Thermal Stability and Decomposition Products of P-Doped Ferrihydrite
Abstract
:1. Introduction
2. Materials and Methods
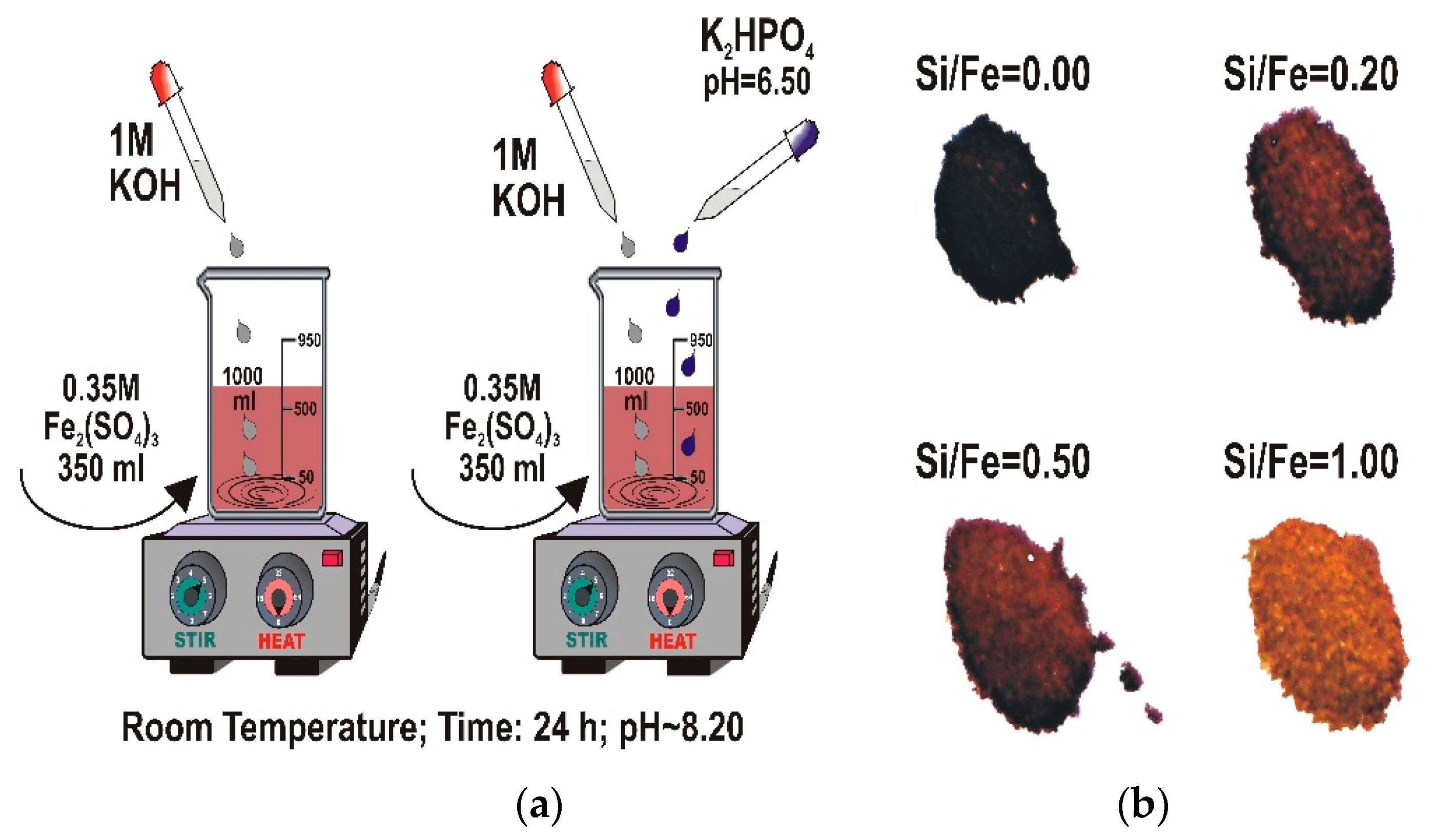
2.1. Synthesis
2.2. Thermal Transformation
2.3. Characterization of P-Doped Ferrihydrites
3. Results and Discussion
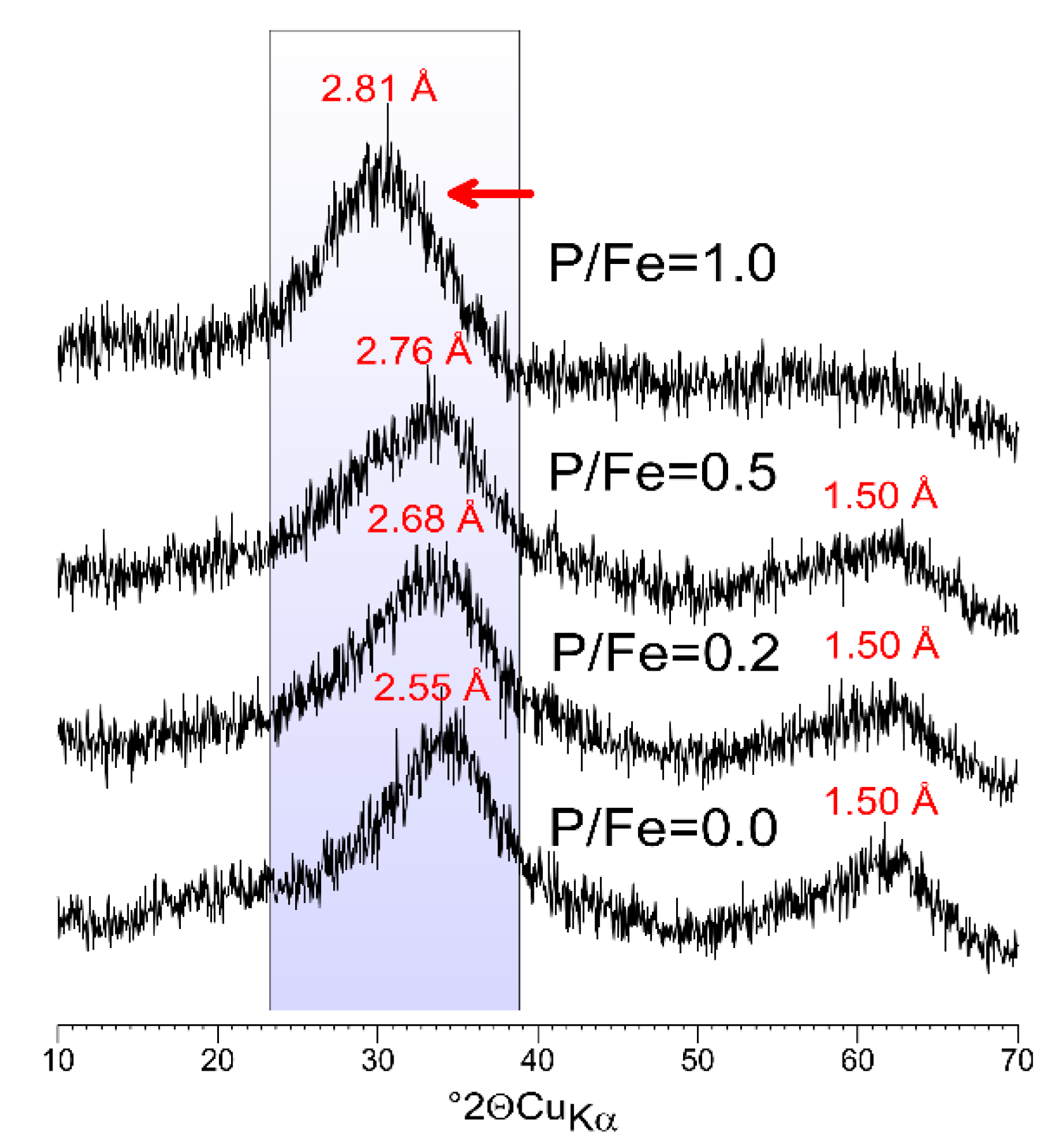
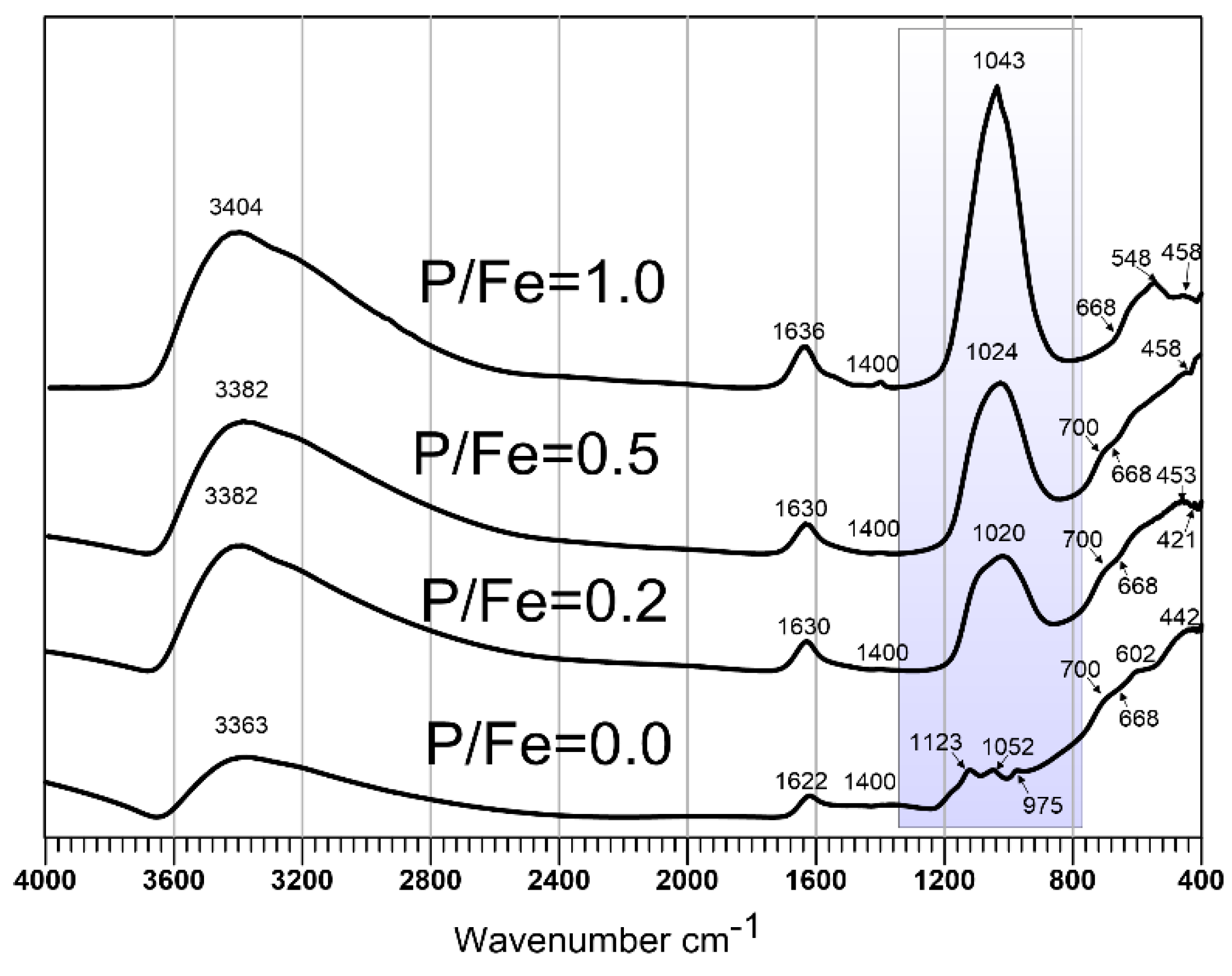
3.1. Properties of P-Doped Ferrihydrites
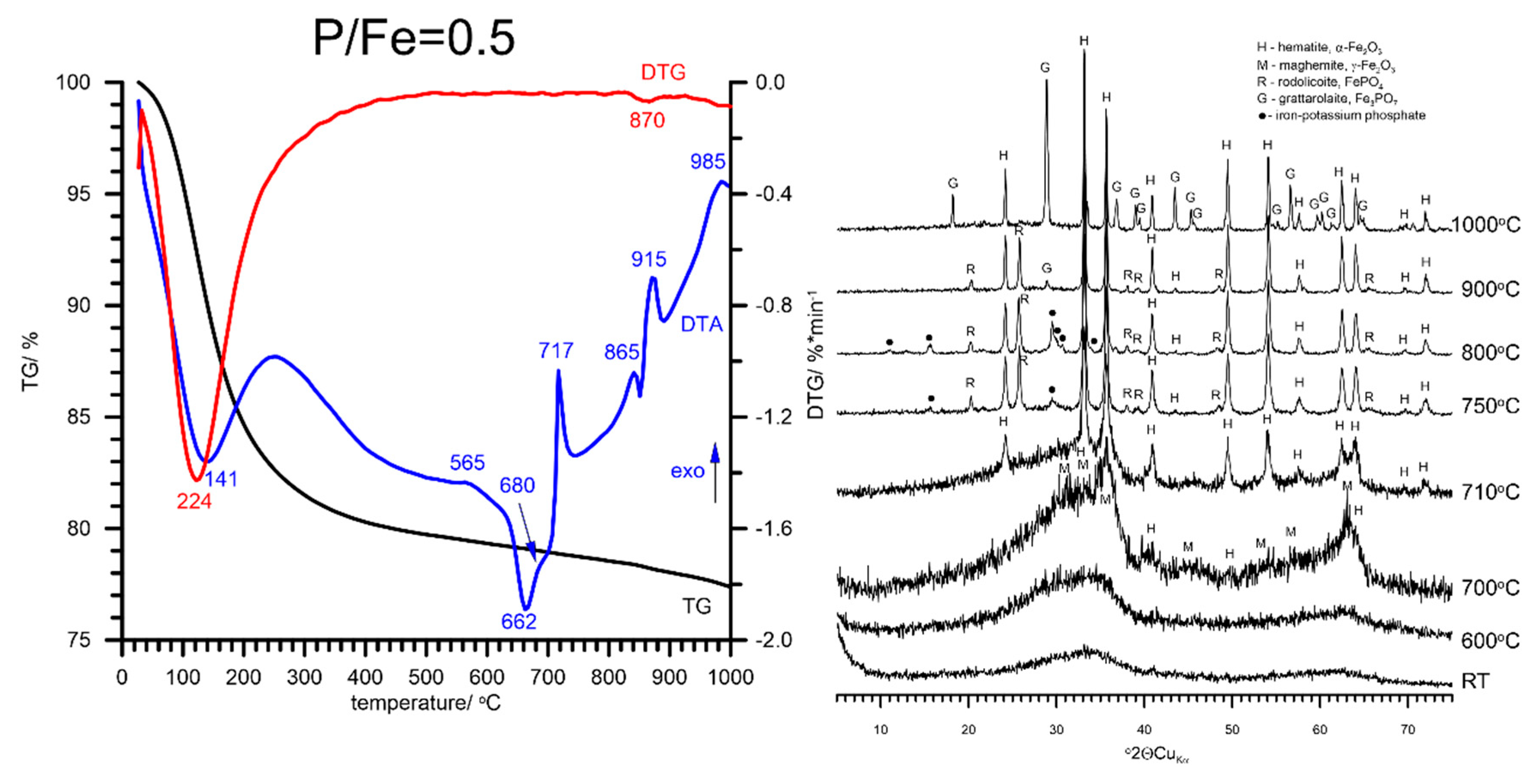
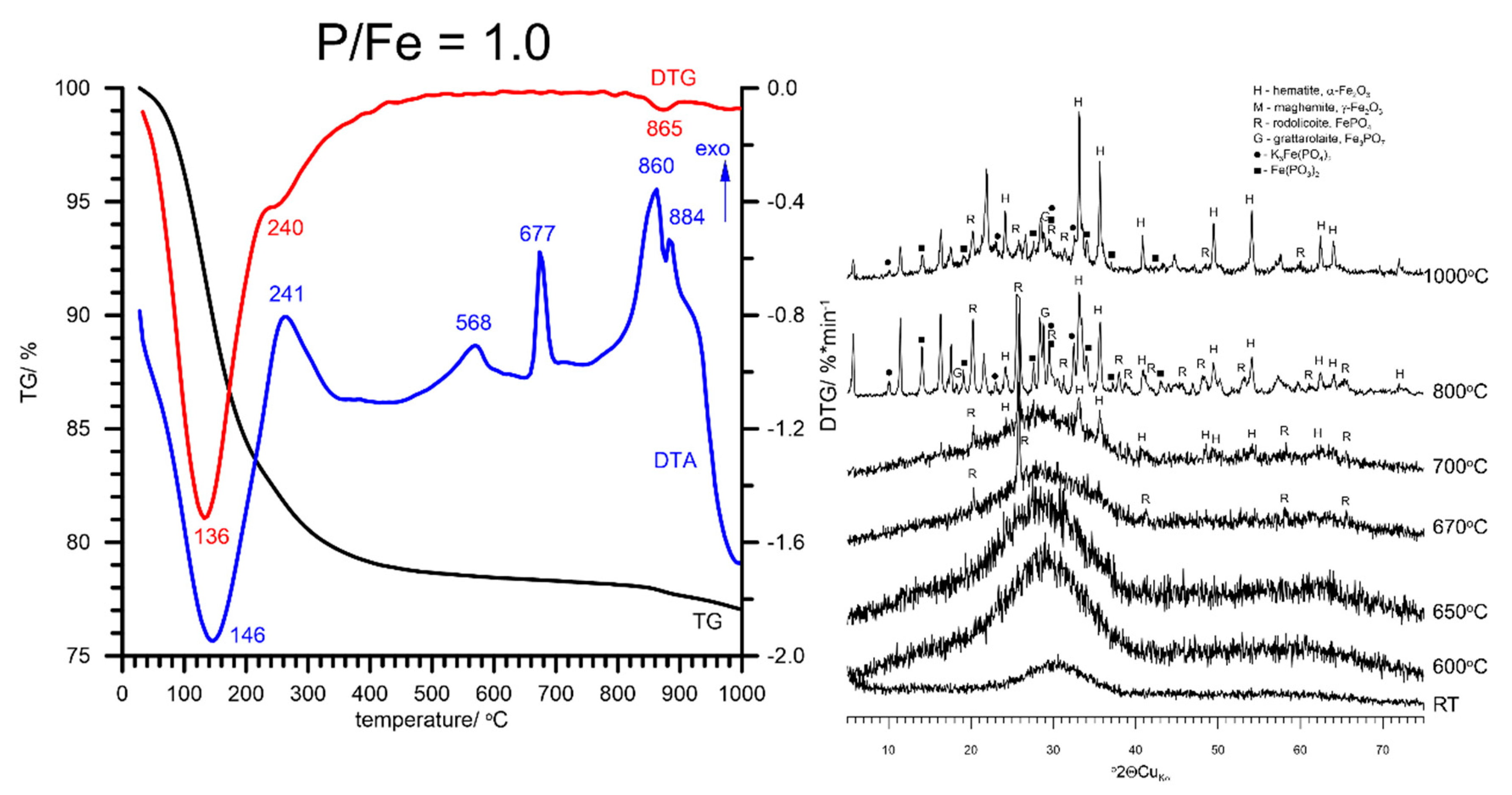
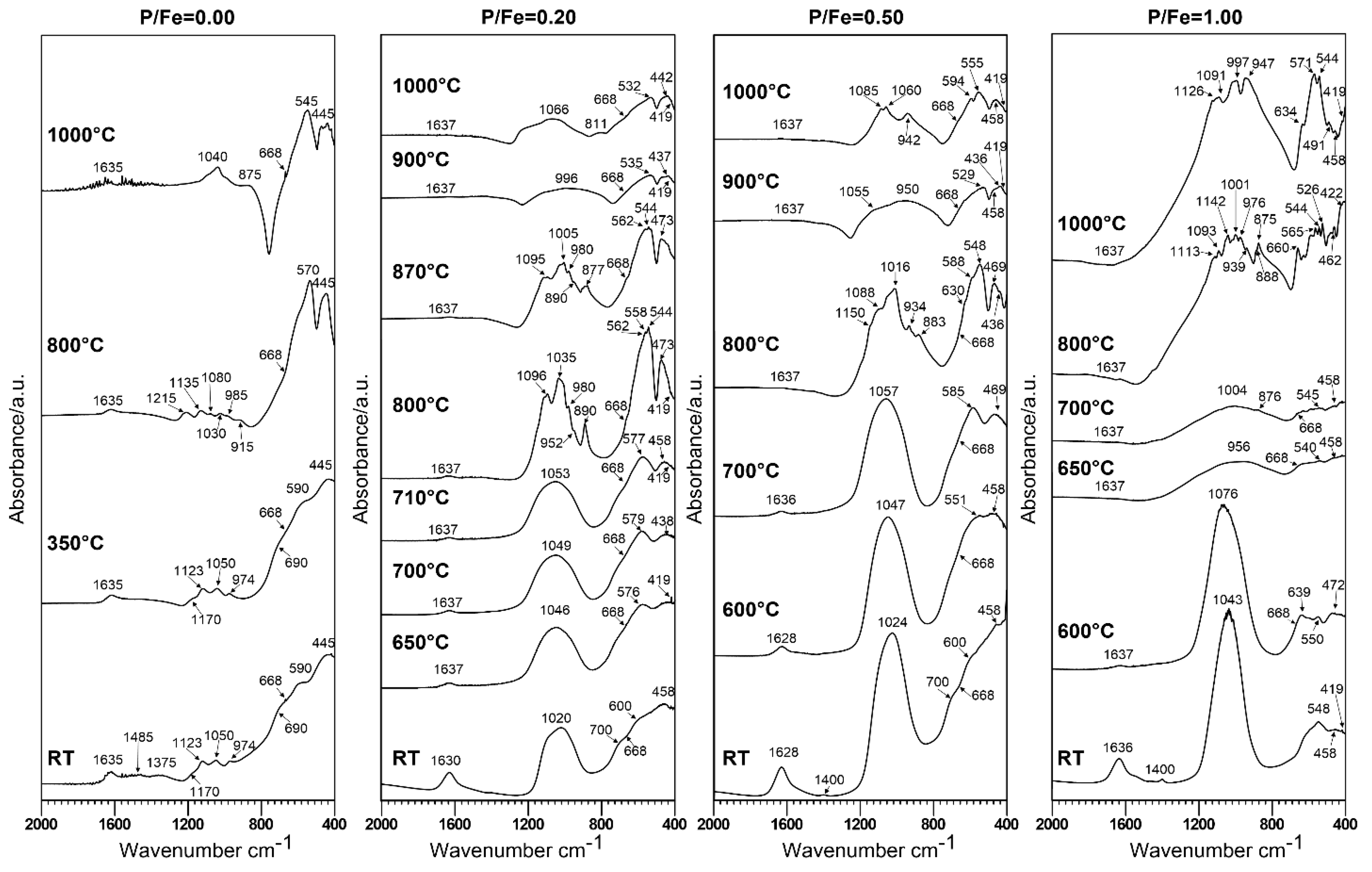
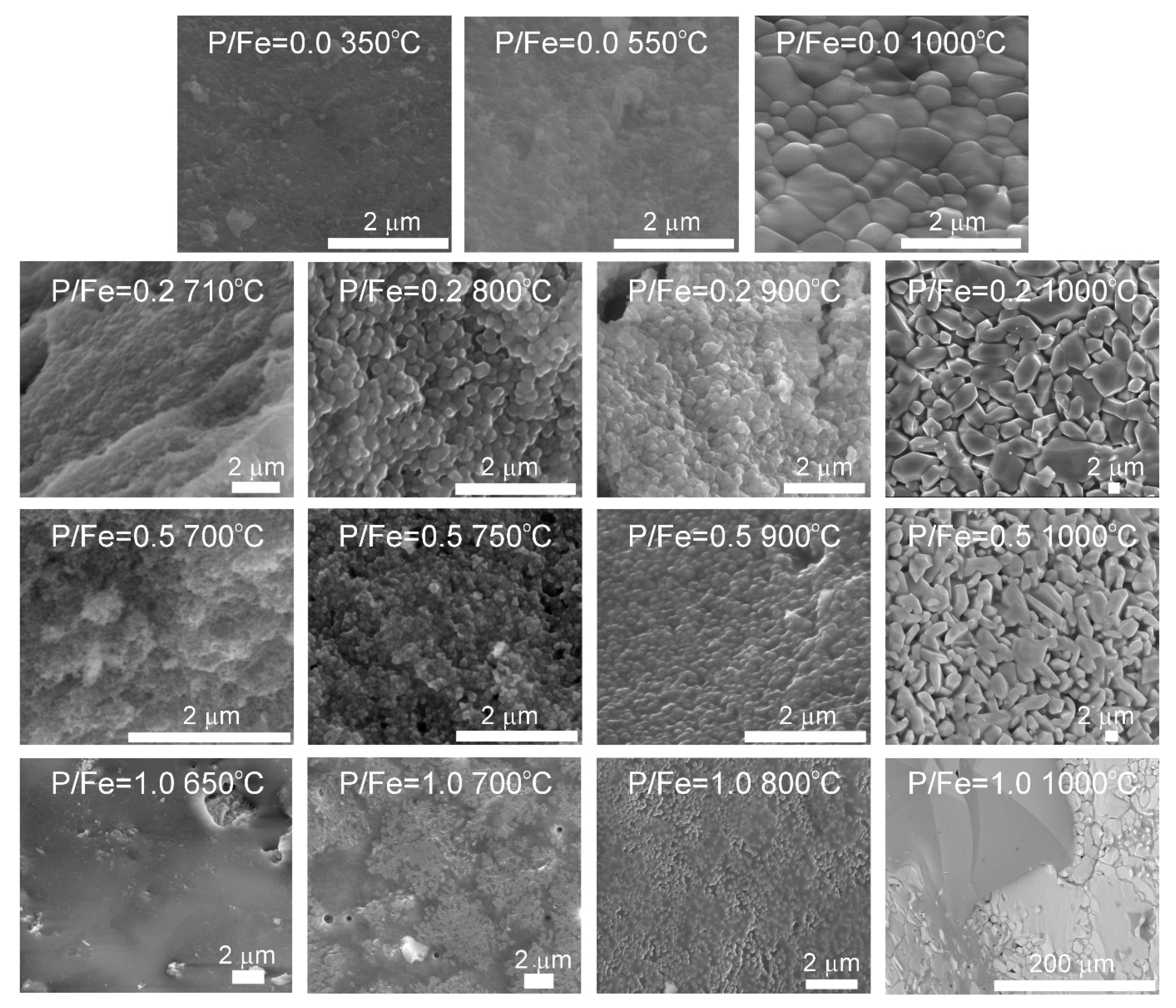
3.2. Thermal Analysis and Identification of the Products of Thermal Transformations
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jambor, J.L.; Dutrizac, J.E. Occurrence and constitution of natural and synthetic ferrihydrite, a widespread iron oxyhydroxide. Chem. Rev. 1998, 98, 2549–2585. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; Weinheim, Wiley: Hoboken, NJ, USA, 2003; ISBN 3-527-30274-3. [Google Scholar]
- Guo, H.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Hiemstra, T. Surface and mineral structure of ferrihydrite. Geochim. Cosmochim. Acta 2013, 105, 316–325. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Surface area of ferrihydrite consistently related to primary surface charge, ion pair formation, and specific ion adsorption. Chem. Geol. 2020, 532, 119304. [Google Scholar] [CrossRef]
- Boily, J.-F.; Song, X. Direct identification of reaction sites on ferrihydrite. Commun. Chem. 2020, 3, 79. [Google Scholar] [CrossRef]
- Hasselöv, M.; von der Krammer, F. Iron oxides as geochemical nanovectors for metal transport in soil-river systems. Elements 2008, 4, 401–406. [Google Scholar] [CrossRef]
