Laser-Assisted Synthesis and Oxygen Generation of Nickel Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
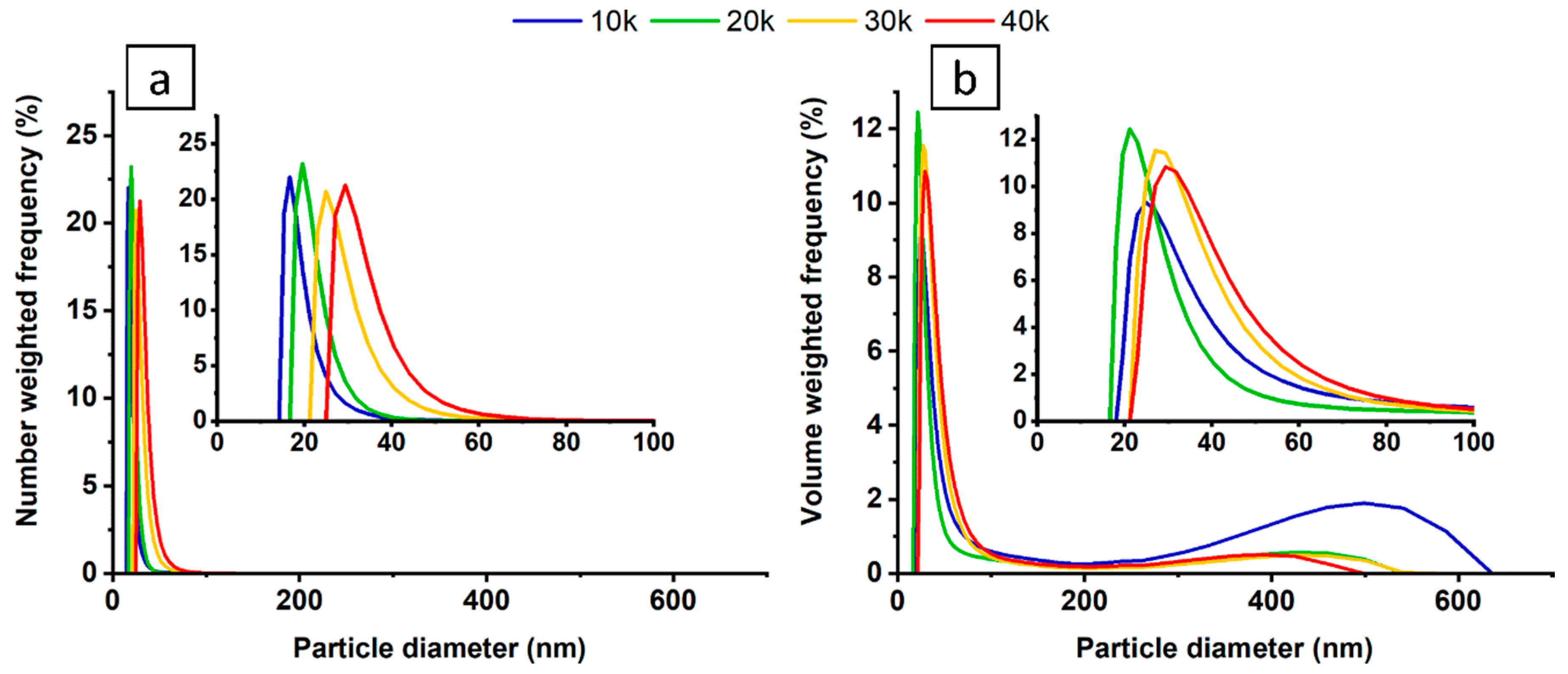
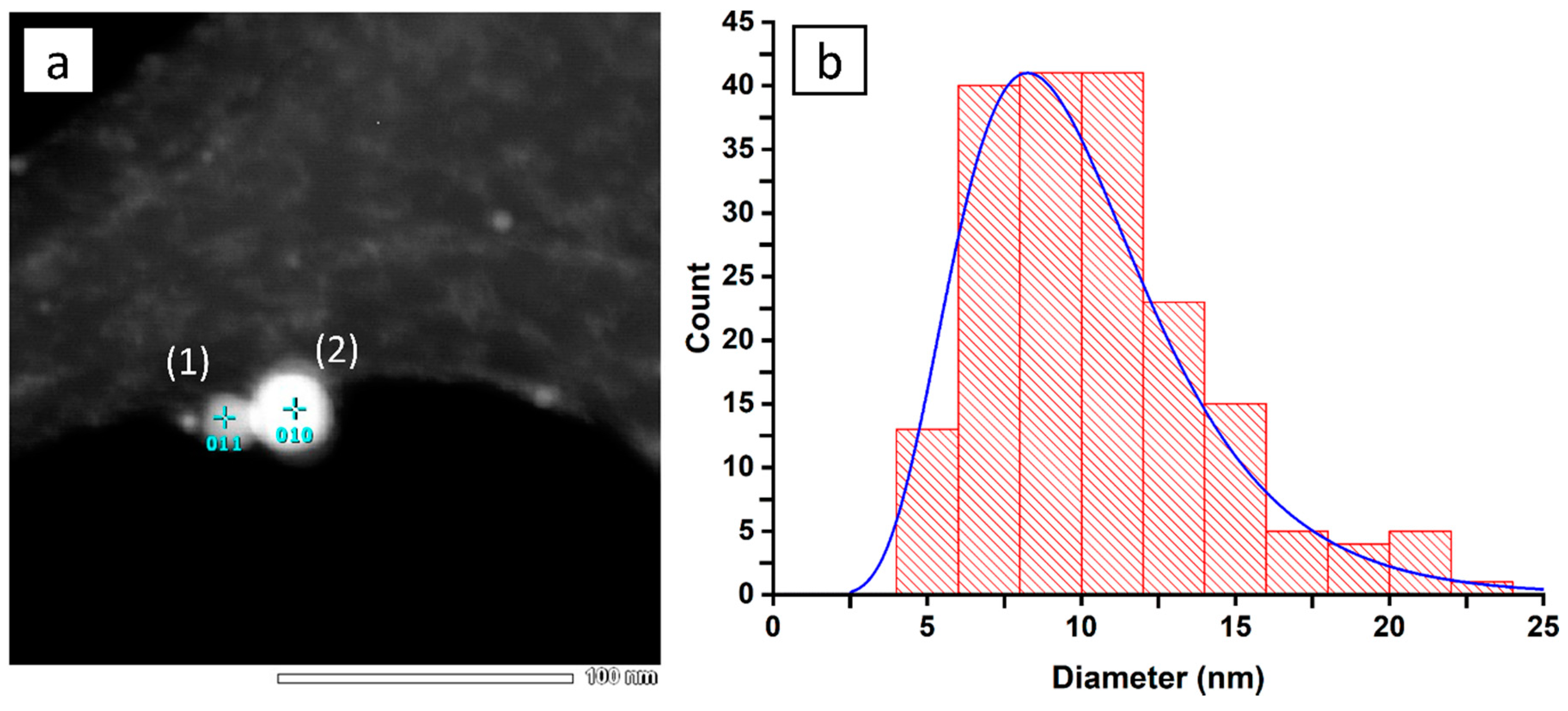
3.1. Colloid Characterisation
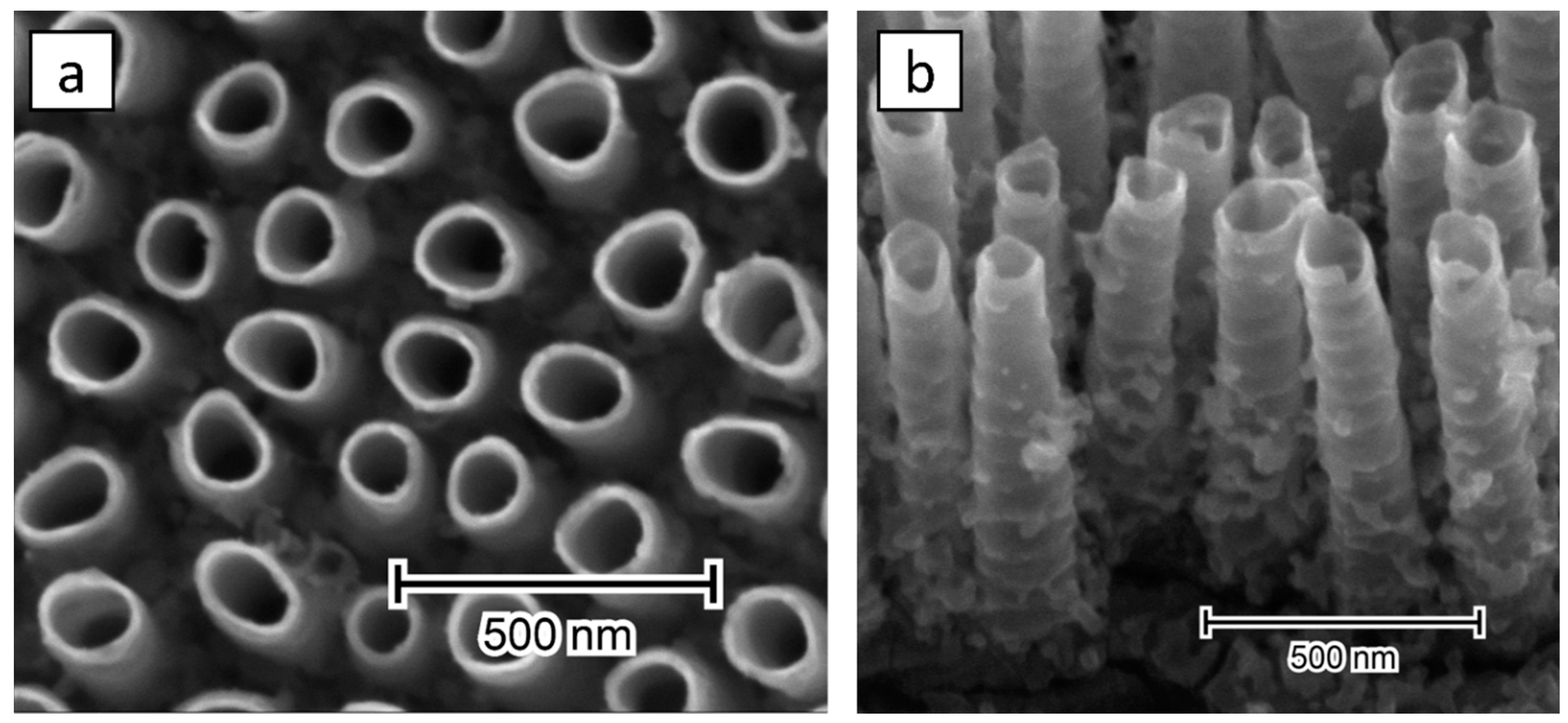
3.2. Electrode Morphology
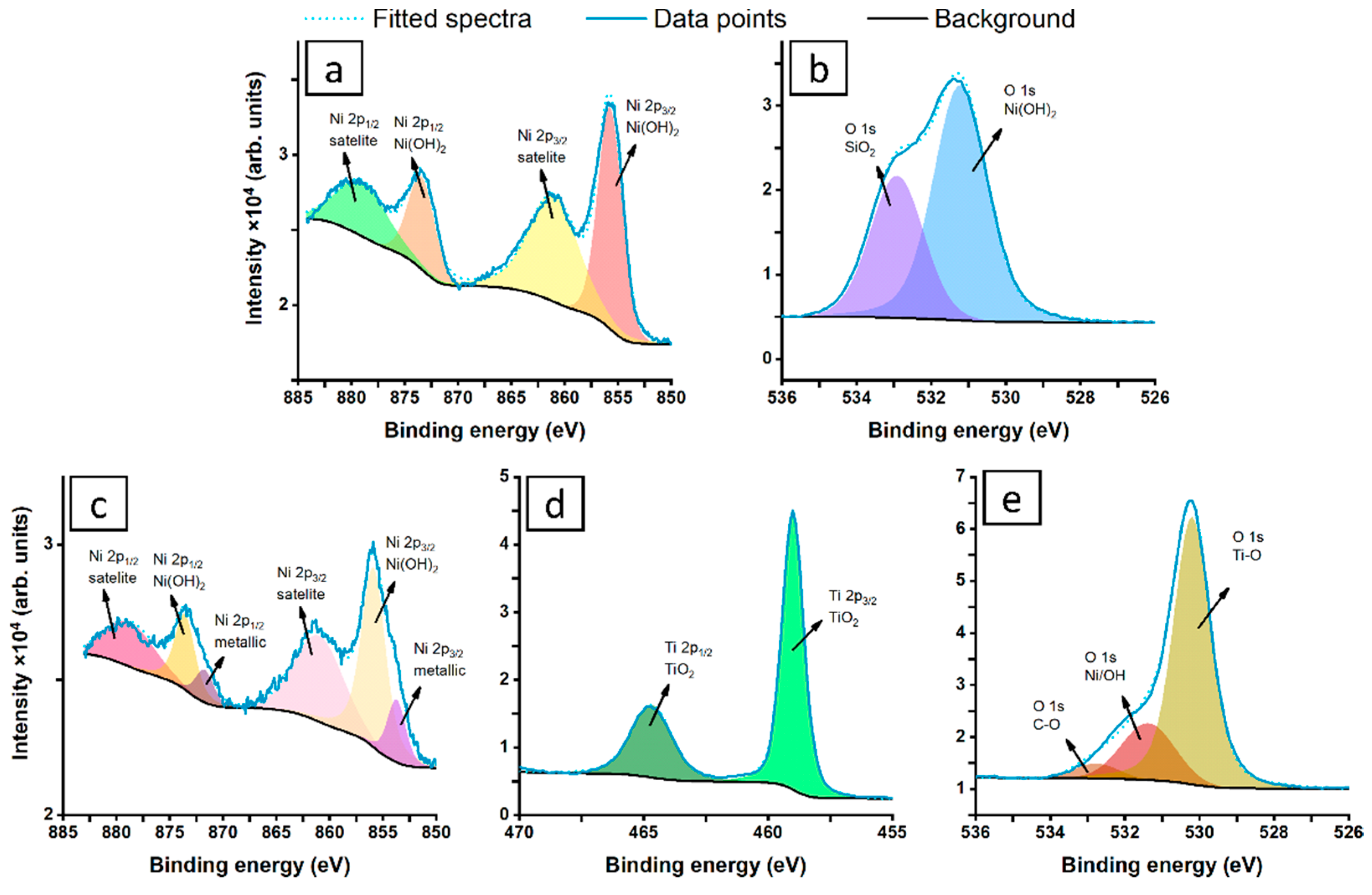
3.3. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reguera, J.; Langer, J.; Jiménez de Aberasturi, D.; Liz-Marzán, L.M. Anisotropic metal nanoparticles for surface enhanced Raman scattering. Chem. Soc. Rev. 2017, 46, 3866–3885. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Yang, L.; Ren, L.; Wang, D.; Ye, J. Metal nanoparticles induced photocatalysis. Natl. Sci. Rev. 2017, 4, 761–780. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Hanske, C.; Sanz-Ortiz, M.N.; Liz-Marzán, L.M. Silica-Coated Plasmonic Metal Nanoparticles in Action. Adv. Mater. 2018, 30, 1707003. [Google Scholar] [CrossRef] [PubMed]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Inorganic and metal nanoparticles and their antimicrobial activity in food packaging applications. Crit. Rev. Microbiol. 2018, 44, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Targeting 2018, 26, 617–632. [Google Scholar] [CrossRef]
- Yan, Y.; Warren, S.C.; Fuller, P.; Grzybowski, B.A. Chemoelectronic circuits based on metal nanoparticles. Nat. Nanotechnol. 2016, 11, 603–608. [Google Scholar] [CrossRef]
- Zeng, H.; Du, X.-W.; Singh, S.C.; Kulinich, S.A.; Yang, S.; He, J.; Cai, W. Nanomaterials via Laser Ablation/Irradiation in Liquid: A Review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Huang, H.; Lai, J.; Lu, J.; Li, Z. Pulsed laser ablation of bulk target and particle products in liquid for nanomaterial fabrication. AIP Adv. 2019, 9, 015307. [Google Scholar] [CrossRef]
- Waag, F.; Gökce, B.; Barcikowski, S. Ablation target cooling by maximizing the nanoparticle productivity in laser synthesis of colloids. Appl. Surf. Sci. 2019, 466, 647–656. [Google Scholar] [CrossRef]
- Resano-Garcia, A.; Champmartin, S.; Battie, Y.; Koch, A.; En Naciri, A.; Ambari, A.; Chaoui, N. Highly-repeatable generation of very small nanoparticles by pulsed-laser ablation in liquids of a high-speed rotating target. Phys. Chem. Chem. Phys. 2016, 18, 32868–32875. [Google Scholar] [CrossRef]
