar] [CrossRef]
- Roy, P.; Kim, D.; Paramasivam, I.; Schmuki, P. Improved efficiency of TiO2 nanotubes in dye sensitized solar cells by decoration with TiO2 nanoparticles. Electrochem. Commun. 2009, 11, 1001–1004. [Google Scholar] [CrossRef]
- Chen, S.; Paulose, M.; Ruan, C.; Mor, G.K.; Varghese, O.K.; Kouzoudis, D.; Grimes, C.A. Electrochemically synthesized CdS nanoparticle-modified TiO2 nanotube-array photoelectrodes: Preparation, characterization, and application to photoelectrochemical cells. J. Photochem. Photobiol. A Chem. 2006, 177, 177–184. [Google Scholar] [CrossRef]
- Assaud, L.; Brazeau, N.; Barr, M.K.S.; Hanbücken, M.; Ntais, S.; Baranova, E.A.; Santinacci, L. Atomic Layer Deposition of Pd Nanoparticles on TiO2 Nanotubes for Ethanol Electrooxidation: Synthesis and Electrochemical Properties. ACS Appl. Mater. Interfaces 2015, 7, 24533–24542. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Han, H.; Riboni, F.; Karlicky, F.; Kment, S.; Goswami, A.; Sudhagar, P.; Yoo, J.; Wang, L.; Tomanec, O.; Petr, M.; et al. α-Fe2O3/TiO2 3D hierarchical nanostructures for enhanced photoelectrochemical water splitting. Nanoscale 2017, 9, 134–142. [Google Scholar] [CrossRef]
- Kurien, U.; Hu, Z.; Lee, H.; Dastoor, A.P.; Ariya, P.A. Radiation enhanced uptake of HgO(g) on iron (oxyhydr)oxide nanoparticles. RSC Adv. 2017, 7, 45010–45021. [Google Scholar] [CrossRef] [Green Version]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting–superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Lin, Y.-G.; Hsu, Y.-K.; Lin, Y.-C.; Chen, Y.-C. Electrodeposited Fe2TiO5 nanostructures for photoelectrochemical oxidation of water. Electrochim. Acta 2016, 213, 898–903. [Google Scholar] [CrossRef]
- Bassi, P.S.; Antony, R.P.; Boix, P.P.; Fang, Y.; Barber, J.; Wong, L.H. Crystalline Fe2O3/Fe2TiO5 heterojunction nanorods with efficient charge separation and hole injection as photoanode for solar water oxidation. Nano Energy 2016, 22, 310–318. [Google Scholar] [CrossRef]
- Hsu, M.-Y.; Van Thang, N.; Wang, C.; Leu, J. Structural and morphological transformations of TiO2 nanotube arrays induced by excimer laser treatment. Thin Solid Films 2012, 520, 3593–3599. [Google Scholar] [CrossRef]
- Kusinski, J.; Kac, S.; Kopia, A.; Radziszewska, A.; Rozmus-Górnikowska, M.; Major, B.; Major, L.; Marczak, J.; Lisiecki, A. Laser modification of the materials surface layer—A review paper. B Pol. Acad. Sci-Tech. 2012, 60, 711–728. [Google Scholar] [CrossRef]
- Haryński, Ł.; Grochowska, K.; Karczewski, J.; Ryl, J.; Siuzdak, K. Scalable Route toward Superior Photoresponse of UV-Laser-Treated TiO2 Nanotubes. ACS Appl. Mater. Interfaces 2020, 12, 3225–3235. [Google Scholar] [CrossRef]
- Dholam, R.; Patel, N.; Adami, M.; Miotello, A. Hydrogen production by photocatalytic water-splitting using Cr- or Fe-doped TiO2 composite thin films photocatalyst. Int. J. Hydrogen Energy 2009, 34, 5337–5346. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Y.; Chen, J.; Lin, J.; Zhang, X.; Wang, Z.; Zhou, J. Enhanced mechanism of the photo-thermochemical cycle based on effective Fe-doping TiO2 films and DFT calculations. Appl. Catal. B Environ. 2017, 204, 324–334. [Google Scholar] [CrossRef]
- Pradubkorn, P.; Maensiri, S.; Swatsitang, E.; Laokul, P. Preparation and characterization of hollow TiO2 nanospheres: The effect of Fe3+ doping on their microstructure and electronic structure. Curr. Appl. Phys. 2020, 20, 178–185. [Google Scholar] [CrossRef]
- Ali, G.; Chen, C.; Yoo, S.H.; Kum, J.M.; Cho, S.O. Fabrication of complete titania nanoporous structures via electrochemical anodization of Ti. Nanoscale Res. Lett. 2011, 6, 332. [Google Scholar] [CrossRef] [Green Version]
- Swift, P. Adventitious carbon—The panacea for energy referencing? Surf. Interface Anal. 1982, 4, 47–51. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-Band Potential of a Semiconductor: Using the Mott–Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Park, B.H.; Li, L.S.; Gibbons, B.J.; Huang, J.Y.; Jia, Q.X. Photovoltaic response and dielectric properties of epitaxial anatase-TiO2 films grown on conductive La0.5Sr0.5CoO3 electrodes. Appl. Phys. Lett. 2001, 79, 2797–2799. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Karczewski, J.; Kupracz, P.; Grochowska, K.; Załęski, K.; Pshyk, O.; Coy, E.; Bartmański, M.; Szkodo, M.; Siuzdak, K. Laser-assisted modification of titanium dioxide nanotubes in a tilted mode as surface modification and patterning strategy. Appl. Surf. Sci. 2020, 508, 145143. [Google Scholar] [CrossRef]
- Werner, W.S.M.; Glantschnig, K.; Ambrosch-Draxl, C. Optical Constants and Inelastic Electron-Scattering Data for 17 Elemental Metals. J. Phys. Chem. Ref. Data 2009, 38, 1013–1092. [Google Scholar] [CrossRef]
- Querry, M.R. Optical Constants, Contractor Report CRDC-CR-85034. 1985. Available online: https://apps.dtic.mil/dtic/tr/fulltext/u2/a158623.pdf (accessed on 9 September 2020).