- Toner, B.M.; Berquó, T.S.; Michel, F.M.; Sorensen, J.V.; Templeton, A.S.; Edwards, K.J. Mineralogy of iron microbial mats from Loihi Seamount. Front. Microbiol. 2012, 3, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Vodyanitskii, Y.N.; Shoba, S.A. Ferrihydrite in soils. Eurasian Soil Sci. 2016, 49, 796–806. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Carbonate adsorption to ferrihydrite: Competitive interaction with phosphate for use in soil systems. ACS Earth Space Chem. 2019, 3, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.C.; Hiemstra, T. Ternary complex formation of phosphate with Ca and Mg ions binding to ferrihydrite: Experiments and mechanisms. ACS Earth Space Chem. 2020, 4, 545–557. [Google Scholar] [CrossRef]
- Kaegi, R.; Voegelin, A.; Folini, D.; Hug, S.J. Effect of phosphate, silicate, and Ca on the morphology, structure and elemental composition of Fe (III)-precipitates formed in aerated Fe (II) and as (III) containing water. Geochim. et Cosmochim. Acta 2010, 74, 5798–5816. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Tyliszczak, T.; Brown, G.E., Jr. Composition and structural aspects of naturally occurring ferrihydrite. Comptes Rendus Geosci. 2011, 343, 210–218. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Levard, C.; Michel, F.M.; Brown, G.E., Jr. Properties of impurity-bearing ferrihydrite II. Insights into the surface structure and composition of pure, Al- and Si-bearing ferrihydrite from Zn(II) sorption experiments and Zn K-edge X-ray absorption spectroscopy. Geochim. Cosmochim Acta 2013, 119, 46–60. [Google Scholar] [CrossRef]
- Cismasu, A.C.; Michel, F.M.; Tcaciuc, A.P.; Brown, G.E., Jr. Properties of impurity-bearing ferrihydrite III. Effects of Si on the structure of 2-line ferrihydrite. Geochim. Cosmochim Acta 2014, 133, 168–185. [Google Scholar] [CrossRef]
- Bolanz, R.M.; Blass, U.; Ackermann, S.; Ciobota, V.; Rösch, P.; Tarcea, N.; Popp, J.; Majzlan, J. The effect of antimonate, arsenate and phosphate on the transformation of ferrihydrite to goethite, hematite, feroxyhite, and tripuhyite. Clays Clay Miner. 2013, 61, 11–25. [Google Scholar] [CrossRef]