- Vadavalli, S.; Valligatla, S.; Neelamraju, B.; Dar, M.H.; Chiasera, A.; Ferrari, M.; Desai, N.R. Optical properties of germanium nanoparticles synthesized by pulsed laser ablation in acetone. Front. Phys. 2014, 2. [Google Scholar] [CrossRef]
- Valverde-Alva, M.A.; García-Fernández, T.; Villagrán-Muniz, M.; Sánchez-Aké, C.; Castañeda-Guzmán, R.; Esparza-Alegría, E.; Sánchez-Valdés, C.F.; Llamazares, J.L.S.; Herrera, C.E.M. Synthesis of silver nanoparticles by laser ablation in ethanol: A pulsed photoacoustic study. Appl. Surf. Sci. 2015, 355, 341–349. [Google Scholar] [CrossRef]
- Abdi, S.; Dorranian, D. Effect of CTAB concentration on the properties of ZnO nanoparticles produced by laser ablation method in CTAB solution. Opt. Laser Technol. 2018, 108, 372–377. [Google Scholar] [CrossRef]
- Khan, M.N.; Bashir, O.; Khan, T.A.; AL-Thabaiti, S.A.; Khan, Z. CTAB capped synthesis of bio-conjugated silver nanoparticles and their enhanced catalytic activities. J. Mol. Liq. 2018, 258, 133–141. [Google Scholar] [CrossRef]
- Biswal, A.K.; Misra, P.K. Biosynthesis and characterization of silver nanoparticles for prospective application in food packaging and biomedical fields. Mater. Chem. Phys. 2020, 250, 123014. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Daqiqeh Rezaei, S.; Tan, Y.N. Simulation guided design of silver nanostructures for plasmon-enhanced fluorescence, singlet oxygen generation and SERS applications. Phys. Chem. Chem. Phys. 2020, 22, 5673–5687. [Google Scholar] [CrossRef] [PubMed]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Kong, F.Y.; Zhang, J.W.; Li, R.F.; Wang, Z.X.; Wang, W.J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, H.; Sun, J.; Liu, P.; Zhang, H.; Xu, B.; Guo, J. Pt nanoparticles decorated high-defective graphene nanospheres as highly efficient catalysts for the hydrogen evolution reaction. Carbon 2018, 137, 405–410. [Google Scholar] [CrossRef]
- Sharma, A.; Hickman, J.; Gazit, N.; Rabkin, E.; Mishin, Y. Nickel nanoparticles set a new record of strength. Nat. Commun. 2018, 9, 4102. [Google Scholar] [CrossRef] [PubMed]
