1. Introduction
Zeolites—one of the most abundant microporous materials—have been widely studied as 3D functional materials and employed as sorbents, catalysts or gas separators due to their various pore sizes and ion-exchange and polar compound adsorption properties [
1,
2,
3,
4]. Over the last 60 years, numerous experiments under ambient and applied temperatures have addressed the fundamental behaviors of zeolites and their potential applications in human life and industry. Experiments under pressure conditions, performed in the last few decades, show zeolites exhibit elastic behaviors and pressure-induced anomalous expansion in response to adopted pressure [
5]. However, numerous high-pressure studies of natrolite (Na
16Al
16Si
24O
80·16 H
2O) have recently been carried out using pressure-induced hydration (PIH) and pressure-induced insertion (PII) accompanying abnormal volume expansion under applied pressures. A notable quantity of studies reported potential for various applications, such as in sequestration of cations and molecules (e.g., Cs
+, Sr
2+, Pb
2+ and CO
2) and trapping of noble gases (e.g., Ar, Kr and Xe), resulting in the insertion of chemical species by widening the window of natrolite pores using pressure; the noble gases then remain trapped by narrowing the channel opening after pressure release [
6,
7,
8,
9,
10,
11,
12,
13]. With respect to the crystal structure, the PIH and PII are a consequence of the auxetic behavior of the natrolite framework, which is visualized using a rotating-squares model of framework topology [
14]. The natrolite framework is composed of a secondary building unit of T
5O
10 (T = Al and Si), which is 3D corner sharing [
15]. This unit consists of alternatively bridged Si- and Al-tetrahedra and forms a helical and elliptical channel along the c-axis [
16]. The PIH phenomenon was first discovered by Lee et al. when pressurized in water containing PTM [
17,
18]. The first PIH occurs around 1.0 GPa to form the paranatrolite phase, (Na
16Al
16Si
24O
80·24 H
2O) accompanying ~6.7% of unit cell volume expansion and subsequent water insertion with ~3.9% volume contraction occurs at approximately 1.2 GPa to form the super-hydrated natrolite phase (Na
16Al
16Si
24O
80·32H
2O). Natrolite shows reversible sequential phase transitions under pressure conditions and is irreversible under simultaneous pressure and temperature conditions. For example, Cs- or Pb-containing natrolite (Cs
16Al
16Si
24O
80·16H
2O and Pb
8Al
16Si
24O
80·16H
2O, respectively) become pollucite (CsAlSi
2O
6·H
2O) after heating to 160 °C at 2 GPa and lawsonite (Pb
4Al
8Si
8O
28·4H
2O) after heating at 200 °C and 4.5 GPa, respectively. The pollucite and lawsonite maintain ~40 wt% of the remaining Cs
+ and Pb
2+ cations and show low leaching rates due to tight coordinate bonding with the framework after irreversible phase transition. The pressure- and temperature-driven processes make natrolite a more suitable form for the sequestration of nuclear waste and as long-term storage material under ambient conditions [
11,
12].
Among the various cation-exchanged natrolites, the silver-exchanged form (Ag-NAT) absorbs water and CO
2 molecules at comparatively low pressures (0.4(1) GPa and 0.8(1) GPa, respectively), whereas natural natrolite absorbs both at 1.0(1) GPa. The onset pressure of pressure-induced insertion (PII) arises from the circular geometry of the channel window, and we have suggested that one of the possible materials for CO
2 storage under crustal conditions [
9]. We investigated the pressure-induced insertion (PII) of N
2, O
2 and CH
4 gases inside microchannels of the Ag-NAT in order to explore potential material for (ir)reversible gas storage by controlling the pressure and temperature. Herein, we report the structural investigation of Ag-NAT in the presence of N
2, O
2 and CH
4 as PTMs under applied pressure using a Diamond Anvil Cell (DAC).