- Senn, A.-C.; Kaegi, R.; Hug, S.J.; Hering, J.G.; Mangold, S.; Voegelin, A. Composition and structure of Fe(III)-precipitates formed by Fe(II) oxidation in water at near-neutral pH: Interdependent effects of phosphate, silicate and Ca. Geochim. Cosmochim. Acta 2015, 162, 220–246. [Google Scholar] [CrossRef] [Green Version]
- Pieczara, G.; Rzepa, G. The effect of Si content on ferrihydrite sorption capacity for Pb(II), Cu(II), Cr(VI) and P(V). Environ. Eng. Manag. J. 2016, 15, 2095–2107. [Google Scholar] [CrossRef]
- Sheng, A.; Li, X.; Arai, Y.; Ding, Y.; Rosso, K.M.; Liu, J. Citrate controls Fe(II)-catalyzed transformation of ferrihydrite by complexation of the labile Fe(III) intermediate. Environ. Sci. Technol. 2020, 54, 7309–7319. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Harrington, R.; Liu, F.; Parise, J.B.; Feng, X.; Sparks, D.L. Effect of ferrihydrite crystallite size on phosphate adsorption reactivity. Environ. Sci. Technol. 2013, 47, 10322–10331. [Google Scholar] [CrossRef]
- Antelo, J.; Arce, F.; Fiol, S. Arsenate and phosphate adsorption on ferrihydrite nanoparticles. Synergetic interactions with calcium ions. Chem. Geol. 2015, 410, 53–62. [Google Scholar] [CrossRef]
- Johnston, C.P.; Chrysochoou, M. Mechanisms of chromate, selenate, and sulfate adsorption on Al-substituted ferrihydrite: Implications for ferrihydrite surface structure and reactivity. Environ. Sci. Technol. 2016, 50, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xiao, D.; Bush, R.T.; Liu, J. Coprecipitated arsenate inhibits thermal transformation of 2-line ferrihydrite: Implications for long-term stability of ferrihydrite. Chemosphere 2015, 122, 88–93. [Google Scholar] [CrossRef]
- Rzepa, G.; Pieczara, G.; Gaweł, A.; Tomczyk, A.; Zalecki, R. The influence of silicate on transformation pathways of synthetic 2-line ferrihydrite. J. Therm. Anal. Calorim. 2016, 125, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Majzlan, J. Thermodynamic stabilization of hydrous ferric oxide by adsorption of phosphate and arsenate. Environ. Sci. Technol. 2011, 45, 4726–4732. [Google Scholar] [CrossRef]