- Elango, G.; Roopan, S.M.; Dhamodaran, K.I.; Elumalai, K.; Al-Dhabi, N.A.; Arasu, M.V. Spectroscopic investigation of biosynthesized nickel nanoparticles and its larvicidal, pesticidal activities. J. Photochem. Photobiol. B 2016, 162, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Eisa, T.; Mohamed, H.O.; Choi, Y.-J.; Park, S.-G.; Ali, R.; Abdelkareem, M.A.; Oh, S.-E.; Chae, K.-J. Nickel nanorods over nickel foam as standalone anode for direct alkaline methanol and ethanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 5948–5959. [Google Scholar] [CrossRef]
- Yang, J.; Qiu, Z.; Zhao, C.; Wei, W.; Chen, W.; Li, Z.; Qu, Y.; Dong, J.; Luo, J.; Li, Z.; et al. In Situ Thermal Atomization To Convert Supported Nickel Nanoparticles into Surface-Bound Nickel Single-Atom Catalysts. Angew. Chem. Int. Ed. 2018, 57, 14095–14100. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Lee, K. Nanostructured materials on 3D nickel foam as electrocatalysts for water splitting. Nanoscale 2017, 9, 12231–12247. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Z.; Geng, F. Radially Aligned Hierarchical Nickel/Nickel–Iron (Oxy)hydroxide Nanotubes for Efficient Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 8585–8593. [Google Scholar] [CrossRef]
- Pateras, A.; Harder, R.; Manna, S.; Kiefer, B.; Sandberg, R.L.; Trugman, S.; Kim, J.W.; de la Venta, J.; Fullerton, E.E.; Shpyrko, O.G.; et al. Room temperature giant magnetostriction in single-crystal nickel nanowires. NPG Asia Mater. 2019, 11, 59. [Google Scholar] [CrossRef]
- Pu, J.; Nishikado, K.; Wang, N.; Nguyen, T.T.; Maki, T.; Qian, E.W. Core-shell nickel catalysts for the steam reforming of acetic acid. Appl. Catal. B 2018, 224, 69–79. [Google Scholar] [CrossRef]
- Nikov, R.G.; Nedyalkov, N.N.; Karashanova, D.B. Laser ablation of Ni in the presence of external magnetic field: Selection of microsized particles. Appl. Surf. Sci. 2020, 518, 146211. [Google Scholar] [CrossRef]
- Torrisi, L.; Torrisi, A. Ni, Ti, and NiTi laser ablation in vacuum and in water to deposit thin films or to generate nanoparticles in solution. Contrib. Plasma Phys. 2020, e202000070. [Google Scholar] [CrossRef]
- Tan, M.I.S.M.H.; Omar, A.F.; Rashid, M.; Hashim, U. VIS-NIR spectral and particles distribution of Au, Ag, Cu, Al and Ni nanoparticles synthesized in distilled water using laser ablation. Results Phys. 2019, 14, 102497. [Google Scholar] [CrossRef]