3. Results and Discussions
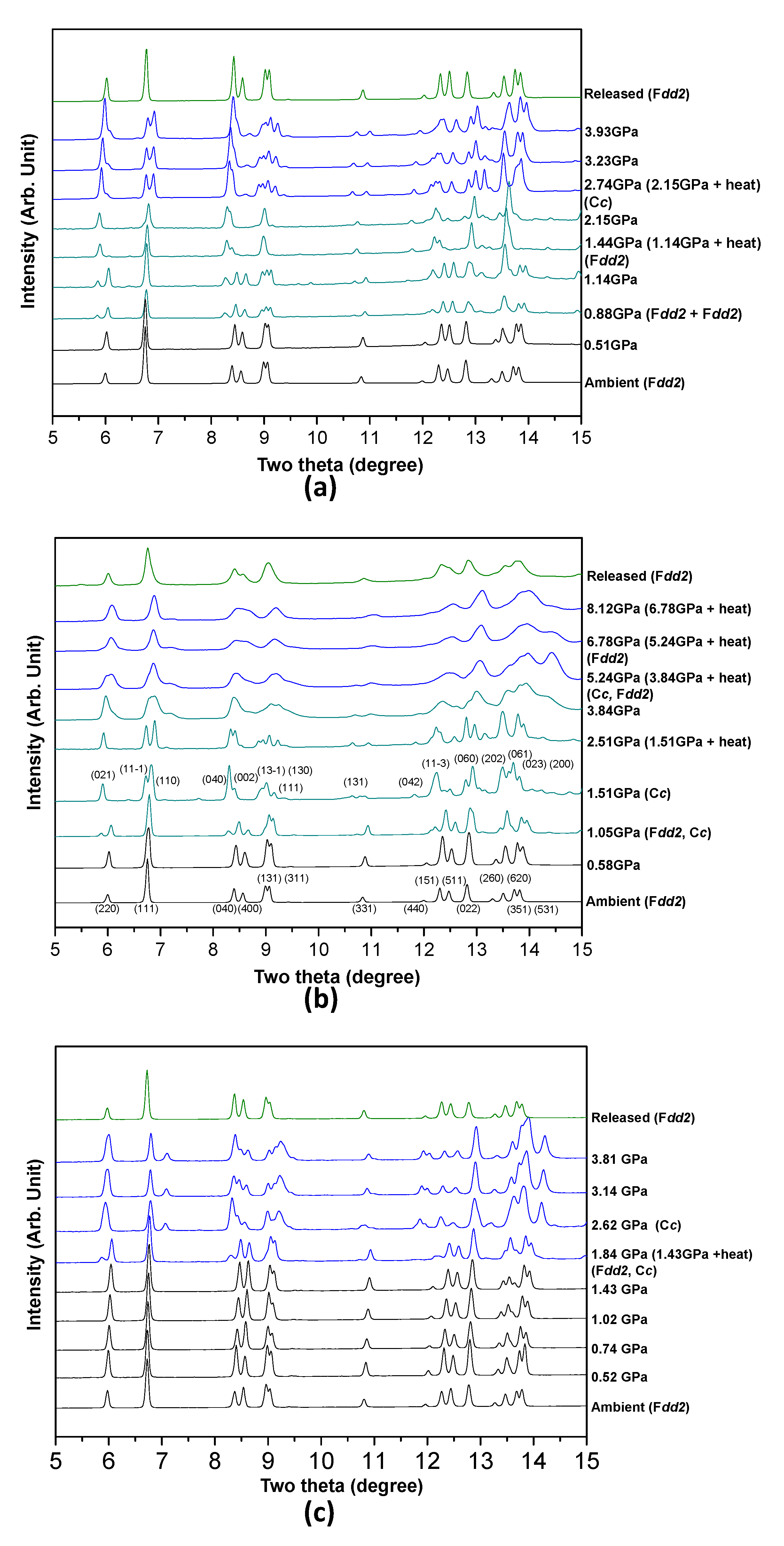
Pressure- and temperature-induced changes in the observed synchrotron X-ray diffraction patterns of the Ag-NAT in the presence of different PTM, N
2, O
2 or CH
4 are shown in
Figure 1. The Bragg peaks of the initial phase are indexed to the orthorhombic space group
Fdd2 under ambient conditions [
19]. In all cases except the CH
4 run, the Ag-NAT expands in two stages by applying pressure and temperature. The Bragg peak of (220) is obviously observed to shift to lower two theta angles in all the diffraction data when PII occurs, indicating that structural changes in the
ab-plane are dominant. The Ag-NAT in the presence of N
2, the first expanded phase is observed at 0.88(5) GPa owing to the starting pressure-induced insertion (PII) of the N
2 molecule. The intensity of peaks that belong to the first expanded phase (space group:
Fdd2) increases up to 1.14(5) GPa, and the ambient phase disappears after heating at 110 °C for an h. The second expanded phase is subsequently observed with a phase transition to monoclinic,
Cc, at 2.15(5) GPa after heating at 200 °C for 1 h (
Figure 1a). The second expanded phase gradually contracts without any further phase change due to pressure and temperature. In the case of O
2 run, the first expansion of the Ag-NAT is accompanied by a transition to monoclinic,
Cc, at 1.05(5) GPa. The second expanded phase (orthorhombic:
Fdd2) was observed at 3.84(5) GPa after heating at 250 °C for 1 h. This phase gradually contracted up to a final pressure of 8.12(5) GPa (
Figure 1b). In the case of the CH
4 run, an expanded phase (space group:
Cc) is observed at 1.43(5) GPa after heating at 150 °C for 1 h and exists up to 3.81(5) GPa (
Figure 1c). In all cases, the Ag-NAT reversibly changes to the initial phase after pressure is released.
A series of whole-profile refinements reveals the details of the compressional changes of the unit cell lengths and volume of the Ag-NAT in the presence of different PTMs (
Figure 2). When we convert to a non-conventional
Fd setting for comparison with the
Fdd2 structure, we find that there are three distinct regions of unit cell parameter changes in the case of N
2 and O
2 as PTMs, while there are two regions of unit cell parameter changes in case of the CH
4 as PTM. In all cases, the
a- and
b-axes increase when the first PII occurs (in order of
a- and
b-axis, 4.1(1)% and 2.6(1)% at 0.88(5) GPa in the N
2 run; 3.6(1)% and 2.4(1)% at 1.05(5) GPa in the O
2 run; 4.1(1)% and 2.4(1)% at 1.84(5) GPa in the CH
4 run). Compared with the
a- and
b-axes, the
c-axis slightly decreases at the pressure of the first PII (−1.0(1)% in the N
2 run; −0.5(1)% in the O
2 run; −0.8(1)% in the CH
4 run). This phenomenon of axes changing when PII occurs is related to expansion and becoming more circular in the channel window [
9]. In the N
2 run, all axes slightly increase within ~0.5% at 2.15(5) GPa, the onset pressure of the second PII. In regard to O
2 run, all axes decrease at 5.24(5) GPa. Regarding our Rietveld refinement results and pressure of second PII, the increase of all axes at 2.15(5) GPa in the case of the N
2 run is dominantly reflected by the PII effect rather than axial contraction by pressure. A decrease in the axes at 5.24(5) GPa and second PII, in the case of O
2 run compressional effect by pressure is more dominant.