- Siefke, T.; Kroker, S.; Pfeiffer, K.; Puffky, O.; Dietrich, K.; Franta, D.; Ohlídal, I.; Szeghalmi, A.; Kley, E.-B.; Tünnermann, A. Materials Pushing the Application Limits of Wire Grid Polarizers further into the Deep Ultraviolet Spectral Range. Adv. Opt. Mater. 2016, 4, 1780–1786. [Google Scholar] [CrossRef]
- Li Bassi, A.; Cattaneo, D.; Russo, V.; Bottani, C.E.; Barborini, E.; Mazza, T.; Piseri, P.; Milani, P.; Ernst, F.O.; Wegner, K.; et al. Raman spectroscopy characterization of titania nanoparticles produced by flame pyrolysis: The influence of size and stoichiometry. J. Appl. Phys. 2005, 98, 074305. [Google Scholar] [CrossRef]
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman spectrum of anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Narayanan, P.S. Raman spectrum of rutile (TiO2). Proc. Indian Acad. Sci. (Math. Sci.) 1950, 32, 279. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Silva, S.V.; de Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- McCarty, K.F. Inelastic light scattering in α-Fe2O3: Phonon vs magnon scattering. Solid State Commun. 1988, 68, 799–802. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): A new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Mao, S.S. Black TiO2 for solar hydrogen conversion. J. Mater. 2017, 3, 96–111. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, J.-G.; Kamiyama, H.; Katada, M.; Ohashi, N.; Moriyoshi, Y.; Ishigaki, T. Pyrogenic Iron(III)-Doped TiO2 Nanopowders Synthesized in RF Thermal Plasma: Phase Formation, Defect Structure, Band Gap, and Magnetic Properties. J. Am. Chem. Soc. 2005, 127, 10982–10990. [Google Scholar] [CrossRef]
- Lipińska, W.; Siuzdak, K.; Ryl, J.; Barski, P.; Śliwiński, G.; Grochowska, K. The optimization of enzyme immobilization at Au-Ti nanotextured platform and its impact onto the response towards glucose in neutral media. Mater. Res. Express 2019, 6, 1150e3. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Yuan, Q.-B.; Duan, D.-H.; Zhang, Z.-L.; Hao, X.-G.; Wei, G.-Q.; Liu, S.-B. Electrochemical activity and stability of core–shell Fe2O3/Pt nanoparticles for methanol oxidation. J. Power Sources 2013, 243, 622–629. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Gilbert, B.; Frandsen, C.; Maxey, E.R.; Sherman, D.M. Band-gap measurements of bulk and nanoscale hematite by soft x-ray spectroscopy. Phys. Rev. B 2009, 79, 035108. [Google Scholar] [CrossRef] [Green Version]
- Lany, S. Band-structure calculations for the 3d transition metal oxides in G W. Phys. Rev. B 2013, 87, 085112. [Google Scholar] [CrossRef] [Green Version]
- Persson, K. Materials Data on TiFeO3 (SG:148) by Materials Project; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2014. [CrossRef]
- Persson, K. Materials Data on Ti(FeO2)2 (SG:15) by Materials Project; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2014. [CrossRef]
- Persson, K. Materials Data on TiFe2O5 (SG:15) by Materials Project; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2020. [CrossRef]
- Macak, J.M.; Tsuchiya, H.; Ghicov, A.; Yasuda, K.; Hahn, R.; Bauer, S.; Schmuki, P. TiO2 nanotubes: Self-organized electrochemical formation, properties and applications. Curr. Opin. Solid State Mater. Sci. 2007, 11, 3–18. [Google Scholar] [CrossRef]
- Schmuki, P. Passivity of Iron in Alkaline Solutions Studied by In Situ XANES and a Laser Reflection Technique. J. Electrochem. Soc. 1999, 146, 2097. [Google Scholar] [CrossRef]
- Neugebauer, H.; Moser, A.; Strecha, P.; Neckel, A. In Situ FTIR Spectroscopy of Iron Electrodes in Alkaline Solutions. J. Electrochem. Soc. 1990, 137, 4. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M.; Rathouský, J.; Zukalb, A. Nanocrystalline TiO2 (Anatase) Electrodes: Surface Morphology, Adsorption, and Electrochemical Properties. J. Electrochem. Soc. 1996, 143, 394. [Google Scholar] [CrossRef]