- Safa, M.; Dorranian, D.; Masoudi, A.A.; Matin, L.F. Characterizing nickel oxide nanostructures produced by laser ablation method: Effects of laser fluence. Appl. Phys. A 2019, 125, 687. [Google Scholar] [CrossRef]
- Paulose, M.; Prakasam, H.E.; Varghese, O.K.; Peng, L.; Popat, K.C.; Mor, G.K.; Desai, T.A.; Grimes, C.A. TiO2 Nanotube Arrays of 1000 μm Length by Anodization of Titanium Foil: Phenol Red Diffusion. J. Phys. Chem. C 2007, 111, 14992–14997. [Google Scholar] [CrossRef]
- Pasikhani, J.V.; Gilani, N.; Pirbazari, A.E. Improvement the wastewater purification by TiO2 nanotube arrays: The effect of etching-step on the photo-generated charge carriers and photocatalytic activity of anodic TiO2 nanotubes. Solid State Sci. 2018, 84, 57–74. [Google Scholar] [CrossRef]
- Ozkan, S.; Nguyen, N.T.; Mazare, A.; Schmuki, P. Optimized spacing between TiO2 nanotubes for enhanced light harvesting and charge transfer. ChemElectroChem 2018, 5, 3183–3190. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ozkan, S.; Hwang, I.; Zhou, X.; Schmuki, P. Spaced TiO2 nanotube arrays allow for a high performance hierarchical supercapacitor structure. J. Mater. Chem., A 2017, 5, 1895–1901. [Google Scholar] [CrossRef]
- Wawrzyniak, J.; Grochowska, K.; Karczewski, J.; Kupracz, P.; Ryl, J.; Dołęga, A.; Siuzdak, K. The geometry of free-standing titania nanotubes as a critical factor controlling their optical and photoelectrochemical performance. Surf. Coat. Technol. 2020, 389, 125628. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Niu, J.; Wang, E.; Niu, G.; Xie, C. Preparation of TiO2 nanotube arrays with efficient photocatalytic performance and super-hydrophilic properties utilizing anodized voltage method. Results Phys. 2019, 14, 102499. [Google Scholar] [CrossRef]
- Yi, Z.; Zeng, Y.; Wu, H.; Chen, X.; Fan, Y.; Yang, H.; Tang, Y.; Yi, Y.; Wang, J.; Wu, P. Synthesis, surface properties, crystal structure and dye-sensitized solar cell performance of TiO2 nanotube arrays anodized under different parameters. Results Phys. 2019, 15, 102609. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Li, R.; Wang, X.; Chen, G.Z. Highly-dispersed nickel nanoparticles decorated titanium dioxide nanotube array for enhanced solar light absorption. Appl. Surf. Sci. 2019, 464, 716–724. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Momeni, M.M.; Faraji, M. Highly active nickel nanoparticles supported on TiO2 nanotube electrodes for methanol electrooxidation. Electroanalysis 2010, 22, 2620–2625. [Google Scholar] [CrossRef]
- Guan, B.; Yu, J.; Guo, S.; Yu, S.; Han, S. Porous nickel doped titanium dioxide nanoparticles with improved visible light photocatalytic activity. Nanoscale Adv. 2020, 2, 1352–1357. [Google Scholar] [CrossRef]
- Sim, L.C.; Ng, K.W.; Ibrahim, S.; Saravanan, P. Preparation of improved p-n junction NiO/TiO2 nanotubes for solar-energy-driven light photocatalysis. Int. J. Photoenergy 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Spanu, D.; Recchia, S.; Mohajernia, S.; Tomanec, O.; Kment, Š.; Zboril, R.; Schmuki, P.; Altomare, M. Templated dewetting–alloying of NiCu bilayers on TiO2 nanotubes enables efficient noble-metal-free photocatalytic H2 evolution. ACS Catal. 2018, 8, 5298–5305. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Sun, L.; Mao, Y.; Wang, M.; Lin, C. An ultrasound-assisted deposition of NiO nanoparticles on TiO2 nanotube arrays for enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 8223. [Google Scholar] [CrossRef]
- Bashah, N.A.A.; Awel, E.; Amri, N.; Alrozi, R.; Yaakob, N.; Zubir, N.A.; Osman, M.S. Role of temperature on colloidal behavior of gold nanoparticles dispersed in organic and aqueous media. AIP Conf. Proc. 2017, 1885, 020281. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions, 1st ed.; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Siebeneicher, S.; Waag, F.; Escobar Castillo, M.; Shvartsman, V.V.; Lupascu, D.C.; Gökce, B. Laser fragmentation synthesis of colloidal bismuth ferrite particles. Nanomaterials 2020, 10, 359. [Google Scholar] [CrossRef]
- Amendola, V.; Meneghetti, M. Laser ablation synthesis in solution and size manipulation of noble metal nanoparticles. Phys. Chem. Chem. Phys. 2009, 11, 3805. [Google Scholar] [CrossRef]