The unit cell volume of the Ag-NAT with the N
2 expands 5.7(1)% at 0.88(5) GPa and 0.8(1)% at 2.15(1) GPa, respectively. Except for the abrupt volume expansion at the pressures of the first and second PII (0.88(5) GPa and 2.15(5) GPa, respectively), the unit cell volume linearly decreases with increasing pressure. The volume changes are mainly caused by changes in the
a- and
b-axes. Related to our Rietveld refinement results, the number of 12.3 N
2 molecules per 80 framework oxygen (O
f) of the Ag-NAT is inserted into the void of the channel at 1.44(5) GPa, after the first PII. At 2.74(5) GPa, 16 N
2 molecules per 80 of are inserted by the second PII. To understand the relationship between molecules by PII and the compressibility of the Ag-NAT in the presence of N
2, O
2 and CH
4, we used the Birch-Murnaghan equation of state with second order and fixed the derivative of the bulk modulus (B
0) to 4. In the case of N
2, the bulk modulus (B
0) of the Ag-NAT is 42(1) GPa before the first PII occurs at 0.88(5) GPa. Between 0.88(5) GPa and 2.15(5) GPa, the bulk modulus of the Ag-NAT changes to 57(5) GPa. After the second PII occurs at 2.15(5) GPa, the bulk modulus is 49(7) GPa. The bulk modulus changes of the Ag-NAT reveal that the Ag-NAT becomes more incompressible due to insertion of N
2 molecules into the NAT framework (
Figure 2d and
Figure 3). In the O
2 run, the unit cell volume of the Ag-NAT also linearly decreases with pressure except an abrupt expansion of 5.5(1)% at 1.05(5) GPa and contraction of 3.0(1)% at 5.24(5) GPa caused by the first and second PII, respectively. The numbers of 14.2 O
2 and 16 O
2 are inserted per 80 of at 2.51(5) GPa and 8.12(5) GPa, after the first and second PII, respectively. The bulk modulus of Ag-NAT in the presence of O
2 is 38(2) GPa before the first PII at 1.05(5) GPa. From 1.05(5) GPa to 5.24(5) GPa, the bulk modulus of the Ag-NAT increases up to 85(5) GPa. After the second PII at 5.24(1) GPa, the bulk modulus is 227(1) GPa. The highest bulk modulus of 227(1) among our results makes it possible to make fourteen coordinate bonds of O
2 molecule and framework oxygen (
Figure 3f) compared to three to eight bonds are formed after PII in other models (
Figure 3a–e). This means that the bonds of O
2 molecules after the second PII sustain the collapsible framework by pressure and therefore the structure becomes more incompressible. Similar to the case of N
2 run, the bulk modulus of the Ag-NAT in O
2 increases after each PII (
Figure 2e and
Figure 3). In the case of CH
4, the volume increases 5.7(1)% in response to insertion of 8 CH
4 per 80 O
f at 1.84(5) GPa. Before and after 1.84(5) GPa, the bulk moduli are 55(3) GPa and 79(2) GPa, respectively. After pressure is reduced to ambient pressure, the unit cell volumes of Ag-NAT in all cases recover to a similar volume of volume at ambient pressure (open symbols in
Figure 2). Overall, the volume changes are accompanied by the PII and the bulk moduli increase after molecule uptake.
For a detailed understanding of the structural changes before and after pressure-induced insertion of each molecule, Rietveld models were established at selected pressure points in each run (
Tables S2 and S3). All models are projected along the [001] direction in
Figure 3, and the ambient model is from our previous study [
19]. The extra-framework cation (EFC), Ag
+ and water molecules in the ambient model are located at the center and side of the NAT channel, respectively, and the extra-framework species show an ordered distribution. Silver cations have six-coordinated bonding with four framework oxygens and two water molecules. The geometry of the channel window is determined by measuring the chain rotation angle of the T
5O
10 (T = Si and Al) secondary building unit, ψ and the elongation ratio between the lengths of the longest and shortest diagonal (
L/S) of the eight-membered rings. The lower degree of the chain rotation angle and elongation ratio indicates a more circular shape of the channel window. The ψ value of the Ag-NAT at ambient pressure is 22.2(1)° and the angle decreases to 19.3(1)°, 18.3(1)° and 20.2(1)° after first PII of N
2, O
2 and CH
4 molecules, respectively (
Figure 3). In the N
2 run, the channel window became more circular with increasing amount of N
2 molecules (12.3 per 80 out of → 16 per 80 O
f) inside the channel after second PII (19.3(1)° at 1.44(5) GPa → 18.9(1)° at 2.74(5) GPa in
Figure 3b,c. The rotation angle of Ag-NAT with O
2 increases after the second PII (14.2 per 80 O
f → 16 per 80; 18.3(1)° at 2.51(5) GPa → 23.5(1)° at 8.12(5) GPa in
Figure 3e,f. The changes in the elongation ratio are quite similar to the changes in the rotation angles under pressure. The ratio decreases from 2.37(1) at ambient to 2.10(1), 2.01(1) and 2.16(1) after the first PII of N
2, O
2 and CH
4 molecules, respectively. After the second PII, the ratio decreases to 2.02(1) in the N
2 run and increases to 2.52(1) in the O
2 run. Considering a comparatively high pressure of 8.12(5) GPa, the compressional force affects the channel window to become more elliptical rather than becoming circular by insertion and increasing the amount of O
2 molecules. The difference Fourier map in the channel shows that two unknown sites are adjacent with interatomic distances of ~1.1 Å and ~1.2 Å in all models of Ag-NAT-N
2 and Ag-NAT-O
2, respectively. We therefore assign unknown sites to N
2 and O
2 and understand that the chemical properties of guest molecules may be retained after inserting into the framework at high pressure.