- Kraal, P.; van Genuchten, C.M.; Behrends, T.; Rose, A.L. Sorption of phosphate and silicate alters dissolution kinetics of poorly crystalline iron (oxyhydr)oxide. Chemosphere 2019, 234, 690–701. [Google Scholar] [CrossRef]
- Paige, C.R.; Snodgrass, W.J.; Nicholson, R.V.; Scharer, J.M.; He, Q.H. The effect of phosphate on the transformation of ferrihydrite into crystalline products in alkaline media. Water Air Soil Pollut. 1997, 97, 397–412. [Google Scholar] [CrossRef]
- He, K.; Ludtke, S.J.; Heller, W.T.; Huang, H.W. Mechanism of alamethicin insertion into lipid bilayers. Biophys. J. 1996, 71, 2669–2679. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Ludtke, S.J.; Worcester, D.L.; Huang, H.W. Neutron scattering in the plane of membranes: Structure of alamethicin pores. Biophys. J. 1996, 70, 2659–2666. [Google Scholar] [CrossRef] [Green Version]
- Gálvez, N.; Barrón, V.; Torrent, J. Effect of phosphate on the crystallization of hematite, goethite and lepidocrocite from ferrihydrite. Clays Clay Miner. 1999, 47, 304–311. [Google Scholar] [CrossRef]
- Feely, R.A.; Trefry, J.H.; Lebon, G.T.; German, C.R. The relationship between P/Fe and V/Fe ratios in hydrothermal precipitates and dissolved phosphate in seawater. Geophys. Res Lett. 1998, 25, 2253–2256. [Google Scholar] [CrossRef]
- Soltis, J.A.; Feinberg, J.M.; Gilbert, B.; Penn, R.L. Phase transformation and particle-mediated growth in the formation of hematite from 2-line ferrihydrite. Cryst. Growth Des. 2016, 16, 922–932. [Google Scholar] [CrossRef] [Green Version]
- Rzepa, G.; Bajda, T.; Gaweł, A.; Debiec, K.; Drewniak, L. Mineral transformations and textural evolution during roasting of bog iron ores. J. Therm. Anal. Calorim. 2016, 123, 615–630. [Google Scholar] [CrossRef] [Green Version]