- Xiang, X.; Zu, X.T.; Zhu, S.; Zhang, C.F.; Wang, L.M. Effect of annealing on the optical absorption of Ni nanoparticles in MgO single crystals. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 250, 229–232. [Google Scholar] [CrossRef]
- Carja, G.; Nakajima, A.; Dranca, C.; Okada, K. Nanoparticles of nickel oxide: Growth and organization on zinc-substituted anionic clay matrix by one-pot route at room temperature. J. Nanopart. Res. 2010, 12, 3049–3056. [Google Scholar] [CrossRef]
- Lee, C.-C.; Cheng, Y.-Y.; Chang, H.Y.; Chen, D.-H. Synthesis and electromagnetic wave absorption property of Ni–Ag alloy nanoparticles. J. Alloys Compd. 2009, 480, 674–680. [Google Scholar] [CrossRef]
- Duque, J.S.; Blandón, J.S.; Riascos, H. Localized Plasmon resonance in metal nanoparticles using Mie theory. J. Phys. Conf. Ser. 2017, 850, 012017. [Google Scholar] [CrossRef]
- Amekura, H.; Kitazawa, H.; Umeda, N.; Takeda, Y.; Kishimoto, N. Nickel nanoparticles in silica glass fabricated by 60 keV negative-ion implantation. Nucl. Instrum. Methods Phys. Res. Sect. B 2004, 222, 114–122. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Pawar, G.S.; Elikkottil, A.; Pesala, B.; Tahir, A.A.; Mallick, T.K. Plasmonic nickel nanoparticles decorated on to LaFeO3 photocathode for enhanced solar hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 578–586. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Sahoo, P.; Misra, D.K.; Salvador, J.; Makongo, J.P.A.; Chaubey, G.S.; Takas, N.J.; Wiley, J.B.; Poudeu, P.F.P. Microstructure and thermal conductivity of surfactant-free NiO nanostructures. J. Solid State Chem. 2012, 190, 29–35. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Nazarov, D.; Ezhov, I.; Mitrofanov, I.; Kim, A.; Rymyantsev, A.; Lyutakov, O.; Popovich, A.; Maximov, M. Atomic Layer Deposition of NiO to Produce Active Material for Thin-Film Lithium-Ion Batteries. Coatings 2019, 9, 301. [Google Scholar] [CrossRef]
- Siuzdak, K.; Szkoda, M.; Lisowska-Oleksiak, A.; Karczewski, J.; Ryl, J. Highly stable organic–inorganic junction composed of hydrogenated titania nanotubes infiltrated by a conducting polymer. RSC Adv. 2016, 6, 33101–33110. [Google Scholar] [CrossRef]
- Sawczak, M.; Sobaszek, M.; Siuzdak, K.; Ryl, J.; Bogdanowicz, R.; Darowicki, K.; Gazda, M.; Cenian, A. Formation of highly conductive boron-doped diamond on TiO2 nanotubes composite for supercapacitor or energy storage devices. J. Electrochem. Soc. 2015, 162, A2085–A2092. [Google Scholar] [CrossRef]