In all cases, guest molecules were located near the Ag
+ cation and coordinated with Ag
+, water molecules and framework oxygen after insertion. The N
2 molecules are bonded with eight and seven framework oxygens after the first and second PII, respectively, in the case of N
2 (
Figure 3b,c). The six bindings of O
2 and framework oxygen are formed after the first PII and the number of bonds is changed to fourteen after PII in the case of O
2 run (
Figure 3e,f) due to sustaining the NAT framework at a comparatively high pressure of 8.12(5) GPa in our pressure range. Three framework oxygen atoms are connected by CH
4 molecules after PII occurs. When CH
4 is inserted at 2.62(5) GPa, the water molecule migrates to the opposite site of CH
4 and forms a coordinate bond with the Ag
+, whereas the atomic positions of the water molecules of the N
2 and O
2 models after the first and second PII are similar to those of the ambient model. In the CH
4 model, CH
4 molecules may push neighboring host water molecules by repulsion during the CH
4 occupies one site in the channel and then the water molecule subsequently migrates to the opposite site of CH
4 to balance the charge distribution inside the channel. Unlike the CH
4 model, the position of the host water molecules in the pressure models of the Ag-NAT with N
2 and O
2 are similar to the position of water molecules in the ambient model because the charge distribution balance is already satisfied due to N
2 and O
2 molecules at both sides of the channel. For a detailed understanding of the insertion of different guest molecules inside the Ag-NAT channel, high-pressure spectroscopy experiments were performed.
Concomitantly with our previous high-pressure studies of the Ag-NAT, we found that the onset pressure of the first PII exponentially increases as a function of the kinetic diameter of molecules as PTM (
Figure 4a) [
9,
13]. In terms of the kinetic diameter, the water molecule can be most permeable in the angstrom scale channel and therefore, the pressure required for an over-hydrated state is as low as 0.4(1) GPa. In the case of the Xe molecule, however, greater mechanical forces, such as pressure, are required in order to open the channel window of the Ag-NAT and subsequently transfer it into the channel. All our experimental results regarding the unit cell volume of the Ag-NAT obtained using the applied pressure are summarized in
Figure 4b. We determined that the Ag-NAT has the limitation of maximum expansion (or contraction) rate of the unit cell volume. Adopting a linear function by the least-square fitting method, the relationship of expanded phases (red symbols) after PII and the as-prepared phases before PII (black symbols) follow Equations (1) and (2), respectively.
where y is the normalized unit cell volume and
x is the pressure.
The comparison of normalized unit cell volumes shows that their maximum degrees of expansion and contraction are approximately 5% and 6%, respectively, in the pressure range from ambient to 8 GPa. The two values of slope in these functions show that the expanded phase would be more incompressible by the evaluated pressure because the inserted molecules sustain the channel to prevent collapse. These accumulated results can provide guidance for similar experiments using the Ag-NAT in the future. For example, we can expect PII will be almost complete if the volume is increased up to ~5% and follow Equation (1). In other words, the Ag-NAT can absorb more gas if the volume contraction trend follows Equation (2). We expect this approach to also be adapted to other porous materials under non-ambient conditions.