- Suber, L.; Foglia, S.; Fiorani, D.; Romero, H.; Montone, A.; Roig, A.; Casas, L. Synthesis, morphological-structural characterization and magnetic properties of amorphous iron (III)-oxyhydroxy-phosphate nanoparticles. J. Solid State Chem. 2004, 177, 2440–2448. [Google Scholar] [CrossRef]
- Cipriani, C.; Mellini, M.; Pratesi, G.; Viti, C. Rodolicoite and grattarolaite, two new phosphate minerals from Santa Barbara Mine, Italy. EJM 1997, 9, 1101–1106. [Google Scholar] [CrossRef]
- Vithana, C.L.; Johnston, S.G.; Dawson, N. Divergent repartitioning of copper, antimony and phosphorus following thermal transformation of schwertmannite and ferrihydrite. Chem. Geol. 2018, 483, 530–543. [Google Scholar] [CrossRef]
- Vempati, R.K.; Loeppert, R.H. Influence of structural and adsorbed Si on the transformation of synthetic ferrihydrite. Clays Clay Miner. 1989, 37, 273–279. [Google Scholar] [CrossRef]
- Vempati, R.K.; Loeppert, R.H.; Cocke, D.L. Mineralogy and reactivity of amorphous Si-ferrihydrites. Solid State Ion. 1990, 38, 53–61. [Google Scholar] [CrossRef]
- Pieczara, G.; Mendsaikhan, N.; Manecki, M.; Rzepa, G. The effect of synthesis method on the physicochemical properties of ferrihydrite and Si-doped ferrihydrite (in Polish with English summary). Przem. Chem. 2015, 94, 1828–1831. [Google Scholar] [CrossRef]
- Ribeiro, J.; Suarez-Ruiz, I.; Ward, C.; Flores, D. Mineralogy of thermally altered coal mining residues in self-burning coal-waste piles in Portugal and Spain. Comun. Geol. 2014, 101, 187–190. [Google Scholar]
- Michel, F.M.; Barrón, V.; Torrent, J.; Morales, M.P.; Serna, C.J.; Boily, J.-F.; Liu, Q.; Ambrosini, A.; Cismasu, A.C.; Brown, G.E., Jr. Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism. Proc. Natl. Acad. Sci. USA 2010, 107, 2787–2792. [Google Scholar] [CrossRef] [Green Version]