- Jacquemin, M.; Genet, M.J.; Gaigneaux, E.M.; Debecker, D.P. Calibration of the X-ray photoelectron spectroscopy binding energy scale for the characterization of heterogeneous catalysts: Is everything really under control? ChemPhysChem 2013, 14, 3618–3626. [Google Scholar] [CrossRef] [PubMed]
- Wysocka, J.; Cieslik, M.; Krakowiak, S.; Ryl, J. Carboxylic acids as efficient corrosion inhibitors of aluminium alloys in alkaline media. Electrochim. Acta 2018, 289, 175–192. [Google Scholar] [CrossRef]
- Hashemi, A.; Bahari, A. Structural and dielectric characteristic of povidone–silica nanocomposite films on the Si (n) substrate. Appl. Phys. A 2017, 123, 535. [Google Scholar] [CrossRef]
- Çopuroğlu, M.; Sezen, H.; Opila, R.L.; Suzer, S. Band-bending at buried SiO2/Si interface as probed by XPS. ACS Appl. Mater. Interfaces 2013, 5, 5875–5881. [Google Scholar] [CrossRef]
- Yang, M.; Huo, L.; Pei, L.; Pan, K.; Gan, Y. Enhanced photoelectrochemical performance and charge transfer properties in self-organized NiOx-doped TiO2 nanotubes. Electrochim. Acta 2014, 125, 288–293. [Google Scholar] [CrossRef]
- Mazare, A.; Dilea, M.; Ionita, D.; Demetrescu, I. Electrochemical behavior in simulated body fluid of TiO2 nanotubes on TiAlNb alloy elaborated in various anodizing electrolyte. Surf. Interface Anal. 2014, 46, 186–192. [Google Scholar] [CrossRef]
- Thasirisap, E.; Vittayakorn, N.; Seeharaj, P. Surface modification of TiO2 particles with the sono-assisted exfoliation method. Ultrason. Sonochem. 2017, 39, 733–740. [Google Scholar] [CrossRef]
- Kupracz, P.; Coy, E.; Grochowska, K.; Karczewski, J.; Rysz, J.; Siuzdak, K. The pulsed laser ablation synthesis of colloidal iron oxide nanoparticles for the enhancement of TiO2 nanotubes photo-activity. Appl. Surf. Sci. 2020, 530, 147097. [Google Scholar] [CrossRef]
- Pol, R.; Guerrero, M.; García-Lecina, E.; Altube, A.; Rossinyol, E.; Garroni, S.; Baró, M.D.; Pons, J.; Sort, J.; Pellicer, E. Ni-, Pt- and (Ni/Pt)-doped TiO2 nanophotocatalysts: A smart approach for sustainable degradation of Rhodamine B dye. Appl. Catal. B 2016, 181, 270–278. [Google Scholar] [CrossRef]