- Bülter, H.; Denuault, G.; Mátéfi-Tempfli, S.; Mátéfi-Tempfli, M.; Dosche, C.; Wittstock, G. Electrochemical analysis of nanostructured iron oxides using cyclic voltammetry and scanning electrochemical microscopy. Electrochim. Acta 2016, 222, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Xie, M.; Travis, J.J.; Wang, G.; Sun, H.; Lian, J.; George, S.M. Pseudocapacitance of amorphous TiO2 thin films anchored to graphene and carbon nanotubes using atomic layer deposition. J. Phys. Chem. C 2013, 117, 44–22497. [Google Scholar] [CrossRef]
- Krysa, J.; Zlamal, M.; Kment, S.; Brunclikova, M.; Hubicka, Z. TiO2 and Fe2O3 Films for Photoelectrochemical Water Splitting. Molecules 2015, 20, 1046–1058. [Google Scholar] [CrossRef]
- Sivula, K. Metal Oxide Photoelectrodes for Solar Fuel Production, Surface Traps, and Catalysis. J. Phys. Chem. Lett. 2013, 4, 1624–1633. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of rapid electrode reactions. Discuss. Faraday Soc. 1947, 1, 11. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Dubale, A.A.; Su, W.-N.; Chen, H.-M.; Hwang, B.-J. Sequentially surface modified hematite enables lower applied bias photoelectrochemical water splitting. Phys. Chem. Chem. Phys. 2017, 19, 20881–20890. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Kong, D.-S.; Wang, Z.; Feng, Y.-Y.; Li, W.-J. Inhibiting effect of carbonate on the photoinduced flatband potential shifts during water photooxidation at TiO2/solution interface. J. Solid State Electrochem. 2017, 21, 1467–1475. [Google Scholar] [CrossRef]
- Jackman, M.J.; Thomas, A.G.; Muryn, C. Photoelectron Spectroscopy Study of Stoichiometric and Reduced Anatase TiO2 (101) Surfaces: The Effect of Subsurface Defects on Water Adsorption at Near-Ambient Pressures. J. Phys. Chem. C 2015, 119, 13682–13690. [Google Scholar] [CrossRef]
- Sołtys-Mróz, M.; Syrek, K.; Pierzchała, J.; Wiercigroch, E.; Malek, K.; Sulka, G.D. Band gap engineering of nanotubular Fe2O3-TiO2 photoanodes by wet impregnation. Appl. Surf. Sci. 2020, 517, 146195. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.; Gupta, A.; Sekhar, P.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Fe-doped TiO2 powders for solar light response and photocatalytic applications. PAC 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Lee, T.; Ryu, H.; Lee, W.-J. Photoelectrochemical properties of iron (III)-doped TiO2 nanorods. Ceram. Int. 2015, 41, 7582–7589. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupracz, P.; Grochowska, K.; Karczewski, J.; Wawrzyniak, J.; Siuzdak, K. The Effect of Laser Re-Solidification on Microstructure and Photo-Electrochemical Properties of Fe-Decorated TiO2 Nanotubes. Materials 2020, 13, 4019. https://doi.org/10.3390/ma13184019
Kupracz P, Grochowska K, Karczewski J, Wawrzyniak J, Siuzdak K. The Effect of Laser Re-Solidification on Microstructure and Photo-Electrochemical Properties of Fe-Decorated TiO2 Nanotubes. Materials. 2020; 13(18):4019. https://doi.org/10.3390/ma13184019
Chicago/Turabian StyleKupracz, Piotr, Katarzyna Grochowska, Jakub Karczewski, Jakub Wawrzyniak, and Katarzyna Siuzdak. 2020. "The Effect of Laser Re-Solidification on Microstructure and Photo-Electrochemical Properties of Fe-Decorated TiO2 Nanotubes" Materials 13, no. 18: 4019. https://doi.org/10.3390/ma13184019
APA StyleKupracz, P., Grochowska, K., Karczewski, J., Wawrzyniak, J., & Siuzdak, K. (2020). The Effect of Laser Re-Solidification on Microstructure and Photo-Electrochemical Properties of Fe-Decorated TiO2 Nanotubes. Materials, 13(18), 4019. https://doi.org/10.3390/ma13184019