- Michel, F.M.; Ehm, L.; Anato, S.M.; Lee, P.L.; Chupas, P.J.; Liu, G.; Strongin, D.R.; Schoonen, M.A.A.; Phillips, B.L.; Parise, J.B. The structure of ferrihydrite, a nanocrystalline material. Science 2007, 316, 1726–1728. [Google Scholar] [CrossRef] [Green Version]
- Kandori, K.; Uchida, S.; Kataoka, S.; Ishikawa, T. Effects of silicate and phosphate ions on the formation of ferric oxide hydroxide particles. J. Mater. Sci. 1992, 27, 719–728. [Google Scholar] [CrossRef]
- Thibault, P.-J.; Rancourt, D.G.; Evans, R.J.; Dutrizac, J.E. Mineralogical confirmation of a near-P:Fe=1:2 limiting stoichiometric ratio in colloidal P-bearing ferrihydrite-like hydrous ferric oxide. Geochim. Cosmochim. Acta 2009, 73, 364–376. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Tang, Y.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci. Nano 2017, 11, 2193–2204. [Google Scholar] [CrossRef]
- Kandori, K.; Kuwae, T.; Ishikawa, T. Control on size and adsorptive properties of spherical ferric phosphate particles. J. Colloid Interface Sci. 2006, 300, 225–231. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Shang, J.; Arai, Y. Ferrihydrite transformation impacted by coprecipitation of phytic acid. Environ. Sci. Technol. 2020, 54, 8837–8847. [Google Scholar] [CrossRef]
- Lienemann, C.-P.; Monnerat, M.; Dominik, J.; Perret, D. Identification of stoichiometric iron-phosphorus colloids produced in a eutrophic lake. Aquat. Sci. 1999, 61, 133–149. [Google Scholar] [CrossRef]
- Boonchom, B.; Danvirutai, C. Thermal decomposition kinetics of FePO4·3H2O precursor to synthetize spherical nanoparticles FePO4. Ind. Eng. Chem. Res. 2007, 46, 9071–9076. [Google Scholar] [CrossRef]
- Al-Hasni, B.M.; Mountjoy, G.; Barney, E. A complete study of amorphous iron phosphate structure. J. Noncryst. Solids 2013, 380, 141–152. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Touny, A.H.; Saleh, M.M. Synthesis of crystalline and amorphous iron phosphate nanoparticles by simple low-temperature method. Mater. Res. Express 2018, 6, 035030. [Google Scholar] [CrossRef]