- Li, S.; Lin, S.; Liao, J.; Pan, N.; Li, D.; Li, J. Nitrogen-doped TiO2 nanotube arrays with enhanced photoelectrochemical property. Int. J. Photoenergy 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Saehana, S.; Muslimin; Abdullah, M. Electrochemical impedance spectroscopy study of TiO2 based solar cells. J. Renew. Sustain. Energy 2014, 6, 023109. [Google Scholar] [CrossRef]
- Gönüllü, Y.; Kelm, K.; Mathur, S.; Saruhan, B. Equivalent circuit models for determination of the relation between the sensing behavior and properties of undoped/Cr doped TiO2 NTs. Chemosensors 2014, 2, 69–84. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Chen, J.; Sun, X.; Xian, T.; Yang, H. In situ construction of CNT/CuS hybrids and their application in photodegradation for removing organic dyes. Nanomaterials 2020, 10, 178. [Google Scholar] [CrossRef] [PubMed]



| Element | Energy (keV) | At.(1) % | At.(2) % |
|---|---|---|---|
| C | 0.227 | 16.46 | 14.89 |
| O | 0.525 | 1.58 | 1.48 |
| Ni | 7.471 | 81.96 | 83.64 |
| Ni2p | Ti2p | O1s | |||
|---|---|---|---|---|---|
| NiO | Ni(OH)2 | TiO2 | Ti-O | Ni/OH | |
| BE (eV) | 853.7 | 855.7 | 459.0 | 530.2 | 531.4 |
| NiNP (%) | -- | 17.8 | -- | -- | 82.2 |
| NiNP@TiO2 (%) | 1.0 | 3.9 | 24.9 | 54.4 | 15.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyniak, J.; Karczewski, J.; Ryl, J.; Grochowska, K.; Siuzdak, K. Laser-Assisted Synthesis and Oxygen Generation of Nickel Nanoparticles. Materials 2020, 13, 4068. https://doi.org/10.3390/ma13184068
Wawrzyniak J, Karczewski J, Ryl J, Grochowska K, Siuzdak K. Laser-Assisted Synthesis and Oxygen Generation of Nickel Nanoparticles. Materials. 2020; 13(18):4068. https://doi.org/10.3390/ma13184068
Chicago/Turabian StyleWawrzyniak, Jakub, Jakub Karczewski, Jacek Ryl, Katarzyna Grochowska, and Katarzyna Siuzdak. 2020. "Laser-Assisted Synthesis and Oxygen Generation of Nickel Nanoparticles" Materials 13, no. 18: 4068. https://doi.org/10.3390/ma13184068
APA StyleWawrzyniak, J., Karczewski, J., Ryl, J., Grochowska, K., & Siuzdak, K. (2020). Laser-Assisted Synthesis and Oxygen Generation of Nickel Nanoparticles. Materials, 13(18), 4068. https://doi.org/10.3390/ma13184068