- Masset, P.; Poinso, J.; Poignet, J. TG/DTA/MS study of the thermal decomposition of FeSO4∙6H2O. J. Therm. Anal. Calorim. 2006, 83, 457–462. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Aleksandrovsky, A.S.; Atuchin, V.V.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestako, N.P.; Andreev, O.V. Exploration of structural, thermal and spectroscopic properties of self-activated sulfate Eu2(SO4)3 with isolated SO4 groups. J. Ind. Eng. Chem. 2018, 68, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Denisenko, Y.G.; Molokeev, M.S.; Krylov, A.S.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Atuchin, V.V.; Azarapin, N.O.; Plyusnin, P.E.; Sal’nikova, E.I.; Andreev, O.V. High-temperature oxidation of europium (II) sulfide. J. Ind. Eng. Chem. 2019, 79, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Jastrzębski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared spectroscopy of different phosphates structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, G.; Cipriani, C.; Giuli, G.; Birch, W.D. Santabarbaraite: A new amorphous phosphate mineral. Eur. J. Miner. 2003, 15, 185–192. [Google Scholar] [CrossRef]
- Rose, J.; Flank, A.-M.; Masion, A.; Bottero, J.-Y.; Elmerich, P. Nucleation and Growth Mechanisms of Fe Oxyhydroxide in the Presence of PO4 Ions. 2. P K-Edge EXAFS Study. Langmuir 1997, 13, 1827–1834. [Google Scholar] [CrossRef]
- Burba, C.M.; Frech, R. Vibrational spectroscopic investigation of structurally-related LiFePO4, NaFePO4, and FePO4 compounds. Spectochim. Acta A 2006, 65, 44–50. [Google Scholar] [CrossRef]
- Modaressi, A.; Courtois, A.; Gerardin, R.; Malaman, B.; Gleitzer, C. Fe3PO7, Un cas de coordinence 5 du fer trivalent, etude structurale et magnetique. J. Solid State Chem. 1983, 47, 245–255. [Google Scholar] [CrossRef]
- Scaccia, S.; Carewska, M.; Di Bartolomeo, A.; Prosini, P.P. Thermoanalytical investigation of iron phosphate obtained by spontaneous precipitation from aqueous solutions. Thermochim. Acta 2002, 383, 145–152. [Google Scholar] [CrossRef]
- Scaccia, S.; Carewska, M.; Prosini, P.P. Thermoanalitical study of iron(III) phosphate obtained by homogeneous precipitation from different media. Thermochim. Acta 2004, 413, 81–86. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer: Berlin, Germany, 2016; p. 119. ISBN 978-3-319-25347-3. [Google Scholar]
- Reale, P.; Scrosati, B.; Delacourt, C.; Wurm, C.; Morcrette, M.; Masquelier, C. Synthesis and thermal behavior of crystalline hydrated iron(III) phosphates of interest as positive electrodes in Li batteries. Chem. Mater. 2003, 15, 5051–5058. [Google Scholar] [CrossRef]
- Glasauer, S.M.; Hug, P.; Weidler, P.G.; Gehring, A.U. Inhibition of sintering by Si during the conversion of Si-rich ferrihydrite to hematite. Clays Clay Miner. 2000, 48, 51–56. [Google Scholar] [CrossRef]
- Zhang, L.; Brow, R.K.; Schlesinger, M.E.; Ghussn, L.; Zanotto, E.D. Glass formation from iron-rich phosphate melts. JNCS 2010, 356, 1252–1257. [Google Scholar] [CrossRef]
- Zhang, L.; Schlesinger, M.E.; Brow, R.K. Phase Equilibria in the Fe2O3-P2O5 system. J. Am. Ceram. Soc. 2011, 94, 1605–1610. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieczara, G.; Manecki, M.; Rzepa, G.; Borkiewicz, O.; Gaweł, A. Thermal Stability and Decomposition Products of P-Doped Ferrihydrite. Materials 2020, 13, 4113. https://doi.org/10.3390/ma13184113
Pieczara G, Manecki M, Rzepa G, Borkiewicz O, Gaweł A. Thermal Stability and Decomposition Products of P-Doped Ferrihydrite. Materials. 2020; 13(18):4113. https://doi.org/10.3390/ma13184113
Chicago/Turabian StylePieczara, Gabriela, Maciej Manecki, Grzegorz Rzepa, Olaf Borkiewicz, and Adam Gaweł. 2020. "Thermal Stability and Decomposition Products of P-Doped Ferrihydrite" Materials 13, no. 18: 4113. https://doi.org/10.3390/ma13184113





